Abstract
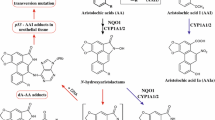
Aristolochic acids (AAs) are a family of natural compounds with AA I and AA II being known carcinogens, whose bioactivation causes DNA adducts formation. However, other congeners have rarely been investigated. This study aimed to investigate genotoxicity of AA IVa, which differs from AA I by a hydroxyl group, abundant in Aristolochiaceae plants. AA IVa reacted with 2'-deoxyadenosine (dA) and 2'-deoxyguanosine (dG) to form three dA and five dG adducts as identified by high-resolution mass spectrometry, among which two dA and three dG adducts were detected in reactions of AA IVa with calf thymus DNA (CT DNA). However, no DNA adducts were detected in the kidney, liver, and forestomach of orally dosed mice at 40 mg/kg/day for 2 days, and bone marrow micronucleus assay also yielded negative results. Pharmacokinetic analyses of metabolites in plasma indicated that AA IVa was mainly O-demethylated to produce a metabolite with two hydroxyl groups, probably facilitating its excretion. Meanwhile, no reduced metabolites were detected. The competitive reaction of AA I and AA IVa with CT DNA, with adducts levels varying with pH of reaction revealed that AA IVa was significantly less reactive than AA I, probably by hydroxyl deprotonation of AA IVa, which was explained by theoretical calculations for reaction barriers, energy levels of the molecular orbits, and charges at the reaction sites. In brief, although it could form DNA adducts in vitro, AA IVa was non-genotoxic in vivo, which was attributed to its low reactivity and biotransformation into an easily excreted metabolite rather than bioactivation.






Similar content being viewed by others
References
Arlt VM, Levová K, Bárta F et al (2011) Role of P450 1A1 and P450 1A2 in bioactivation versus detoxication of the renal carcinogen aristolochic acid I: studies in Cyp1a1-/-, Cyp1a2-/-, and Cyp1a1/1a2-/- mice. Chem Res Toxicol 24(10):1710–1719. https://doi.org/10.1021/tx200259y
Balachandran P, Wei F, Lin RC, Khan IA, Pasco DS (2005) Structure activity relationships of aristolochic acid analogues: toxicity in cultured renal epithelial cells. Kidney Int 67(5):1797–1805. https://doi.org/10.1111/j.1523-1755.2005.00277.x
Chan W, Cui L, Xu G, Cai Z (2006) Study of the phase I and phase II metabolism of nephrotoxin aristolochic acid by liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom 20(11):1755–1760. https://doi.org/10.1002/rcm.2513
Chan CK, Liu Y, Pavlovic N, Chan W (2019) Aristolochic acids: newly identified exposure pathways of this class of environmental and food-borne contaminants and its potential link to chronic kidney diseases. Toxics 7(1):14. https://doi.org/10.3390/toxics7010014
Chen CH, Dickman KG, Moriya M et al (2012) Aristolochic acid-associated urothelial cancer in Taiwan. Proc Natl Acad Sci USA 109(21):8241–8246. https://doi.org/10.1073/pnas.1119920109
Chen R, Zhou C, Cao Y et al (2020b) Assessment of Pig-a, micronucleus, and comet assay endpoints in Tg RasH2 mice carcinogenicity study of aristolochic acid I. Environ Mol Mutagen 61(2):266–275. https://doi.org/10.1002/em.22325
Chen R, You X, Cao Y et al (2020a) Benchmark dose analysis of multiple genotoxicity endpoints in gpt delta mice exposed to aristolochic acid I. Mutagenesis. https://doi.org/10.1093/mutage/geaa034
Dedı Ková A, Bárta F, Martínek V et al (2020) In vivo metabolism of aristolochic acid I and II in rats is influenced by their coexposure. Chem Res Toxicol 33(11):2804–2818. https://doi.org/10.1021/acs.chemrestox.0c00198
