Abstract
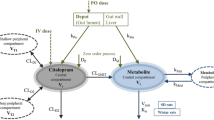
The principal aim of this study was to develop, validate, and demonstrate a physiologically based pharmacokinetic (PBPK) model to predict and characterize the absorption, distribution, metabolism, and excretion of acetaminophen (APAP) in humans. A PBPK model was created that included pharmacologically and toxicologically relevant tissue compartments and incorporated mechanistic descriptions of the absorption and metabolism of APAP, such as gastric emptying time, cofactor kinetics, and transporter-mediated movement of conjugated metabolites in the liver. Through the use of a hierarchical Bayesian framework, unknown model parameters were estimated using a large training set of data from human pharmacokinetic studies, resulting in parameter distributions that account for data uncertainty and inter-study variability. Predictions from the model showed good agreement to a diverse test set of data across several measures, including plasma concentrations over time, renal clearance, APAP absorption, and pharmacokinetic and exposure metrics. The utility of the model was then demonstrated through predictions of cofactor depletion, dose response of several pharmacokinetic endpoints, and the relationship between APAP biomarker levels in the plasma and those in the liver. The model addressed several limitations in previous PBPK models for APAP, and it is anticipated that it will be useful in predicting the pharmacokinetics of APAP in a number of contexts, such as extrapolating across doses, estimating internal concentrations, quantifying population variability, assessing possible impacts of drug coadministration, and, when coupled with a suitable pharmacodynamic model, predicting toxicity.








Similar content being viewed by others
References
Ameer B, Greenblatt DJ, Divoll M, Abernethy DR, Shargel L (1981) High-performance liquid chromatographic determination of acetaminophen in plasma: single-dose pharmacokinetic studies. J Chromatogr 226:224–230
Ben-Shachar R, Chen Y, Luo S, Hartman C, Reed M, Nijhout HF (2012) The biochemistry of acetaminophen hepatotoxicity and rescue: a mathematical model. Theor Biol Med Model 9:55
Bois FY (2009) GNU MCSim: Bayesian statistical inference for SBML-coded systems biology models. Bioinformatics 25:1453–1454
Brown RP, Delp MD, Lindstedt SL, Rhomberg LR, Beliles RP (1997) Physiological parameter values for physiologically based pharmacokinetic models. Toxicol Ind Health 13:407–484
Campbell JL, Clewell RA, Gentry PR, Andersen ME, Clewell HJ (2012) Physiologically based pharmacokinetic/toxicokinetic modeling. Methods Mol Biol 929:439–499
Chan MT, Anderson PJ, Chan JC, Lau GS, Critchley JA (1997) Single-dose pharmacokinetics of paracetamol and its conjugates in Chinese non-insulin-dependent diabetic patients with renal impairment. Eur J Clin Pharmacol 52:285–288
Chen L, Mohr SN, Yang CS (1996) Decrease of plasma and urinary oxidative metabolites of acetaminophen after consumption of watercress by human volunteers. Clin Pharmacol Ther 60:651–660
Chiew A, Day P, Salonikas C, Naidoo D, Graudins A, Thomas R (2010) The comparative pharmacokinetics of modified-release and immediate-release paracetamol in a simulated overdose model. Emerg Med Aust 22:548–555
Chiu WA, Okino MS, Evans MV (2009) Characterizing uncertainty and population variability in the toxicokinetics of trichloroethylene and metabolites in mice, rats, and humans using an updated database, physiologically based pharmacokinetic (PBPK) model, and Bayesian approach. Toxicol Appl Pharmacol Elsevier BV 241:36–60
