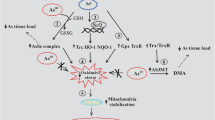
Abstract
Manifestation of methylmercury (MeHg) toxicity depends on individual susceptibility to MeHg, as well as MeHg burden level. Therefore, biomarkers that reflect the protective capacity against MeHg are needed. The critical role of oxidative stress in the pathogenesis of MeHg cytotoxicity has been demonstrated. Because MeHg has high affinity for selenohydryl groups, sulfhydryl groups, and selenides, and causes posttranscriptional defects in selenoenzymes, proteins with selenohydryl and sulfhydryl groups should play a critical role in mediating MeHg-induced oxidative stress. Here, plasma oxidative stress markers and selenoproteins were investigated in MeHg-intoxicated rats showing neuropathological changes after 4 weeks of MeHg exposure. The thiol antioxidant barrier (–SHp) level significantly decreased 2 weeks after MeHg exposure, which is an early stage at which no systemic oxidative stress, histopathological changes, or clinical signs were detected. Diacron reactive oxidant metabolite (d-ROM) levels significantly increased 3 weeks after MeHg exposure, indicating the occurrence of systemic oxidative stress. Rats treated with lead acetate or cadmium chloride showed no changes in levels of –SHp and d-ROM. Selenoprotein P1 abundance significantly decreased in MeHg-treated rats, whereas it significantly increased in rats treated with Pb or Cd. Plasma selenium-dependent glutathione peroxidase (GPx3) activity also significantly decreased after MeHg exposure, whereas plasma non-selenoenzyme glutathione reductase activity significantly increased in MeHg-treated rats. The results suggest that decreased capacity of –SHp and selenoproteins (GPx3 and selenoprotein P) can be useful biomarkers of ongoing MeHg cytotoxicity and the individual protective capacity against the MeHg body burden.







Similar content being viewed by others
References
Abdalla FH, Cardoso AM, Pereira LB et al (2013) Neuroprotective effect of quercetin in ectoenzymes and acetylcholinesterase activities in cerebral cortex synaptosomes of cadmium-exposed rats. Mol Cell Biochem 381(1–2):1–8. doi:10.1007/s11010-013-1659-x
Alcaraz-Contreras Y, Garza-Ocanas L, Carcano-Diaz K, Ramirez-Gomez XS (2011) Effect of glycine on lead mobilization, lead-induced oxidative stress, and hepatic toxicity in rats. J Toxicol 2011:430539. doi:10.1155/2011/430539
Burk RF, Hill KE (2005) Selenoprotein P: an extracellular protein with unique physical characteristics and a role in selenium homeostasis. Annu Rev Nutr 25:215–235. doi:10.1146/annurev.nutr.24.012003.132120
Burk RF, Hill KE (2009) Selenoprotein P-expression, functions, and roles in mammals. Biochim Biophys Acta 1790(11):1441–1447. doi:10.1016/j.bbagen.2009.03.026
Chen J, Berry MJ (2003) Selenium and selenoproteins in the brain and brain diseases. J Neurochem 86(1):1–12
Chen J, Shaikh ZA (2009) Activation of Nrf2 by cadmium and its role in protection against cadmium-induced apoptosis in rat kidney cells. Toxicol Appl Pharmacol 241(1):81–89. doi:10.1016/j.taap.2009.07.038
Ferramola ML, Anton RI, Anzulovich AC, Gimenez MS (2011) Myocardial oxidative stress following sub-chronic and chronic oral cadmium exposure in rats. Environ Toxicol Pharmacol 32(1):17–26. doi:10.1016/j.etap.2011.03.002
Fujimura M, Usuki F (2014) Low in situ expression of antioxidative enzymes in rat cerebellar granular cells susceptible to methylmercury. Arch Toxicol 88(1):109–113. doi:10.1007/s00204-013-1089-2