Fan Y, Li Z, Xi J (2020) Recent developments in detoxication techniques for aristolochic acid-containing traditional Chinese medicines. RSC Adv 10(3):1410–1425. https://doi.org/10.1039/C9RA08327H
Frisch MJ, Trucks G, Schlegel HB et al (2009) Gaussian 09 (Revision A.1). Gaussian Inc, Wallingford CT
Fukui K (1981) The path of chemical reactions—the IRC approach. Acc Chem Res. https://doi.org/10.1021/ar00072a001
Goodenough AK, Schut HA, Turesky RJ (2007) Novel LC-ESI/MS/MS(n) method for the characterization and quantification of 2’-deoxyguanosine adducts of the dietary carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b] pyridine by 2-D linear quadrupole ion trap mass spectrometry. Chem Res Toxicol 20(2):263–276. https://doi.org/10.1021/tx0601713
Gupta R (1996) 32P-Postlabeling for detection of DNA adducts. In: Pfeifer GP (ed) Technologies for detection of DNA damage and mutations. Springer US, Boston
Heinrich M, Chan J, Wanke S, Neinhuis C, Simmonds MS (2009) Local uses of Aristolochia species and content of nephrotoxic aristolochic acid 1 and 2–a global assessment based on bibliographic sources. J Ethnopharmacol 125(1):108–144. https://doi.org/10.1016/j.jep.2009.05.028
IARC (2012) Pharmaceuticals volume 100 A. A review of human carcinogens. IARC Monogr Eval Carcinog Risks Hum 100(Pt A):1–401
Ji HJ, Li JY, Wu SF et al (2020) Two new aristolochic acid analogues from the roots of Aristolochia contorta with significant cytotoxic activity. Molecules 26(1):44–55. https://doi.org/10.3390/molecules26010044
Kathuria P, Sharma P, Abendong MN, Wetmore SD (2015) Conformational preferences of DNA following damage by aristolochic acids: structural and energetic insights into the different mutagenic potential of the ALI and ALII-N(6)-dA adducts. Biochemistry 54(15):2414–2428. https://doi.org/10.1021/bi501484m
Klaus V, Bastek H, Damme K et al (2017) Time-matched analysis of DNA adduct formation and early gene expression as predictive tool for renal carcinogenesis in methylazoxymethanol acetate treated Eker rats. Arch Toxicol 91(10):3427–3438. https://doi.org/10.1007/s00204-017-1953-6
Kupchan SM, Merianos JJ (1968) The isolation and structural elucidation of novel derivatives of aristolochic acid from Aristolochia indica. J Org Chem 33(10):3735–3738. https://doi.org/10.1021/jo01274a011
Laing C, Hamour S, Sheaff M, Miller R, Woolfson R (2006) Chinese herbal uropathy and nephropathy. Lancet 368(9532):338. https://doi.org/10.1016/S0140-6736(06)69079-X
Liu Y, Chan CK, Jin L, Wong SK, Chan W (2019) Quantitation of DNA adducts in target and nontarget organs of aristolochic acid I-exposed rats: correlating DNA adduct levels with organotropic activities. Chem Res Toxicol 32(3):397–399. https://doi.org/10.1021/acs.chemrestox.8b00359
Marenich AV, Cramer CJ, Truhlar DG (2009) Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J Phys Chem B 113(18):6378–6396. https://doi.org/10.1021/jp810292n
Michl J, Jennings HM, Kite GC, Ingrouille MJ, Simmonds MS, Heinrich M (2013) Is aristolochic acid nephropathy a widespread problem in developing countries? A case study of Aristolochia indica L. in Bangladesh using an ethnobotanical-phytochemical approach. J Ethnopharmacol 149(1):235–244. https://doi.org/10.1016/j.jep.2013.06.028
Michl J, Ingrouille MJ, Simmonds MS, Heinrich M (2014) Naturally occurring aristolochic acid analogues and their toxicities. Nat Prod Rep 31(5):676–693. https://doi.org/10.1039/c3np70114j