Clements JA, Heading RC, Nimmo WS, Prescott LF (1978) Kinetics of acetaminophen absorption and gastric emptying in man. Clin Pharmacol Ther 24:420–431
Clements JA, Critchley JA, Prescott LF (1984) The role of sulphate conjugation in the metabolism and disposition of oral and intravenous paracetamol in man. Br J Clin Pharmacol 18:481–485
Critchley JA, Nimmo GR, Gregson CA, Woolhouse NM, Prescott LF (1986) Inter-subject and ethnic differences in paracetamol metabolism. Br J Clin Pharmacol 22:649–657
Critchley JA, Critchley LA, Anderson PJ, Tomlinson B (2005) Differences in the single-oral-dose pharmacokinetics and urinary excretion of paracetamol and its conjugates between Hong Kong Chinese and Caucasian subjects. J Clin Pharm Ther 30:179–184
Edginton AN, Schmitt W, Willmann S (2006) Development and evaluation of a generic physiologically based pharmacokinetic model for children. Clin Pharmacokinet 45:1013–1034
Esteban A, Calvo R, Pérez-Mateo M (1996) Paracetamol metabolism in two ethnically different Spanish populations. Eur J Drug Metab Pharmacokinet 21:233–239
Fong BM, Siu TS, Tam S (2011) Persistently increased acetaminophen concentrations in a patient with acute liver failure. Clin Chem 57:9–11
Forrest JA, Clements JA, Prescott LF (1982) Clinical pharmacokinetics of paracetamol. Clin Pharmacokinet 7:93–107
Geenen S, Yates JWT, Kenna JG, Bois FY, Wilson ID, Westerhoff HV (2013) Multiscale modelling approach combining a kinetic model of glutathione metabolism with PBPK models of paracetamol and the potential glutathione-depletion biomarkers ophthalmic acid and 5-oxoproline in humans and rats. Integr Biol (Camb) 5:877–888
Gelman A, Shirley K (2011) Inference from simulations and monitoring convergence. In: Brooks S, Gelman A, Jones GL, Meng X-L (eds) Handb. Markov Chain Monte Carlo. Chapman and Hall, UK, pp 163–174
Gwilt J, Robertson A, McChesney E (1963) Determination of blood and other tissue concentrations of paracetamol in dog and man. J Pharm Pharmacol 15:440–444
Hjelle JJ, Klaassen CD (1984) Glucuronidation and biliary excretion of acetaminophen in rats. J Pharmacol Exp Ther 228:407–413
Hjelle JJ, Hazelton GA, Klaassen CD (1985) Acetaminophen decreases adenosine 3′-phosphate 5′-phosphosulfate and uridine diphosphoglucuronic acid in rat liver. Drug Metab Dispos 13:35–41
Hunter JD (2007) Matplotlib: a 2D graphics environment. Comput Sci Eng 9:90–95
Iida S, Mizuma T, Sakuma N, Hayashi M, Awazu S (1989) Transport of acetaminophen conjugates in isolated rat hepatocytes. Drug Metab Dispos 17:341–344
Itoh H, Nagano T, Takeyama M (2002) Effect of nizatidine on paracetamol and its metabolites in human plasma. J Pharm Pharmacol 54:869–873
Jayasinghe KS, Roberts CJ, Read AE (1986) Is biliary excretion of paracetamol significant in man? Br J Clin Pharmacol 22:363–366
Jensen LS, Valentine J, Milne RW, Evans AM (2004) The quantification of paracetamol, paracetamol glucuronide and paracetamol sulphate in plasma and urine using a single high-performance liquid chromatography assay. J Pharm Biomed Anal 34:585–593
Jones E, Oliphant TE, Peterson P (2001) SciPy: open source scientific tools for Python. http://www.scipy.org/
Kamali F (1993) The effect of probenecid on paracetamol metabolism and pharmacokinetics. Eur J Clin Pharmacol 45:551–553
Kim D-W, Tan EY, Jin Y, Park S, Hayes M, Demirhan E et al (2011) Effects of imatinib mesylate on the pharmacokinetics of paracetamol (acetaminophen) in Korean patients with chronic myelogenous leukaemia. Br J Clin Pharmacol 71:199–206