Fujimura M, Usuki F, Kawamura M, Izumo S (2011) Inhibition of the Rho/ROCK pathway prevents neuronal degeneration in vitro and in vivo following methylmercury exposure. Toxicol Appl Pharmacol 250(1):1–9. doi:10.1016/j.taap.2010.09.011
Gurer H, Ozgunes H, Neal R, Spitz DR, Ercal N (1998) Antioxidant effects of N-acetylcysteine and succimer in red blood cells from lead-exposed rats. Toxicology 128(3):181–189
Harada M, Akagi H, Tsuda T, Kizaki T, Ohno H (1999) Methylmercury level in umbilical cords from patients with congenital Minamata disease. Sci Total Environ 234(1–3):59–62
Hunter D, Russell DS (1954) Focal cerebellar and cerebellar atrophy in a human subject due to organic mercury compounds. J Neurol Neurosurg Psychiatry 17(4):235–241
Imura N (1986) The role of micronutrient, selenium, in the manifestation of toxicity of heavy metals. Dev Toxicol Environ Sci 12:115–123
Jones DP, Carlson JL, Mody VC, Cai J, Lynn MJ, Sternberg P (2000) Redox state of glutathione in human plasma. Free Radic Biol Med 28(4):625–635
Kiran Kumar B, Prabhakara Rao Y, Noble T et al (2009) Lead-induced alteration of apoptotic proteins in different regions of adult rat brain. Toxicol Lett 184(1):56–60. doi:10.1016/j.toxlet.2008.10.023
Komatsu F, Kudoh H, Kagawa Y (2007) Evaluation of oxidative stress and effectiveness of low-dose glucocorticoid therapy on exacerbation of chronic obstructive pulmonary disease. J Gerontol A Biol Sci Med Sci 62(4):459–464
Leinonen JS, Ahonen JP, Lonnrot K et al (2000) Low plasma antioxidant activity is associated with high lesion volume and neurological impairment in stroke. Stroke 31(1):33–39
Meister A (1991) Glutathione deficiency produced by inhibition of its synthesis, and its reversal; applications in research and therapy. Pharmacol Ther 51(2):155–194
Moriarty PM, Reddy CC, Maquat LE (1998) Selenium deficiency reduces the abundance of mRNA for Se-dependent glutathione peroxidase 1 by a UGA-dependent mechanism likely to be nonsense codon-mediated decay of cytoplasmic mRNA. Mol Cell Biol 18(5):2932–2939
Nkabyo YS, Gu LH, Jones DP, Ziegler TR (2006) Thiol/disulfide redox status is oxidized in plasma and small intestinal and colonic mucosa of rats with inadequate sulfur amino acid intake. J Nutr 136(5):1242–1248
Nobunaga T, Satoh H, Suzuki T (1979) Effects of sodium selenite on methylmercury embryotoxicity and teratogenicity in mice. Toxicol Appl Pharmacol 47(1):79–88
Park ST, Lim KT, Chung YT, Kim SU (1996) Methylmercury-induced neurotoxicity in cerebral neuron culture is blocked by antioxidants and NMDA receptor antagonists. Neurotoxicology 17(1):37–45
Partl S, Herbst H, Schaeper F, Mohnhaupt A, Stoltenburg-Didinger G (1998) GFAP gene expression is altered in young rats following developmental low level lead exposure. Neurotoxicology 19(4–5):547–551
Sakamoto M, Yasutake A, Kakita A et al (2013) Selenomethionine protects against neuronal degeneration by methylmercury in the developing rat cerebrum. Environ Sci Technol 47(6):2862–2868. doi:10.1021/es304226h
Satoh H, Yasuda N, Shimai S (1985) Development of reflexes in neonatal mice prenatally exposed to methylmercury and selenite. Toxicol Lett 25(2):199–203
Selvin-Testa A, Loidl CF, Lopez-Costa JJ, Lopez EM, Pecci-Saavedra J (1994) Chronic lead exposure induces astrogliosis in hippocampus and cerebellum. Neurotoxicology 15(2):389–401
Shagirtha K, Muthumani M, Prabu SM (2011) Melatonin abrogates cadmium induced oxidative stress related neurotoxicity in rats. Eur Rev Med Pharmacol Sci 15(9):1039–1050