Michl J, Kite GC, Wanke S et al (2016) LC–MS- and (1)H NMR-based metabolomic analysis and in vitro toxicological assessment of 43 Aristolochia species. J Nat Prod 79(1):30–37. https://doi.org/10.1021/acs.jnatprod.5b00556
Michl J, Bello O, Kite GC, Simmonds MSJ, Heinrich M (2017) Medicinally used Asarum Species: high-resolution LC-MS analysis of aristolochic acid analogs and in vitro toxicity screening in HK-2 cells. Front Pharmacol 8:215. https://doi.org/10.3389/fphar.2017.00215
Nair AB, Jacob S (2016) A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm 7(2):27–31. https://doi.org/10.4103/0976-0105.177703
Nortier JL, Martinez MC, Schmeiser HH et al (2000) Urothelial carcinoma associated with the use of a Chinese herb (Aristolochia fangchi). N Engl J Med 342(23):1686–1692. https://doi.org/10.1056/NEJM200006083422301
Pailer M, Bergthaller P, Schaden G (1965) Über die Isolierung und Charakterisierung von vier neuen Aristolochiasäuren (aus Aristolochia clematitis L.). Monatsh Chem Verw Teile Anderer Wiss 96(3):863–883. https://doi.org/10.1007/BF00919160
Petrescu AM, Lukinich-Gruia AT, Paunescu V, Ilia G (2019) A theoretical study of the molecular coupled structures of aristolochic acids and humic acid. Potential Environ Contam Chem Biodivers. https://doi.org/10.1002/cbdv.201900406
Sato N, Takahashi D, Chen SM et al (2004) Acute nephrotoxicity of aristolochic acids in mice. J Pharm Pharmacol 56(2):221–229. https://doi.org/10.1211/0022357023051
Schmeiser HH, Frei E, Wiessler M, Stiborova M (1997) Comparison of DNA adduct formation by aristolochic acids in various in vitro activation systems by 32P-post-labelling: evidence for reductive activation by peroxidases. Carcinogenesis 18(5):1055–1062. https://doi.org/10.1093/carcin/18.5.1055
Shibutani S, Bonala RR, Rosenquist T et al (2010) Detoxification of aristolochic acid I by O-demethylation: less nephrotoxicity and genotoxicity of aristolochic acid Ia in rodents. Int J Cancer 127(5):1021–1027. https://doi.org/10.1002/ijc.25141
Sidorenko VS, Yeo JE, Bonala RR, Johnson F, Schärer OD, Grollman AP (2012) Lack of recognition by global-genome nucleotide excision repair accounts for the high mutagenicity and persistence of aristolactam-DNA adducts. Nucleic Acids Res 40(6):2494–2505. https://doi.org/10.1093/nar/gkr1095
Sidorenko VS, Attaluri S, Zaitseva I et al (2014) Bioactivation of the human carcinogen aristolochic acid. Carcinogenesis 35(8):1814–1822. https://doi.org/10.1093/carcin/bgu095
Stiborová M, Levová K, Bárta F et al (2012) Bioactivation versus detoxication of the urothelial carcinogen aristolochic acid I by human cytochrome P450 1A1 and 1A2. Toxicol Sci 125(2):345–358. https://doi.org/10.1093/toxsci/kfr306
Stiborová M, Arlt VM, Schmeiser HH (2017) DNA adducts formed by aristolochic acid are unique biomarkers of exposure and explain the initiation phase of upper urothelial cancer. Int J Mol Sci 18(10):2144. https://doi.org/10.3390/ijms18102144
Vanherweghem JL, Depierreux M, Tielemans C et al (1993) Rapidly progressive interstitial renal fibrosis in young women: association with slimming regimen including Chinese herbs. Lancet 341(8842):387–391. https://doi.org/10.1016/0140-6736(93)92984-2
Wen YJ, Su T, Tang JW et al (2006) Cytotoxicity of phenanthrenes extracted from Aristolochia contorta in human proximal tubular epithelial cell line. Nephron Exp Nephrol 103(3):e95–e102. https://doi.org/10.1159/000092194
Xing G, Qi X, Chen M et al (2012) Comparison of the mutagenicity of aristolochic acid I and aristolochic acid II in the gpt delta transgenic mouse kidney. Mutat Res 743(1–2):52–58. https://doi.org/10.1016/j.mrgentox.2011.12.021