Laine JE, Auriola S, Pasanen M, Juvonen RO (2009) Acetaminophen bioactivation by human cytochrome P450 enzymes and animal microsomes. Xenobiotica 39:11–21
Lau GS, Critchley JA (1994) The estimation of paracetamol and its major metabolites in both plasma and urine by a single high-performance liquid chromatography assay. J Pharm Biomed Anal 12:1563–1572
Levitt DG (2013) Quantitation of small intestinal permeability during normal human drug absorption. BMC Pharmacol Toxicol 14:34
Lin JH (1994) Dose-dependent pharmacokinetics: experimental observations and theoretical considerations. Biopharm Drug Dispos 15:1–31
Lin J, Lu A (1997) Role of pharmacokinetics and metabolism in drug discovery and development. Pharmacol Rev 49:403–449
Lyons MA, Yang RSH, Mayeno AN, Reisfeld B (2008) Computational toxicology of chloroform: reverse dosimetry using Bayesian inference, Markov chain Monte Carlo simulation, and human biomonitoring data. Environ Health Perspect 116:1040–1046
Marangoni AG (2002) Enzyme kinetics. Wiley, USA
Martin U, Temple RM, Winney RJ, Prescott LF (1993) The disposition of paracetamol and its conjugates during multiple dosing in patients with end-stage renal failure maintained on haemodialysis. Eur J Clin Pharmacol 45:141–145
Morris ME, Levy G (1984) Renal clearance and serum protein binding of acetaminophen and its major conjugates in humans. J Pharm Sci 73:1038–1041
Mutlib AE, Goosen TC, Bauman JN, Williams JA, Kulkarni S, Kostrubsky S (2006) Kinetics of acetaminophen glucuronidation by UDP-glucuronosyltransferases 1A1, 1A6, 1A9 and 2B15. Potential implications in acetaminophen-induced hepatotoxicity. Chem Res Toxicol 19:701–709
Nagar S, Walther S, Blanchard RL (2006) Sulfotransferase (SULT) 1A1 polymorphic variants *1, *2, and *3 are associated with altered enzymatic activity, cellular phenotype, and protein degradation. Mol Pharmacol 69:2084–2092
Naritomi Y, Terashita S, Kagayama A, Sugiyama Y (2003) Utility of hepatocytes in predicting drug metabolism: comparison of hepatic intrinsic clearance in rats and humans in vivo and in vitro. Drug Metab Dispos 31:580–588
Navid A, Ng DM, Stewart BJ, Wong SE, Lightstone FC (2013) Quantitative In Silico analysis of transient metabolism of acetaminophen and associated causes of hepatotoxicity in humans. In Silico Pharmacol 1:14
Péry ARR, Brochot C, Zeman FA, Mombelli E, Desmots S, Pavan M et al (2013) Prediction of dose-hepatotoxic response in humans based on toxicokinetic/toxicodynamic modeling with or without in vivo data: a case study with acetaminophen. Toxicol Lett Elsevier Ireland Ltd. 220:26–34
Peters SA (2012) Physiologically-based pharmacokinetic (PBPK) modeling and simulations. Wiley, USA
Prescott LF (1980) Kinetics and metabolism of paracetamol and phenacetin. Br J Clin Pharmacol 10(Suppl 2):291S–298S
Prescott LF, Speirs GC, Critchley JA, Temple RM, Winney RJ (1989) Paracetamol disposition and metabolite kinetics in patients with chronic renal failure. Eur J Clin Pharmacol 36:291–297
Rawlins MD, Henderson DB, Hijab AR (1977) Pharmacokinetics of paracetamol (acetaminophen) after intravenous and oral administration. Eur J Clin Pharmacol 11:283–286
Reisfeld B, Mayeno AN, Lyons MA, Yang RSH (2007) Computational toxicology. In: Ekins S (ed) Wiley, USA, pp. 33–69
Riches Z, Bloomer J, Patel a, Nolan a, Coughtrie M (2009) Assessment of cryopreserved human hepatocytes as a model system to investigate sulfation and glucuronidation and to evaluate inhibitors of drug conjugation. Xenobiotica 39:374–381
