Abstract
Summary
Early life nutrition affects peak bone mass attainment. In this prospective cohort study, children with high adherence to a “dairy and whole grains” pattern in infancy had higher bone mineral density at the age of 6 years. Although the observed effects are small, our study provides insight into mechanisms linking early nutrition to bone acquisition in childhood.
Introduction
Nutrition in early life may affect peak bone mass attainment. Previous studies on childhood nutrition and skeletal health mainly focused on individual nutrients, which does not consider the cumulative effects of nutrients. We investigated the associations between dietary patterns in infancy and childhood bone health.
Methods
This study included 2850 children participating in a population-based prospective cohort study. Dietary information was obtained from a food frequency questionnaire at the age of 13 months. Using principal component analysis, three major dietary patterns were extracted, explaining in total 30 % of the variation in dietary intake. At the age of 6 years, a total body dual-energy X-ray absorptiometry (DXA) scan was performed, and bone mineral density (BMD), bone mineral content (BMC), area-adjusted BMC (aBMC), and bone area (BA) were analyzed.
Results
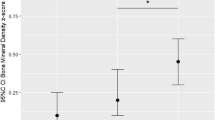
Higher adherence score to a “dairy and whole grains” pattern was positively associated with BMD and aBMC, but not with BMC and BA. Accordingly, children in the highest quartile of the “dairy and whole grains” pattern had higher BMD (difference 3.98 mg/cm2, 95 % confidence interval (CI) 0.36 to 7.61) and aBMC (difference 4.96 g, 95 % CI 1.27 to 8.64) than children in the lowest quartile. Stratification for vitamin D supplementation showed that the positive associations between the “dairy and whole grains” pattern and bone outcomes were only observed in children who did not receive vitamin D supplementation. A “potatoes, rice, and vegetables” and a “refined grains and confectionery” pattern were not consistently associated with bone outcomes.
Conclusions
An infant dietary pattern characterized by high intakes of dairy and cheese, whole grains, and eggs is positively associated with bone development in childhood. Further research is needed to investigate the consequences for bone health in later life.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Peak bone mass is reached during young adulthood and is influenced by genetics as well as modifiable lifestyle factors, including nutrition [1, 2]. Hence, nutrition during early life may have important consequences for peak bone mass attainment and future fracture risk. Previous studies on childhood nutrition and bone health have mainly focused on individual nutrients, including calcium and vitamin D, or specific foods, such as dairy products, fruits, and vegetables [3–6]. However, foods and nutrients can have high level of intercorrelation or interaction, which is not fully considered when examining their individual effects on health outcomes. Evaluating dietary patterns, which include the combination of dietary factors, may better overcome confounding by other dietary factors and account for the cumulative and interactive effects of multiple nutrients [7]. Previous studies in adults have examined the associations between dietary patterns and bone health. These studies reported that so-called healthy, prudent, or nutrient-dense dietary patterns (i.e., rich in fruits, vegetables, grains, fish, and low in meat) may positively affect bone health in adults [8], women [9–11], and elderly [12], whereas dietary patterns rich in refined or energy-dense foods (i.e., meats, fried foods, soft drinks, confectionery) appear to be inversely associated with bone health [8, 10–12].
Since a significant proportion of adult bone mass is accrued during childhood and adolescence, gaining an understanding of the role of dietary components on bone mass in early life is important to identify interventions favorable to bone health. Nevertheless, except for a few previous [13–16] studies in (small groups of) children or adolescents, not much is known on the relation between dietary patterns and bone mass, particularly in young children. Therefore, we investigated the associations between dietary patterns assessed in infancy and bone health (bone mineral density, bone mineral content, and bone area) at the age of 6 years in 2850 children participating in a population-based prospective cohort study. In addition, we aimed to evaluate whether the associations differ by the child’s sex, birth weight, gestational age at birth, ethnicity, body mass index, or vitamin supplement use.
Methods
Design
This study was embedded in the Generation R Study, a population-based prospective cohort study from pregnancy onward in the city of Rotterdam, the Netherlands, which has been described previously in detail [17]. Pregnant mothers enrolled between 2001 and 2005. The study protocol was approved by the Medical Ethical Committee of Erasmus Medical Center, Rotterdam. All participants provided written consent.
A questionnaire on infant nutrition was implemented from 2003 onward and was sent to 5088 mothers who provided consent for postnatal follow-up and were capable of understanding the Dutch language (Fig. 1). In total, 3650 (72 %) of these mothers returned the questionnaire [18]. Information on nutrition was available in 3629 children, of which 3550 were singleton live births. Of those, 2922 children participated in the 6-year visit, and dual-energy X-ray absorptiometry (DXA) scans were performed successfully in 2850 children (Fig. 1).