Xu YQ, Li XW, Liu GX et al (2013) Comparative study of the contents of analogues of aristolochic acid in two kinds of Aristolochiae fructus by high-performance liquid chromatography. J Nat Med 67(1):113–122. https://doi.org/10.1007/s11418-012-0664-9
Yuan JB, Huang Q, Ren G et al (2014) Acute and subacute toxicity of the extract of Aristolochiae fructus and honey-fried Aristolochiae fructus in rodents. Biol Pharm Bull 37(3):387–393. https://doi.org/10.1248/bpb.b13-00736
Yuan J, Ren G, Liang J et al (2017) Comparative studies on the multi-component pharmacokinetics of Aristolochiae fructus and honey-fried Aristolochiae fructus extracts after oral administration in rats. BMC Complement Altern Med 17(1):107. https://doi.org/10.1186/s12906-017-1626-2
Yun BH, Rosenquist TA, Sidorenko V et al (2012) Biomonitoring of aristolactam-DNA adducts in human tissues using ultra-performance liquid chromatography/ion-trap mass spectrometry. Chem Res Toxicol 25(5):1119–1131. https://doi.org/10.1021/tx3000889
Zhang C, Wang X, Shang M et al (2006) Simultaneous determination of five aristolochic acids and two aristololactams in Aristolochia plants by high-performance liquid chromatography. Biomed Chromatogr 20(4):309–318. https://doi.org/10.1002/bmc.565
Zhang J, Chan CK, Ham YH, Chan W (2020) Identifying cysteine, N-Acetylcysteine, and glutathione conjugates as novel metabolites of aristolochic acid I: emergence of a new detoxification pathway. Chem Res Toxicol 33(6):1374–1381. https://doi.org/10.1021/acs.chemrestox.9b00488
Zhao Y, Truhlar D (2008) The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06 functionals and 12 other functionals. Theoret Chem Acc 120:215–241. https://doi.org/10.1007/s00214-007-0310-x
Zuo L, Yao S, Wang W, Duan W (2008) An efficient method for demethylation of aryl methyl ethers. Tetrahedron Lett 49:4054–4056. https://doi.org/10.1016/j.tetlet.2008.04.070
Acknowledgements
We appreciate Dr. Tianpei Xie and Dr. Yong Qian for their helpful discussions and constructive suggestions. We thank Shanghai Standard Technology Co., Ltd. for providing the AA I and AA IVa.
Funding
This study was supported by the grants from the National Natural Science Foundation of China (Grant No. 81873081).
Author information
Authors and Affiliations
Contributions
JW performed experiment; collected and analyzed data; wrote the initial draft and revised the manuscript; prepared and created the published work. YL conceived and supervised the project; designed methodology (biology studies); verified the replication and reproducibility of results; reviewed and edited the manuscript; funding acquisition. XZ supervised the project; designed methodology (analytical chemistry studies); verified the replication and reproducibility of results; reviewed and edited the manuscript. ZY performed high-resolution mass spectrometric data collection in our studies. RC, JX, and YC participated in the experimental operation. YC performed density functional theory calculations of the study.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The study design has been approved by the appropriate ethics committee and does not contain clinical studies or patient data.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wan, J., Chen, R., Yang, Z. et al. Aristolochic acid IVa forms DNA adducts in vitro but is non-genotoxic in vivo. Arch Toxicol 95, 2839–2850 (2021). https://doi.org/10.1007/s00204-021-03077-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-021-03077-1