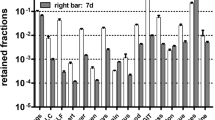
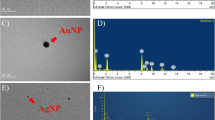
Abstract
Silver (Ag) and its compounds are priority contaminants, for which toxicological effects are well documented, but their toxicokinetics are not fully documented for a proper risk assessment. While the toxicokinetics of insoluble Ag nanoparticles (Ag NPs) was recently documented, there is a lack of data on the kinetic behavior of the soluble form, such as one of the mostly used silver nitrate (AgNO3) form. This study aimed to better document the toxicokinetics of Ag element following inhalation of soluble AgNO3 for comparison with a previous study on the kinetics of inhaled Ag NPs using a similar experimental design. We exposed male Sprague–Dawley rats to AgNO3 during 6 continuous hours (typical of a daily worker exposure) to determine the kinetic time courses of Ag element in blood, tissues, and excreta over a 14-day period post-exposure. Only a small fraction of Ag was found in lungs following the onset of the 6-h inhalation of AgNO3 (on average (± SD) 0.3 ± 0.1% at the end of the 6-h inhalation). Blood profiles of Ag element showed peak levels right after the end of the 6-h inhalation period and levels decreased rapidly thereafter. Toxicokinetic parameter values calculated from the average blood-concentration profiles showed a mean residence time (MRT) of 135 h and mean half-life (t1/2) of 94 h, with AUC of 2.5 mg/L × h and AUMC of 338 mg/L × h2. In terms of percent of inhaled dose, highest levels of Ag in extrapulmonary organs were found in liver, which represented on average (± SD) 1.6 ± 0.6% of calculated inhaled dose followed by the kidney with 0.1 ± 0.08%. Peak levels in the GI tract (including contents) were found at the end of the 6-h inhalation and represented 20 ± 15.6% of the inhaled dose. The dominant excretion route of Ag was through feces. The time course of Ag element in the GI tract and feces following AgNO3 inhalation is also compatible with an intestinal reabsorption of Ag. When compared to results of Ag NPs of a prior study with the same design, this study showed differences in the kinetics of soluble AgNO3 compared to insoluble Ag NPs, with higher levels in blood, GI tract, and extrapulmonary tissues but lower levels in lungs following AgNO3 exposure.



Similar content being viewed by others
References
ACGIH (2021) TLVs and BEIs based on the documentation of the threshold limit values for chemical substances and physical agents & biological exposure indices. American Conference of Governmental Industrial Hygienists, Cincinnati, OH
Andriamasinoro SN, Dieme D, Marie-Desvergne C et al (2021) Kinetic time courses of inhaled silver nanoparticles in rats. Arch Toxicol. https://doi.org/10.1007/s00204-021-03191-0
Arai Y, Miyayama T, Hirano S (2015) Difference in the toxicity mechanism between ion and nanoparticle forms of silver in the mouse lung and in macrophages. Toxicology 328:84–92. https://doi.org/10.1016/j.tox.2014.12.014
DFG (2021) MAK and BAT values list 2021 maximum permissible concentrations at the place of work and biological tolerance values for working materials. German Medical Science, Germany
Drake PL, Hazelwood KJ (2005) Exposure-related health effects of silver and silver compounds: a review. Ann Occup Hyg 49(7):575–585. https://doi.org/10.1093/annhyg/mei019%
Fehaid A, Hamed MF, Abouelmagd MM, Taniguchi A (2016) Time-dependent toxic effect and distribution of silver nanoparticles compared to silver nitrate after intratracheal instillation in rats. J Am J Nanomater 4:12–19
Gibaldi M, Perrier D (1982) Drugs and the pharmaceutical sciences. In: Dekker M (ed) Pharmacokinetics, 2nd edn. Wiley, New York, pp 445–449
Graham UM, Jacobs G, Yokel RA et al (2017) From dose to response: in vivo nanoparticle processing and potential toxicity. Adv Exp Med Biol 947:71–100. https://doi.org/10.1007/978-3-319-47754-1_4
Hadrup N, Lam HR (2014) Oral toxicity of silver ions, silver nanoparticles and colloidal silver - a review. Regul Toxicol Pharm 68(1):1–7
Hayes AW (2007) Principles and methods of toxicology, 5th edn. Taylor & Francis, Boca Raton
Humphreys SD, Routledge PA (1998) The toxicology of silver nitrate. Adverse Drug React Toxicol Rev 17(2–3):115–143