Dietary patterns
Mothers received a food frequency questionnaire (FFQ) for their child at a median age of 12.9 months (95 % range 12.2 to 19.0). The FFQ was developed for children in the second year of life and consisted of 211 food items, as described previously in detail [18]. The FFQ was validated against 3-day 24-h recalls in a representative sample of Dutch children aged 14 months (n = 32) [18]. Average daily nutritional values were calculated using the Dutch Food Composition Database 2006 [19].
Within all children with available dietary data (n = 3629), the 211 food items from the FFQ were classified into 21 food groups based on nutrient profile and food preferences (Supplementary Table 1). Subsequently, we applied principal component analysis (PCA) to the food groups to construct dietary patterns [7], using the varimax rotation method to obtain uncorrelated factors. Briefly, PCA aggregates food groups on the basis of the degree to which the food groups in the dataset are correlated with one another. Food groups that correlate high are grouped into a factor (dietary pattern), which explains a proportion of the variance in dietary data [7]. To determine the number of factors to retain, we used several criteria that included eigenvalue ≥1.5, position above the bend in the scree plot (a graphical presentation of eigenvalues), and interpretability of the patterns. This identified three major dietary patterns (Supplementary Table 1). For each individual food group, a factor loading was calculated, representing the extent to which the food group correlates to a particular factor/dietary pattern. Food groups with factor loadings >0.2 were considered to have a strong correlation with that factor and were used to describe and label each dietary pattern. Accordingly, each subject was assigned an adherence score (Z-score) for each dietary pattern, which was calculated by multiplying the factor loadings with the corresponding standardized intake for each food group and summing across the food items. A higher adherence score indicates a higher adherence to the respective dietary pattern.
Bone mineral density measurements
Bone measurements in childhood were conducted in a dedicated research center in Erasmus Medical Center, Rotterdam. The mean age of the children at examination was 6.0 ± 0.2 years. Total body bone mineral density was measured using a DXA scan (iDXA, General Electrics—Lunar, 2008, Madison, WI, USA), using procedures previously described [20]. Scans were analyzed using enCORE version 13.6. As recommended by the International Society for Clinical Densitometry for the measurement in children, we used total body less head (TBLH) instead of total body bone mineral density as a region of interest for all analyses [21]. Bone mineral density (BMD) measured by DXA was expressed as bone mineral content (BMC, in g) per projected bone area (in cm2). BMC was calculated from BMD using the projected bone area. Area-adjusted BMC (aBMC) was derived as a measure of volumetric BMD by using linear regression to adjust BMC for bone area and adding the residuals to the mean BMC [22].
Covariates
Information on maternal age, educational level, parity, and folic acid supplementation use was obtained by a questionnaire at enrolment. Maternal smoking and alcohol consumption during pregnancy were assessed by questionnaires in each trimester. Maternal height and weight at enrolment were measured without shoes and heavy clothing, and body mass index (kg/m2) was calculated. Information on infant sex, birth weight, and gestational age at birth was available from midwife and hospital registries. Information on child ethnicity was obtained by a questionnaire at enrolment and was defined according to the classification of Statistics Netherlands based on country of birth of the parents [23]. We categorized child ethnicity into European descent (Dutch, Turkish, other European, American, Oceanic); African descent (Moroccan, other African, Antillean, Surinamese-Creole, and Cape Verdean); and Asian descent (Indonesian, other Asians, and Surinamese-Hindu) according to the largest ethnic groups. Postnatal growth was repeatedly measured at the community health centers, and SD scores for weight were obtained using Dutch reference growth charts [24]. Catch-up growth in the first year of life was defined as change in SD score for weight >0.67 between the age of 1 and 11 months. Information on breastfeeding was obtained from delivery reports and postnatal questionnaires at 2, 6, and 12 months after delivery and was categorized as never breastfeeding, any partial breastfeeding in the first 4 months of life, and exclusive breastfeeding in the first 4 months of life. Information on (multi)vitamin supplement use was available from the FFQ at 14 months and was categorized into vitamin D supplementation (vitamin D alone, vitamin A + D, or multivitamin) and other vitamin supplementation (fluoride, iron, vitamin A only, or other). Duration of TV watching during weekdays and weekend days, as an indicator of sedentary behavior, was assessed by questionnaires at the child’s ages of 2, 3, 4, and 6 years. Participation in sports, as an indicator of physical activity, was obtained by a questionnaire at the child’s age of 6 years. At the 6-year visit, child weight was measured to the nearest 0.2 kg, and child height was measured to the nearest 0.1 cm, without shoes and heavy clothing. Measures of total body fat and lean mass (kg) were derived from the DXA scans.
