Abstract
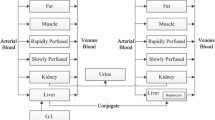
Multiple oximes have been synthesized and evaluated for use as countermeasures against chemical warfare nerve agents. The current U.S. military and civilian oxime countermeasure, 2-[(hydroxyimino)methyl]-1-methylpyridin-1-ium chloride (2-PAM), is under consideration for replacement with a more effective acetylcholinesterase reactivator, 1,1’-methylenebis{4-hydroxyiminomethyl}pyridinium dimethanesulfonate (MMB-4). Kinetic data in the scientific literature for MMB-4 are limited; therefore, a physiologically based pharmacokinetic (PBPK) model was developed for a structurally related oxime, 1,1’-trimethylenebis{4-hydroximinomethyl}pyridinium dibromide. Based on a previous model structure for the organophosphate diisopropylfluorophosphate, the model includes key sites of acetylcholinesterase inhibition (brain and diaphragm), as well as fat, kidney, liver, rapidly perfused tissues and slowly perfused tissues. All tissue compartments are diffusion limited. Model parameters were collected from the literature, predicted using quantitative structure–property relationships or, when necessary, fit to available pharmacokinetic data from the literature. The model was parameterized using rat plasma, tissue and urine time course data from intramuscular administration, as well as human blood and urine data from intravenous and intramuscular administration; sensitivity analyses were performed. The PBPK model successfully simulates rat and human data sets and has been evaluated by predicting intravenous mouse and intramuscular human data not used in the development of the model. Monte Carlo analyses were performed to quantify human population kinetic variability in the human evaluation data set. The model identifies potential pharmacokinetic differences between rodents and humans, indicated by differences in model parameters between species. The PBPK model can be used to optimize the dosing regimen to improve oxime therapeutic efficacy in a human population.













Similar content being viewed by others
References
Arms AD, Travis CC (1988) Reference physiological parameters in pharmacokinetic modeling. U.S. Environmental Protection Agency, Office of Health and Environmental Assessment, Washington, DC, EPA/600/6-88/004, NTIS PB88-196019
Bosse JA, Wassermann O (1970) On the blood content of guinea-pig tissues. Pharmacology 4:273–277
Brown RP, Delp MD, Lindstedt SL, Rhomberg LR, Beliles RP (1997) Physiological parameter values for physiologically based pharmacokinetic models. Toxicol Ind Health 13:407–484
Calesnick B, Christensen RM (1967) Human toxicity of various oximes. 2-Pyridine aldoxime methyl chloride, its methane sulfonate salt, and 1,1’-trimethylenebis-(4-formylpyridinium chloride). Arch Environ Health 15:599–608
CHEMM (2011) Nerve agent treatment—autoinjector instructions. U.S. Department of Health and Human Services, Chemical Hazards Emergency Medical Management, Washington, DC. Updated 25 Jun 2011. Accessed 9 Aug 2012. http://chemm.nlm.nih.gov/antidote_nerveagents.htm
Chen K, Seng KY (2012) Calibration and validation of a physiologically based model for Soman intoxication in the rat, marmoset, guinea pig and pig. J Appl Toxicol 32(9):673–686
Choi MK, Song IS (2008) Organic cation transporters and their pharmacokinetic and pharmacodynamic consequences. Drug Metab Pharmacokinet 23:243–253
Clewell HJ, Gentry PR, Gearhart JM, Allen BC, Andersen ME (2001) Comparison of cancer risk estimates for vinyl chloride using animal and human data with a PBPK model. Sci Total Environ 274:37–66
Copp SW, Hageman KS, Behnke BJ, Poole DC, Musch TI (2010) Effects of Type II diabetes on exercising skeletal muscle blood flow in the rat. J Appl Physiol 109:1347–1353
Covington TR, Robinan GP, Van Landingham CB, Andersen ME, Kester JE, Clewell HJ (2007) The use of Markov chain Monte Carlo uncertainty analysis to support a public health goal for perchloroethylene. Regul Toxicol Pharmacol 47:1–18
Craigmill AL (2003) A physiologically based pharmacokinetic model for oxytetracycline residues in sheep. J Vet Pharmacol Therap 26(1):55–63
Davies B, Morris T (1993) Physiological parameters in laboratory animals and humans. Pharm Res 10:1093–1095