Juling S, Bachler G, von Gotz N et al (2016) In vivo distribution of nanosilver in the rat: the role of ions and de novo-formed secondary particles. Food Chem Toxicol 97:327–335
Keller JG, Graham UM, Koltermann-Jülly J et al (2020) Predicting dissolution and transformation of inhaled nanoparticles in the lung using abiotic flow cells: the case of barium sulfate. Sci Rep 10(1):1–15
Kim JK, Kim HP, Park JD et al (2021) Lung retention and particokinetics of silver and gold nanoparticles in rats following subacute inhalation co-exposure. Part Fibre Toxicol 18(1):5. https://doi.org/10.1186/s12989-021-00397-z
Kone BC, Kaleta M, Gullans SR (1988) Silver ion (Ag+)-Induced increases in cell membrane K+ and Na+ permeability in the renal proximal tubule: reversal by thiol reagents. J Membr Biol 102(1):11–19. https://doi.org/10.1007/BF01875349
Kuethe DO, Behr VC, Begay S (2002) Volume of rat lungs measured throughout the respiratory cycle using 19F NMR of the inert gas SF6. Magn Reson Med 48(3):547–549. https://doi.org/10.1002/mrm.10245
Liu J, Wang Z, Liu FD, Kane AB, Hurt RH (2012) Chemical transformations of nanosilver in biological environments. ACS Nano 6(11):9887–9899. https://doi.org/10.1021/nn303449n
Loeschner K, Hadrup N, Qvortrup K et al (2011) Distribution of silver in rats following 28 days of repeated oral exposure to silver nanoparticles or silver acetate. Part Fibre Toxicol 8:18
Oberdörster G, Kuhlbusch TAJ (2018) In vivo effects: methodologies and biokinetics of inhaled nanomaterials. NanoImpact 10:38–60. https://doi.org/10.1016/j.impact.2017.10.007
Oller AR, Costa M, Oberdörster G (1997) Carcinogenicity assessment of selected nickel compounds. Toxicol Appl Pharmacol 143(1):152–166. https://doi.org/10.1006/taap.1996.8075
Osier M, Oberdörster G (1997) Intratracheal inhalation vs intratracheal instillation: differences in particle effects. J Fundam Appl Toxicol 40(2):220–227
Phalen RF, Morrow PE (1973) Experimental inhalation of metallic silver. J Health Physics 24(5):509–518
Pujalté I, Dieme D, Haddad S, Serventi AM, Bouchard M (2017) Toxicokinetics of titanium dioxide (TiO(2)) nanoparticles after inhalation in rats. Toxicol Lett 265:77–85. https://doi.org/10.1016/j.toxlet.2016.11.014
Ratte HT (1999) Bioaccumulation and toxicity of silver compounds: a review. J Environ Toxicol Chem Int J 18(1):89–108
Recordati C, De Maglie M, Bianchessi S et al (2016) Tissue distribution and acute toxicity of silver after single intravenous administration in mice: nano-specific and size-dependent effects. Part Fibre Toxicol 13:12. https://doi.org/10.1186/s12989-016-0124-x
Rosenman KD, Moss A, Kon S (1979) Argyria: clinical implications of exposure to silver nitrate and silver oxide. J Occup Med 21(6):430–435
Smith JN, Thomas DG, Jolley H et al (2018) All that is silver is not toxic: silver ion and particle kinetics reveals the role of silver ion aging and dosimetry on the toxicity of silver nanoparticles. Part Fibre Toxicol 15(1):47. https://doi.org/10.1186/s12989-018-0283-z
Takenaka S, Karg E, Roth C et al (2001) Pulmonary and systemic distribution of inhaled ultrafine silver particles in rats. Environ Health Perspect 109(Suppl 4):547–551
van der Zande M, Vandebriel RJ, Van Doren E et al (2012) Distribution, elimination, and toxicity of silver nanoparticles and silver ions in rats after 28-day oral exposure. ACS Nano 6(8):7427–7442
Weldon BA, Faustman ME, Oberdorster G et al (2016) Occupational exposure limit for silver nanoparticles: considerations on the derivation of a general health-based value. Nanotoxicology 10(7):945–956
Williams BD, Denhom JA (2009) An assessment of the environmental toxicity of silver iodide-with reference to a cloud seeding trial in the snowy mountains of Australia. J Weather Modif 41(1):75–96
Funding
This work was funded by the Chair in Toxicological Risk Assessment and Management of the University of Montreal.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Andriamasinoro, S.N., Dieme, D., Haddad, S. et al. Toxicokinetics of silver element following inhalation of silver nitrate in rats. Arch Toxicol 97, 663–670 (2023). https://doi.org/10.1007/s00204-022-03424-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-022-03424-w