Statistical analysis
Dietary pattern adherence scores were used in the analyses both as continuous variables (where 1 unit increase equals an increase of 1 SD) and categorized into quartiles, with the lowest quartile as the reference category. We compared participant and dietary characteristics among the highest quartiles of adherence scores to the dietary patterns. We performed multivariable linear regression models to evaluate the associations for dietary pattern adherence scores with BMD, BMC, aBMC, and bone area (BA). Potential confounders were selected based on previous literature or a strong association with the outcome and were kept in the multivariable model in case of a ≥10 % alteration in effect estimates. Crude models were adjusted for child’s sex, total energy intake, age at 6-year visit, and time interval between dietary assessment and visit. The latter covariate was included because the effect of dietary patterns on bone outcomes may depend on the time interval between dietary assessment and assessment of bone mass. Multivariable models were additionally adjusted for ethnicity, birth weight Z-score (i.e., sex and gestational age adjusted), height at visit, weight at visit, and maternal BMI at enrolment. We adjusted for weight at visit by adding lean mass plus fat mass (as measured by DXA) to the model, thereby excluding the contribution of bone mass to the child’s weight. To test the independent effect of each dietary pattern, the three dietary pattern adherence scores were included in the models simultaneously. Adjustments for maternal age, educational level, parity, smoking, alcohol consumption, folic acid supplement use during pregnancy, child’s catch-up growth in the first year of life, history of breastfeeding, TV watching, and participation in sports did not materially alter effect estimates and were not retained in the final models. To assess whether the associations between dietary patterns and bone mass differed by the child’s sex, birth weight, gestational age at birth, ethnicity, BMI at 6-year visit, vitamin D supplementation, or other vitamin supplementation, statistical interaction was evaluated by adding the product term of the dietary pattern adherence scores and the covariate and as an independent variable to the multivariable models. Stratified analyses were conducted in case of a significant interaction term (P < 0.05). Because the FFQ has been validated in Dutch children, we performed a sensitivity analysis in Dutch children only. Therefore, we first performed a PCA in this group of children to extract dietary patterns, and we subsequently examined the associations with bone outcomes. To reduce potential bias associated with missing data, we performed multiple imputations of missing covariates (<9 % missing values) based on the correlation between the variable with missing values and other subject characteristics [25]. Data were imputed (n = 5 imputations) according to the Markov Chain Monte Carlo method [26], assuming no monotone missing pattern. Analyses were performed in the original dataset and in the imputed datasets. Because we observed similar effect estimates, we only present the results based on imputed datasets. Statistical analyses were performed using SPSS version 21 for Windows (SPSS Inc., Chicago, IL, USA).
Results
Subject characteristics and dietary patterns
Maternal and child characteristics are presented in Table 1. Most mothers had a high educational level (57.2 %). Of all children, 82.6 % were of European background, and 51.2 % participated in sports. Mean BMD was 540 ± 45 mg/cm2.
Three dietary patterns were identified, which were labeled according to the food groups with the highest positive factor loadings (>0.2) (Supplementary Table 1). The first dietary pattern was labeled as a “potatoes, rice, and vegetables” pattern and was characterized by high loadings for potatoes, pasta and rice, vegetables, meat and meat products, fish and shellfish, oils, and condiments and sauces. The “refined grains and confectionery” pattern was associated with high intakes of refined grains, solid fats, confectionery, snack bar products and savory snacks, and sugar-containing beverages. The third pattern was labeled as the “dairy and whole grains” pattern and had high loadings for whole grains, dairy and cheese, and eggs, and a negative loading for breast milk and infant formula (Supplementary Table 1). In total, the three patterns explained 30 % of the variation in food intake. The correlations between the three dietary patterns were low (i.e., Pearson correlation coefficients ranging from −0.001 to −0.059).
Mean intakes of foods and nutrients (absolute and expressed relative to energy intake) for the highest quartiles of adherence to the three dietary patterns are presented in Supplementary Table 2. Children with high adherence to the “refined grains and confectionery” pattern had on average higher intakes of total energy and total carbohydrates and lower relative intakes of total protein, dietary fiber, beta-carotene, and folate, as compared to children with high adherence to the “potatoes, rice, and vegetables” pattern. As compared to children with high adherence to the “potatoes, rice, and vegetables” pattern, children with high adherence to the “dairy and whole grains” pattern appeared to have a more “nutrient-dense” diet, as reflected by their lower mean intake of total energy, but higher relative intakes of total protein, animal protein, vitamin B12, calcium, magnesium, phosphorus, and sodium. In contrast, their relative intakes of vitamin C, vitamin D, vitamin E, and iron were lower. In addition, they more often used vitamin D supplements.