de Miranda P, Feitknecht UF, Gibbon SL, Burrows GE, Way JL (1972) Urinary metabolite of 1, 1’-trimethylene-bis (4-aldoximinopyridinium) dibromide (TMB-4) in the rat. I. Trimethylene-1-(4-aldoximinopyridinium)-1’-(4-cyanopyridinium) dibromide. J Pharmacol Exp Ther 180:435–445
Dresser MJ, Gray AT, Giacomini KM (2000) Kinetic and selectivity differences between rodent, rabbit, and human organic cation transporters (OCT1). J Pharmacol Exp Ther 292(3):1146–1152
Eddleston M, Buckley NA, Eyer P, Dawson AH (2008) Management of acute organophosphorus pesticide poisoning. Lancet 371:597–607
Eyer P (2003) The role of oximes in the management of organophosphorus pesticide poisoning. Toxicol Rev 22:165–190
Eyer PA, Worek F (2007) Oximes. In: Marrs TC, Maynard RL, Sidell FR (eds) Chemical warfare agents toxicology and treatment, 2nd edn. Wiley, West Sussex, pp 305–329
Firemark H, Barlow CF, Roth LJ (1964) The penetration of 2-PAM-C14 into brain and the effect of cholinesterase inhibitors on its transport. J Pharmacol Exp Ther 145:252–265
Garrigue H, Maurizis JC, Madelmont JC, Nicolas C, Meyniel JM, Louvel A, Demerseman P, Sentenac-Roumanou H, Veyre A (1991) Disposition and metabolism of acetylcholinesterase reactivators 2PAM-I, TMB4 and R665 in rats submitted to organophosphate poisoning. Xenobiotica 21:583–595
Gearhart JM, Jepson GW, Clewell HJ, Andersen ME, Conolly RB (1990) Physiologically based pharmacokinetic and pharmacodynamic model for the inhibition of acetylcholinesterase by diisopropylfluorophosphate. Toxicol Appl Pharmacol 106:295–310
Gearhart JM, Jepson GW, Clewell HJ, Andersen ME, Conolly RB (1994) Physiologically based pharmacokinetic model for the inhibition of acetylcholinesterase by organophosphate esters. Environ Health Perspect 102(suppl 11):51–60
Joosen MJ, van der Schans MJ, van Dijk CG, Kuijpers WC, Wortelboer HM, van Helden HP (2011) Increasing oxime efficacy by blood-brain barrier modulation. Toxicol Lett 206:67–71
Kayouka M, Houze P, Baud FJ, Cisternino S, Debray M, Risede P, Schinkel AH, Warnet JM (2011) Does modulation of organic cation transporters improve pralidoxime activity in an animal model of organophosphate poisoning? Crit Care Med 39:803–811
Kohn MC (2002) Use of sensitivity analysis to assess reliability of metabolic and physiological models. Risk Anal 22:623–631
Koplovitz I, Schulz SM, Railer RF, Sigler M, Lee RB (2007) Effect of atropine and diazepam on the efficacy of oxime treatment of nerve agent intoxication. J Med CBR Def 5:1–15
Kovarik Z, Calic M, Sinko G, Bosak A (2007) Structure-activity approach in the reactivation of tabun-phosphorylated human acetylcholinesterase with bispyridinium para-aldoximes. Arh Hig Rada Toksikol 58:201–209
Loizou G, Spendiff M, Barton HA, Bessems J, Bois FY, d’Yvoire MB, Buist H, Clewell HJ, Meek B, Gundert-Remy U, Goerlitz G, Schmitt W (2008) Development of good modelling practice for physiologically based pharmacokinetic models for use in risk assessment: the first steps. Regul Toxicol Pharmacol 50:400–411
Lundy PM, Hand BT, Broxup BR, Yipchuck G, Hamilton MG (1990) Distribution of the bispyridinium oxime 14C HI-6 in male and female rats. Arch Toxicol 64:377–382
Lundy PM, Hamilton MG, Sawyer TW, Mikler J (2011) Comparative protective effects of HI-6 and MMB-4 against organophosphorous nerve agent poisoning. Toxicology 285:90–96
Maurizis JC, Ollier M, Nicolas C, Madelmont JC, Garrigue H, Veyre A (1992) In vitro binding of oxime acetylcholinesterase reactivators to proteoglycans synthesized by cultured chondrocytes and fibroblasts. Biochem Pharmacol 44:1927–1933
Maxwell DM, Brecht KM (2001) Carboxylesterase: specificity and spontaneous reactivation of an endogenous scavenger for organophosphorus compounds. J Appl Toxicol 21(suppl 1):S103–S107
Milic B, Maksimovic M, Nedelijkovic M (1996) Trimedoxime and HI-6: kinetic comparison after intravenous administration to mice. Pharmacol Toxicol 78:269–272
Miller DS, Bauer B, Hartz AM (2008) Modulation of P-glycoprotein at the blood-brain barrier: opportunities to improve central nervous system pharmacotherapy. Pharmacol Rev 60(2):196–209
Morgan RL, Burrows GE, Yen MH, Way JL (1982) Metabolic disposition of the alkylphosphate antagonist, 1,1’-trimethylenebis(4-aldoximinopyridinium) ion (TMB-4), in the rat. III. Trimethylene-1-(4-aldoximinopyridinium)-1’-(4-carboxamidopyridinium) ion. Drug Metab Dispos 10:491–494