Rodgers T, Rowland M (2006) Physiologically based pharmacokinetic modelling 2: predicting the tissue distribution of acids, very weak bases, neutrals and zwitterions. J Pharm Sci 95:1238–1257
Rumack BH (2002) Acetaminophen hepatotoxicity: the first 35 years. J Toxicol Clin Toxicol 40:3–20
Sahajwalla CG, Ayres JW (1991) Multiple-dose acetaminophen pharmacokinetics. J Pharm Sci 80:855–860
Shinoda S, Aoyama T, Aoyama Y, Tomioka S, Matsumoto Y, Ohe Y (2007) Pharmacokinetics/pharmacodynamics of acetaminophen analgesia in Japanese patients with chronic pain. Biol Pharm Bull 30:157–161
Souliman S, Blanquet S, Beyssac E, Cardot J-M (2006) A level A in vitro/in vivo correlation in fasted and fed states using different methods: applied to solid immediate release oral dosage form. Eur J Pharm Sci 27:72–79
Srinivasan RS, Bourne DW, Putcha L (1994) Application of physiologically based pharmacokinetic models for assessing drug disposition in space. J Clin Pharmacol 34:692–698
Tan Q, Zhu R, Li H, Wang F, Yan M, Dai L (2012) Simultaneous quantitative determination of paracetamol and its glucuronide conjugate in human plasma and urine by liquid chromatography coupled to electrospray tandem mass spectrometry: application to a clinical pharmacokinetic study. J Chromatogr B Analyt Technol Biomed Life Sci Elsevier BV 893–894:162–7
Tone Y, Kawamata K, Murakami T, Higashi Y, Yata N (1990) Dose-dependent pharmacokinetics and first-pass metabolism of acetaminophen in rats. J Pharmacobiodyn 13:327–335
Tonoli D, Varesio E, Hopfgartner G (2012) Quantification of acetaminophen and two of its metabolites in human plasma by ultra-high performance liquid chromatography–low and high resolution tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci Elsevier BV 904:42–50
Vajjah P, Isbister GK, Duffull SB (2012) Introduction to pharmacokinetics in clinical toxicology. Methods Mol Biol 929:289–312
Van der Walt S, Colbert SC, Varoquaux G (2011) The NumPy array: a structure for efficient numerical computation. Comput Sci Eng 13:22–30
Volak LP, Hanley MJ, Masse G, Hazarika S, Harmatz JS, Badmaev V et al (2013) Effect of a herbal extract containing curcumin and piperine on midazolam, flurbiprofen and paracetamol (acetaminophen) pharmacokinetics in healthy volunteers. Br J Clin Pharmacol 75:450–462
Wishart DS, Jewison T, Guo AC, Wilson M, Knox C, Liu Y et al (2013) HMDB 3.0—The Human Metabolome Database in 2013. Nucleic Acids Res 41:D801–D807
Bormann I. DigitizeIt. Available at http://www.digitizeit.de. Accessed 15 Nov 2013
Python Software Foundation. Python Language Reference, version 2.7. Available at http://www.python.org. Accessed 10 Nov 2013
Yin OQ, Tomlinson B, Chow AH, Chow MS (2001) Pharmacokinetics of acetaminophen in Hong Kong Chinese subjects. Int J Pharm 222:305–308
Zhu T, Ding L, Guo X, Yang L, Wen A (2007) Simultaneous determination of tramadol and acetaminophen in human plasma by LC–ESI–MS. Chromatographia 66:171–178
Acknowledgments
The authors wish to thank the staff at the Franklin A. Graybill Statistical Laboratory at Colorado State University for their advice and consultation on several issues related to statistical analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zurlinden, T.J., Reisfeld, B. Physiologically based modeling of the pharmacokinetics of acetaminophen and its major metabolites in humans using a Bayesian population approach. Eur J Drug Metab Pharmacokinet 41, 267–280 (2016). https://doi.org/10.1007/s13318-015-0253-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13318-015-0253-x