Dietary patterns and bone mass
Covariate-adjusted associations between infant dietary patterns and childhood bone mass are presented in Table 2. No associations were observed for the “potatoes, rice, and vegetables” pattern and the “refined grains and confectionery” pattern with BMD, BMC, aBMC, or BA. However, higher adherence to the “dairy and whole grains” pattern was positively associated with BMD and aBMC (P = 0.03 and P < 0.01, respectively). In accordance, the highest quartile of adherence to the “dairy and whole grains” pattern was positively associated with BMD (difference 3.98 mg/cm2, 95 % confidence interval (CI) 0.36 to 7.61) and aBMC (difference 4.96 g, 95 % CI 1.27 to 8.64), as compared to the lowest quartile. No associations were observed for this dietary pattern with BMC or BA. Supplementary Table 3 presents the crude associations (only adjusted for child’s sex, total energy intake, age at visit, and time interval between dietary assessment and visit) between dietary patterns and childhood bone mass, showing positive associations between the adherence score to the “refined and grains” pattern and aBMC (P = 0.03). Also, as compared to the lowest quartile, the highest quartile of adherence to this pattern was positively associated with BMD, BMC, and BA. These associations changed after adjustment for important maternal and child confounders, especially child weight and height. Crude associations for the “dairy and whole grains” pattern with BMD and aBMC were larger than the covariate-adjusted associations, but patterns of associations were similar. In addition, positive associations were observed for adherence to this pattern with BMC and BA (P < 0.01 and P = 0.02, respectively).
Subgroup and sensitivity analyses
The associations between dietary patterns and bone outcomes did not significantly differ by the child’s sex, birth weight, gestational age at birth, BMI at 6-year visit, or multivitamin supplement use. In the analyses with BMD, a significant interaction was observed between the “potatoes, rice, and vegetables” pattern and ethnicity (P interaction = 0.02). Stratified analyses showed that the adherence score to this dietary pattern tended to be positively associated with BMD only in the European children (P = 0.08) (results not shown). In the analyses with BA, we observed a significant interaction between the “dairy and whole grains” pattern and vitamin D supplementation (P interaction = 0.04). Further stratified analyses showed that the “dairy and whole grains” pattern was positively associated with aBMC and inversely associated with BA, only in the group of children who did not receive vitamin D supplementation (n = 1473). No associations were observed between the “dairy and whole grains” pattern and bone outcomes in the children who did receive vitamin D supplementation (Table 3). In a sensitivity analysis with Dutch children only (n = 1956), we obtained dietary patterns that resembled the three patterns that were identified in the total group, and we observed similar associations with bone outcomes (results not shown).
Discussion
In this large population-based cohort study from early life onward, we observed that children with a dietary pattern characterized by high intakes of dairy and cheese, whole grains, and eggs, during early infancy, have a higher BMD during childhood. These associations were only observed in children who did not receive vitamin D supplementation. We found no evidence for consistent associations between a “potatoes, rice, and vegetables” pattern and bone mass in our population of school-age children. Furthermore, the associations between a “refined grains and confectionery” pattern and bone mass appeared to be explained by confounding factors.
Calcium, phosphorus, and protein are important components of bone; hence, adequate intake of these nutrients is required for normal bone development. Other nutrients, including vitamin D, vitamin K, magnesium, zinc, and fluoride, are also involved in bone metabolism [2, 27]. Many of these nutrients are intercorrelated and act in a synergistic way. For example, vitamin D is involved in the absorption of calcium, and magnesium plays an important role in vitamin D and calcium metabolism [1, 2]. Moreover, whereas the effects of single nutrients are often small, evaluating dietary patterns may enable detection of the cumulative effects of nutrients and nutrient interactions on health outcomes [7].