Newmark J (2009) Nerve agents. In: Dobb MR (ed) Clinical neurotoxicology: syndromes, substances, environments. Saunders Elsevier, Philadelphia, PA, pp 646–659
Office of the Campus Veterinarian (OCV) (2011) Guidelines for handling, restraint, injections and blood collection from small laboratory animals. Washington State University, Pullman WA. http://campusvet.wsu.edu/infofac/handling.htm. Accessed 3 Aug 2011
Rahal A, Malik JK (2011) Pharmacokinetics, urinary excretion and plasma protein binding of pralidoxime in goats. Sm Rumin Res 95:179–183
Saxena A, Luo C, Chilukuri N, Maxwell DM, Doctor BP (2008) Novel approaches to medical protection against chemical warfare nerve agents. In: Romano JA, Lukey BJ, Salem H (eds) Chemical warfare agents: chemistry, pharmacology, toxicology and therapeutics. CRC Press, New York, pp 145–173
Schmitt W (2008) General approach for the calculation of tissue to plasma partition coefficients. Toxicol In Vitro 22:457–467
Shih TM, Skovira JW, O’Donnell JC, McDonough JH (2009) Evaluation of nine oximes on in vivo reactivation of blood, brain, and tissue cholinesterase activity inhibited by organophosphorus nerve agents at lethal dose. Toxicol Mech Methods 19:386–400
Shu Y (2011) Research progress in the organic cation transporters. Zhong Nan Da Xue Xue Bao Yi Xue Ban 36(10):913–926
Sidell FR, Groff WA (1971) Intramuscular and intravenous administration of small doses of 2-pyridinium aldoxime methochloride to man. J Pharm Sci 60:1224–1228
Srivastava AK, Malik JK (1988) Biodistribution and plasma protein binding of 2-formyl-1-methyl pyridinium methiodide (2-PAM) in sheep (Ovis aries). Indian J Exp Biol 26:643–644
Stemler FW, Tezak-Reid TM, McCluskey MP, Kaminskis A, Corcoran KD, Shih ML, Stewart JR, Wade JV, Hayward IJ (1991) Pharmacokinetics and pharmacodynamics of oximes in unanesthetized pigs. Fundam Appl Toxicol 16:548–558
Swartz RD, Sidell FR (1974) Renal tubular secretion of pralidoxime in man. Proc Soc Exp Biol Med 146:419–424
USAF (1996) Self aid and buddy care instructor handbook. Air Force Handbook 36-2218, vol 1. United States Air Force, Washington, DC. http://www.e-publishing.af.mil/shared/media/epubs/AFH36-2218V1.pdf
Voicu VA, Bajgar J, Medvedovici A, Radulescu FS, Miron DS (2010) Pharmacokinetics and pharmacodynamics of some oximes and associated therapeutic consequences: a critical review. J Appl Toxicol 30:719–729
Vojvodic VB (1970) Blood levels, urinary excretion and potential toxicity of N, N’-trimethylenebis (pyridinium-4-aldoxime) dichloride (TMB-4) in healthy man following intramuscular injection of the oxime. Pharmacol Clin 2:216–220
Worek F, Szinicz L, Eyer P, Thiermann H (2005) Evaluation of oxime efficacy in nerve agent poisoning: development of a kinetic-based dynamic model. Toxicol Appl Pharmacol 209:193–202
Worek F, Wille T, Aurbek N, Eyer P, Thiermann H (2010) Reactivation of organophosphate-inhibited human, Cynomolgus monkey, swine and guinea pig acetylcholinesterase by MMB-4: a modified kinetic approach. Toxicol Appl Pharmacol 249(3):231–237
Wu AHB (2006) Tietz clinical guide to laboratory tests, 4th edn. Saunders Elsevier, St. Louis, MO
Yang F, Liu HW, Li M, Ding HZ, Huang XH, Zeng ZL (2012) Use of a Monte Carlo analysis within a physiologically based pharmacokinetic model to predict doxycycline residue withdrawal time in edible tissues in swine. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 29(1):73–84
Zhang L, Brett CM, Giacomini KM (1998) Role of organic cation transporters in drug absorption and elimination. Annu Rev Pharmacol Toxicol 38:431–460
Zhao P, Zhang L, Grillo JA, Liu Q, Bullock JM, Moon YJ, Song P, Brar SS, Madabushi R, Wu TC, Booth BP, Rahman NA, Reynolds KS, Gil BE, Lesko LJ, Huang SM (2011) Applications of physiologically based pharmacokinetic (PBPK) modeling and simulation during regulatory review. Clin Pharmacol Ther 89:259–267
Acknowledgments
This project received support from the Defense Threat Reduction Agency-Joint Science and Technology Office, Basic and Supporting Sciences Division (1.E0056.08.AHB.C).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sterner, T.R., Ruark, C.D., Covington, T.R. et al. A physiologically based pharmacokinetic model for the oxime TMB-4: simulation of rodent and human data. Arch Toxicol 87, 661–680 (2013). https://doi.org/10.1007/s00204-012-0987-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-012-0987-z