Shanker G, Aschner M (2003) Methylmercury-induced reactive oxygen species formation in neonatal cerebral astrocytic cultures is attenuated by antioxidants. Brain Res Mol Brain Res 110(1):85–91
Soltaninejad K, Kebriaeezadeh A, Minaiee B et al (2003) Biochemical and ultrastructural evidences for toxicity of lead through free radicals in rat brain. Hum Exp Toxicol 22(8):417–423
Sugiura Y, Tamai Y, Tanaka H (1978) Selenium protection against mercury toxicity: high binding affinity of methylmercury by selenium-containing ligands in comparison with sulfur-containing ligands. Bioinorg Chem 9(2):167–180
Tandon SK, Singh S, Prasad S et al (2003) Reversal of cadmium induced oxidative stress by chelating agent, antioxidant or their combination in rat. Toxicol Lett 145(3):211–217
Tanito M, Kaidzu S, Takai Y, Ohira A (2012) Status of systemic oxidative stresses in patients with primary open-angle glaucoma and pseudoexfoliation syndrome. PLoS ONE 7(11):e49680. doi:10.1371/journal.pone.0049680
Turell L, Carballal S, Botti H, Radi R, Alvarez B (2009) Oxidation of the albumin thiol to sulfenic acid and its implications in the intravascular compartment. Braz J Med Biol Res 42(4):305–311
Usuki F, Ishiura S (1998) Expanded CTG repeats in myotonin protein kinase increase susceptibility to oxidative stress. NeuroReport 9(10):2291–2296
Usuki F, Takahashi N, Sasagawa N, Ishiura S (2000) Differential signaling pathways following oxidative stress in mutant myotonin protein kinase cDNA-transfected C2C12 cell lines. Biochem Biophys Res Commun 267(3):739–743. doi:10.1006/bbrc.1999.2026
Usuki F, Yasutake A, Umehara F et al (2001) In vivo protection of a water-soluble derivative of vitamin E, Trolox, against methylmercury-intoxication in the rat. Neurosci Lett 304(3):199–203
Usuki F, Yasutake A, Umehara F, Higuchi I (2004) Beneficial effects of mild lifelong dietary restriction on skeletal muscle: prevention of age-related mitochondrial damage, morphological changes, and vulnerability to a chemical toxin. Acta Neuropathol 108(1):1–9. doi:10.1007/s00401-004-0844-0
Usuki F, Fujita E, Sasagawa N (2008) Methylmercury activates ASK1/JNK signaling pathways, leading to apoptosis due to both mitochondria- and endoplasmic reticulum (ER)-generated processes in myogenic cell lines. Neurotoxicology 29(1):22–30. doi:10.1016/j.neuro.2007.08.011
Usuki F, Yamashita A, Fujimura M (2011) Post-transcriptional defects of antioxidant selenoenzymes cause oxidative stress under methylmercury exposure. J Biol Chem 286(8):6641–6649. doi:10.1074/jbc.M110.168872
Yee S, Choi BH (1994) Methylmercury poisoning induces oxidative stress in the mouse brain. Exp Mol Pathol 60(3):188–196. doi:10.1006/exmp.1994.1017
Yee S, Choi BH (1996) Oxidative stress in neurotoxic effects of methylmercury poisoning. Neurotoxicology 17(1):17–26
Acknowledgments
This work was partly supported by the Japan Society for the Promotion of Science KAKENHI (Grant No. 25460402). The authors thank Ms. Kyoko Yoshihara, Ayumi Onitsuka, Eri Kawano, Michiko Fuchigami, and Miwa Matsunaga for excellent technical assistance.
Conflict of interest
The authors declare that there are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Usuki, F., Fujimura, M. Decreased plasma thiol antioxidant barrier and selenoproteins as potential biomarkers for ongoing methylmercury intoxication and an individual protective capacity. Arch Toxicol 90, 917–926 (2016). https://doi.org/10.1007/s00204-015-1528-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-015-1528-3