Previous studies in adults observed that dietary patterns rich in refined, energy-dense foods were inversely associated with bone health [8, 10–12]. We did not observe associations for the “refined grains and confectionery” pattern with bone mass. Also, no associations were observed between the “potatoes, rice, and vegetables” pattern and bone health. A few previous studies have examined dietary patterns in relation to bone health in children or adolescence, indicating that nutrition in early life or in adolescence may influence bone health. In 325 American children followed from age 4 to age 8 years, two dietary patterns were identified that were both positively associated with longitudinal bone mass development. The first pattern was rich in refined grains, processed meat, cheese, eggs, fried potatoes, and sweetened beverages, while the second pattern was characterized by high intakes of whole grains, processed meats, vegetables, and fruits [16]. In a cross-sectional study in 196 Korean adolescents aged 12–15 years, high adherence to a “milk and cereal” pattern was positively associated with lumbar spine BMD, whereas the “traditional Korean,” “fast food,” and “snacks” dietary patterns were not associated with bone mass [15]. A study in Dutch adolescents aged 9–15 years showed that those who previously followed a macrobiotic diet, characterized by high intakes of cereals, pulses, and vegetables and low intakes of meat, chicken, and dairy, had a lower relative bone mass than subjects who followed an omnivorous diet [14]. Finally, in 559 children of mothers participating in the Southampton Women’s Survey, an “infant feeding guidelines” pattern in infancy, characterized by high intakes of vegetables, fruits, meat/fish, home-prepared foods, and breast milk, was not associated with bone mass at the age of 4 years [13]. Other evidence that early life nutritional factors may influence childhood bone health come from studies on maternal diet [28, 29]. A previous study also embedded in the Generation R Study observed that maternal intakes of protein, phosphorus, and calcium and blood concentrations of vitamin B12 in pregnancy were positively related to childhood bone mass, whereas maternal carbohydrate intake and blood concentrations of homocysteine were inversely associated with childhood bone mass [20].
In the present study, a “dairy and whole grains” pattern in infancy was associated with higher BMD and aBMC in childhood. Parameter estimates were small, but significant. This dietary pattern was characterized by high intakes of whole grains, dairy and cheese, and eggs, which together contain a number of ingredients that may have beneficial effects on bone outcomes. Key nutrients supplied by dairy foods are calcium, magnesium, vitamin D (especially if fortified), and high-quality proteins. Whole grain products contain magnesium, iron, B vitamins, and other bioactive compounds (i.e., phytochemicals, antioxidants), which may benefit bone health [2, 27]. It goes beyond the aim of the present study to disentangle the relative individual contributions of different dietary components. Nevertheless, we performed exploratory analyses to evaluate the associations for specific foods/nutrients (i.e., dairy products, whole grains, eggs, calcium, vitamin D, B vitamins) with bone outcomes and observed that these associations were not consistent and/or smaller in magnitude, as compared to the associations for the “dairy and whole grains” pattern (results not shown). This suggests that the associations between the “dairy and whole grains” pattern and bone outcomes are largely driven by the combination of food groups and nutrients that make part of this pattern as a whole. Nevertheless, further research is needed to confirm this.
We observed that children with the highest adherence to the “dairy and whole grains” pattern had on average higher relative intakes of total protein, animal protein, calcium, phosphorus, magnesium, and vitamin B12. These are nutrients that have been positively related to bone health in children and/or adults [2, 27, 30]. In contrast, these children had a lower mean intake of vitamin D, as compared to children in the highest quartiles of the other two dietary patterns. In the Netherlands, most dairy products are not fortified with vitamin D. Hence, other foods such as fish, meat, margarines, baking fats, and infant formula might be more important sources for children. In addition, since the year 2000, the use of vitamin D supplements has been recommended for children up to the age of 4 years. We observed that vitamin D supplements were used more often in children with a high adherence to the “dairy and whole grains” pattern, which may counterbalance their relatively lower dietary intake of vitamin D. Interestingly, however, we observed that the beneficial effect on bone health of the “dairy and whole grains” pattern was more explicit in the children who did not receive vitamin D supplementation. This could suggest that the importance of the consumption of specific dietary factors diminishes in case of vitamin D supplementation or, alternatively, that the consumption of dairy and whole grain products may compensate for the absence of vitamin D supplementation.
We observed a significant interaction between the “potatoes, rice, and vegetables” pattern and ethnicity in the analyses with BMD. Differential effects of dietary patterns on bone may be related to racial/ethnic differences in the metabolism of specific nutrients, such as calcium and vitamin D [31]. Furthermore, risk factors associated with BMD and fracture risk may be ethnic-specific [32], suggesting that specific dietary exposures might beneficially affect bone development in some ethnic groups, but not in others.
Next to providing specific nutrients, the bone-promoting effect of the “dairy and whole grains” pattern may also act through insulin growth factor 1 (IGF-1). Cow’s milk is known to increase IGF-1 levels [33] (reviewed by [34]), which stimulates bone mass accrual [34]. Furthermore, dietary patterns could also indirectly affect bone mass, through changes in fat and/or lean mass that are driven by the diet. For example, body weight/body fat is a known factor that is positively related to bone mass [1, 35]. To take this into account, we adjusted all our analyses for total energy intake and child anthropometrics to obtain estimates that were uncorrelated with body size or energy intake.
In analyses stratified for vitamin D supplementation, we observed positive associations for the “dairy and whole grains” pattern with aBMC, but inverse associations with bone area. Further investigations using 3D assessments (e.g., quantitative CT) are needed to determine whether these findings are arising from differences in skeletal frame size and/or bone geometry.
The observed effect estimates are small and may not reflect individual, clinically relevant, differences. Nevertheless, the present study provides insight into mechanisms linking early life nutrition to bone acquisition during childhood. Prospective studies with follow-up into adulthood can determine whether such dietary exposures during childhood can be translated to risk differences later in life.
Methodological considerations
An important strength of this study is the population-based cohort design from early life onward, with detailed measurements of dietary intake and bone mass in a large number of children. We collected information on many potential confounding variables. However, as in any observational study, residual confounding due to unmeasured variables such as physical activity levels might still be an issue.
Information on bone health was available for 80 % of all singleton children with information on dietary intake. Children who participated in the DXA measurement at the age of 6 years on average had a lower total energy intake and a lower adherence score to the “refined grains and confectionery” pattern compared with children who did not participate. Selection bias due to selective loss to follow-up is of concern if the associations between dietary patterns and bone health differ between those included and not included in the study. Although this seems unlikely, it cannot be excluded.
Dietary patterns were assessed at the approximate age of 14 months. Dietary habits may change during childhood, and these dietary changes could also affect childhood bone health. Unfortunately, no further data on childhood diet was available to examine this. Previous studies in (young) children have shown evidence of tracking of dietary habits [36–39], which suggests that information on infant nutrition may be a reasonable indicator of childhood diet. Nevertheless, more research on tracking of dietary intakes is needed to confirm this.
Dietary pattern analysis involves several decisions that may influence the findings, such as the composition of food groups, numbers of factors to extract, and labeling of the components [7]. Nevertheless, we used common criteria to select the factors. Although the amount of variance (30 %) explained by the extracted dietary patterns was small, it is comparable with previous studies [10, 15, 36].
Conclusion
In this prospective cohort study, we observed that children with a dietary pattern characterized by high intakes of dairy and cheese, whole grains, and eggs during early infancy have a higher BMD during childhood. These associations were only observed in children who did not receive vitamin D supplementation. Adherence to a dietary pattern rich in potatoes, rice, and vegetables and to a dietary pattern rich in refined grains and confectionery was not consistently associated with bone outcomes. Given the small parameter estimates, further research is needed to replicate the findings and to investigate whether the observed differences have consequences for bone health in later life.
Abbreviations
- aBMC:
-
Area-adjusted bone mineral content
- BA:
-
Bone area
- BMC:
-
Bone mineral content
- BMD:
-
Bone mineral density
- DXA:
-
Dual-energy X-ray absorptiometry
- FFQ:
-
Food frequency questionnaire
- PCA:
-
Principal component analysis
References
Heaney RP, Abrams S, Dawson-Hughes B, Looker A, Marcus R, Matkovic V, Weaver C (2000) Peak bone mass. Osteoporos Int 11(12):985–1009
Ilich JZ, Kerstetter JE (2000) Nutrition in bone health revisited: a story beyond calcium. J Am Coll Nutr 19(6):715–737
Huncharek M, Muscat J, Kupelnick B (2008) Impact of dairy products and dietary calcium on bone-mineral content in children: results of a meta-analysis. Bone 43(2):312–321
McGartland CP, Robson PJ, Murray LJ, Cran GW, Savage MJ, Watkins DC, Rooney MM, Boreham CA (2004) Fruit and vegetable consumption and bone mineral density: the Northern Ireland Young Hearts Project. Am J Clin Nutr 80(4):1019–1023
Mouratidou T, Vicente-Rodriguez G, Gracia-Marco L, Huybrechts I, Sioen I, Widhalm K, Valtuena J, Gonzalez-Gross M, Moreno LA (2013) Associations of dietary calcium, vitamin D, milk intakes, and 25-hydroxyvitamin D with bone mass in Spanish adolescents: the HELENA study. J Clin Densitom 16(1):110–117
Vatanparast H, Baxter-Jones A, Faulkner RA, Bailey DA, Whiting SJ (2005) Positive effects of vegetable and fruit consumption and calcium intake on bone mineral accrual in boys during growth from childhood to adolescence: the University of Saskatchewan Pediatric Bone Mineral Accrual Study. Am J Clin Nutr 82(3):700–706
Hu FB (2002) Dietary pattern analysis: a new direction in nutritional epidemiology. Curr Opin Lipidol 13(1):3–9
Langsetmo L, Poliquin S, Hanley DA, Prior JC, Barr S, Anastassiades T, Towheed T, Goltzman D, Kreiger N, CaMos Research G (2010) Dietary patterns in Canadian men and women ages 25 and older: relationship to demographics, body mass index, and bone mineral density. BMC Musculoskelet Disord 11:20
Kontogianni MD, Melistas L, Yannakoulia M, Malagaris I, Panagiotakos DB, Yiannakouris N (2009) Association between dietary patterns and indices of bone mass in a sample of Mediterranean women. Nutrition 25(2):165–171
Hardcastle AC, Aucott L, Fraser WD, Reid DM, Macdonald HM (2011) Dietary patterns, bone resorption and bone mineral density in early post-menopausal Scottish women. Eur J Clin Nutr 65(3):378–385
Okubo H, Sasaki S, Horiguchi H, Oguma E, Miyamoto K, Hosoi Y, Kim MK, Kayama F (2006) Dietary patterns associated with bone mineral density in premenopausal Japanese farmwomen. Am J Clin Nutr 83(5):1185–1192
Tucker KL, Chen H, Hannan MT, Cupples LA, Wilson PW, Felson D, Kiel DP (2002) Bone mineral density and dietary patterns in older adults: the Framingham Osteoporosis Study. Am J Clin Nutr 76(1):245–252
Harvey NC, Robinson SM, Crozier SR, Marriott LD, Gale CR, Cole ZA, Inskip HM, Godfrey KM, Cooper C, Southampton Women's Survey Study G (2009) Breast-feeding and adherence to infant feeding guidelines do not influence bone mass at age 4 years. Br J Nutr 102(6):915–920
Parsons TJ, van Dusseldorp M, van der Vliet M, van de Werken K, Schaafsma G, van Staveren WA (1997) Reduced bone mass in Dutch adolescents fed a macrobiotic diet in early life. J Bone Miner Res 12(9):1486–1494
Shin S, Hong K, Kang SW, Joung H (2013) A milk and cereal dietary pattern is associated with a reduced likelihood of having a low bone mineral density of the lumbar spine in Korean adolescents. Nutr Res 33(1):59–66
Wosje KS, Khoury PR, Claytor RP, Copeland KA, Hornung RW, Daniels SR, Kalkwarf HJ (2010) Dietary patterns associated with fat and bone mass in young children. Am J Clin Nutr 92(2):294–303
Jaddoe VW, van Duijn CM, Franco OH, van der Heijden AJ, van Iizendoorn MH, de Jongste JC, van der Lugt A, Mackenbach JP, Moll HA, Raat H, Rivadeneira F, Steegers EA, Tiemeier H, Uitterlinden AG, Verhulst FC, Hofman A (2012) The Generation R Study: design and cohort update 2012. Eur J Epidemiol 27(9):739–756
Kiefte-de Jong JC, de Vries JH, Bleeker SE, Jaddoe VW, Hofman A, Raat H, Moll HA (2013) Socio-demographic and lifestyle determinants of ‘Western-like’ and ‘Health conscious’ dietary patterns in toddlers. Br J Nutr 109(1):137–147
Netherlands Nutrition Centre (2006) NEVO: Dutch food composition database 2006. Netherlands Nutrition Centre, The Hague
Heppe DH, Medina-Gomez C, Hofman A, Franco OH, Rivadeneira F, Jaddoe VW (2013) Maternal first-trimester diet and childhood bone mass: the Generation R Study. Am J Clin Nutr 98(1):224–232
Bianchi ML, Baim S, Bishop NJ, Gordon CM, Hans DB, Langman CB, Leonard MB, Kalkwarf HJ (2010) Official positions of the International Society for Clinical Densitometry (ISCD) on DXA evaluation in children and adolescents. Pediatr Nephrol 25(1):37–47
Macdonald-Wallis C, Tobias JH, Smith GD, Lawlor DA (2010) Relation of maternal prepregnancy body mass index with offspring bone mass in childhood: is there evidence for an intrauterine effect? Am J Clin Nutr 92(4):872–880
Statistics Netherlands (2004) Migrants in the Netherlands [in Dutch]
Durmus B, van Rossem L, Duijts L, Arends LR, Raat H, Moll HA, Hofman A, Steegers EA, Jaddoe VW (2011) Breast-feeding and growth in children until the age of 3 years: the Generation R Study. Br J Nutr 105(11):1704–1711
Sterne JA, White IR, Carlin JB, Spratt M, Royston P, Kenward MG, Wood AM, Carpenter JR (2009) Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ 338:b2393
Horton N, Lipsitz S (2001) Multiple imputation in practice: comparison of software packages for regression models with missing variables. Am Stat 55(3):244–254
Vicente-Rodriguez G, Ezquerra J, Mesana MI, Fernandez-Alvira JM, Rey-Lopez JP, Casajus JA, Moreno LA (2008) Independent and combined effect of nutrition and exercise on bone mass development. J Bone Miner Metab 26(5):416–424
Jones G (2011) Early life nutrition and bone development in children. Nestle Nutr Work Ser Pediatr Program 68:227–233, discussion 233-226
Prentice A, Schoenmakers I, Laskey MA, de Bono S, Ginty F, Goldberg GR (2006) Nutrition and bone growth and development. Proc Nutr Soc 65(4):348–360
Swart KM, van Schoor NM, Lips P (2013) Vitamin B12, folic acid, and bone. Curr Osteoporos Rep 11(3):213–218
Walker MD, Novotny R, Bilezikian JP, Weaver CM (2008) Race and diet interactions in the acquisition, maintenance, and loss of bone. J Nutr 138(6):1256S–1260S
Leslie WD (2012) Clinical review: ethnic differences in bone mass—clinical implications. J Clin Endocrinol Metab 97(12):4329–4340
Cadogan J, Eastell R, Jones N, Barker ME (1997) Milk intake and bone mineral acquisition in adolescent girls: randomised, controlled intervention trial. BMJ 315(7118):1255–1260
Hoppe C, Molgaard C, Michaelsen KF (2006) Cow’s milk and linear growth in industrialized and developing countries. Annu Rev Nutr 26:131–173
Sayers A, Tobias JH (2010) Fat mass exerts a greater effect on cortical bone mass in girls than boys. J Clin Endocrinol Metab 95(2):699–706
Robinson S, Marriott L, Poole J, Crozier S, Borland S, Lawrence W, Law C, Godfrey K, Cooper C, Inskip H, Southampton Women's Survey Study G (2007) Dietary patterns in infancy: the importance of maternal and family influences on feeding practice. Br J Nutr 98(5):1029–1037
Wang Y, Bentley ME, Zhai F, Popkin BM (2002) Tracking of dietary intake patterns of Chinese from childhood to adolescence over a six-year follow-up period. J Nutr 132(3):430–438
Mikkila V, Rasanen L, Raitakari OT, Pietinen P, Viikari J (2005) Consistent dietary patterns identified from childhood to adulthood: the cardiovascular risk in Young Finns Study. Br J Nutr 93(6):923–931
Singer MR, Moore LL, Garrahie EJ, Ellison RC (1995) The tracking of nutrient intake in young children: the Framingham Children’s Study. Am J Public Health 85(12):1673–1677
Acknowledgments
EHH, JCKJ, and OHF work in ErasmusAGE, a center for aging research across the life course funded by Nestlé Nutrition (Nestec Ltd.), Metagenics Inc., and AXA. Nestlé Nutrition (Nestec Ltd.), Metagenics Inc., and AXA had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. The Generation R Study is conducted by the Erasmus Medical Center Rotterdam in close collaboration with the School of Law and Faculty of Social Sciences of the Erasmus University Rotterdam, the Municipal Health Service Rotterdam area, the Rotterdam Homecare Foundation, and the Stichting Trombosedienst & Artsenlaboratorium Rijnmond, Rotterdam. The general design of the Generation R Study is made possible by financial support from the Erasmus University Medical Center Rotterdam, the Erasmus University Rotterdam, the Netherlands Organization for Health Research and Development (ZonMw), the Netherlands Organisation for Scientific Research (NWO), and the Ministry of Health, Welfare and Sport. We gratefully acknowledge all participants and the contribution of general practitioners, hospitals, midwives, and pharmacies in Rotterdam. Acquisition of nutritional data was funded by an unrestricted grant from Europe Container Terminals received by Prof. Dr. H.A. Moll. Dr. Jaddoe and Dr. Rivadeneira received additional grants from the Netherlands Organization for Health Research and Development (VIDI 016.136.361 and VIDI 016.136.367, respectively).
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
F. Rivadeneira and O. H. Franco shared last author.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 44 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
van den Hooven, E.H., Heppe, D.H.M., Kiefte-de Jong, J.C. et al. Infant dietary patterns and bone mass in childhood: the Generation R Study. Osteoporos Int 26, 1595–1604 (2015). https://doi.org/10.1007/s00198-015-3033-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-015-3033-1