Abstract
Summary
The combination of cytokines present in the circulation of patients with active rheumatoid arthritis might contribute to the generalized bone loss that commonly occurs in these patients, by directly inhibiting osteoblast proliferation and differentiation, but especially by enhancing endogenous cytokine (i.e., receptor activator of nuclear factor-kappa B ligand (RANKL) and interleukin-6 (IL)-6) production by osteoblasts, thereby stimulating osteoclastogenesis.
Introduction
Generalized bone loss, as occurs in patients with rheumatoid arthritis (RA), is related to elevated levels of circulating cytokines. Individual cytokines have deleterious effects on proliferation and differentiation of osteoblast cell lines, but little is known about the effect of the interaction between inflammatory factors in the circulation of patients with active RA on human osteoblast function, including their communication towards other bone cells. We investigated whether serum from patients with active RA enhances cytokine production by osteoblasts, thereby effectively altering osteoblast-stimulated osteoclastogenesis.
Methods
Serum was obtained from 20 patients with active RA (active RA sera) and from the same patients in clinical remission (remission RA sera). To determine osteoclastogenesis, RA serum-pretreated primary human osteoblast cultures were established in direct contact with human osteoclast precursors in the presence or absence of osteoprotegerin (OPG) or IL-6 inhibitor.
Results
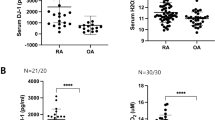
Compared to remission RA sera, active RA sera inhibited osteoblast proliferation and differentiation in vitro as demonstrated by a reduced DNA content and gene expression of KI-67, collagen type 1, osteopontin, and osteocalcin. Active RA sera inhibited OPG expression and enhanced RANKL and IL-6 expression but did not alter IL-8 expression in osteoblasts. IL-1β, IL-17, and tumor necrosis factor-α (TNF-α) expression were undetectable. In coculture, active RA sera treatment of osteoblasts stimulated while addition of OPG or IL-6 inhibitory antibodies significantly reduced the number of osteoclasts.
Conclusion
Active RA sera contain circulating factors, likely cytokines and chemokines, that might contribute to bone loss by directly inhibiting osteoblast proliferation and differentiation, but especially, these factors modulate endogenous cytokine production by osteoblasts, thereby affecting osteoclastogenesis.




Similar content being viewed by others
References
Mielants H, Van den Bosch F (2009) Extra-articular manifestations. Clin Exp Rheumatol 27(Suppl 55):S56–S61
Vis M, Güler-Yüksel M, Lems WF (2013) Can bone loss in rheumatoid arthritis be prevented? Osteoporos Int 10:2541–2553
van Staa L, Geusens P, Bijlsma JWJ, Cooper C (2006) Clinical assessment of the long-term risk of fracture in patients with rheumatoid arthritis. Arthritis Rheum 54:3104–3112
Orstavik RE, Haugeberg G, Mowinckel P, Hoiseth A, Uhlig T, Falch JA (2004) Vertebral deformities in rheumatoid arthritis: a comparison with population based controls. Arch Int Med 164:420–425
Gough AK, Lilley J, Eyre S, Holder RL, Emery P (1994) Generalized bone loss in patients with early rheumatoid arthritis. Lancet 344:23–27
Cooper C (1997) The crippling consequences of fractures and their impact on quality of life. Am J Med 103:12S–17S
van der Heijde DM (1995) Joint erosions and patients with early rheumatoid arthritis. Br J Rheumatol 34(Suppl 2):74–78
Schett G (2006) Rheumatoid arthritis inflammation and bone loss. Wien Med Wochenschr 156:34–41
Eggelmeijer F, Papapoulos SE, Westedt ML, Van Paassen HC, Dijkmans BA, Breedveld FC (1993) Bone metabolism in rheumatoid arthritis: relation to disease activity. Br J Rheumatol 32:387–391
Brennan FM, McInnes IB (2008) Evidence that cytokines play a role in rheumatoid arthritis. J Clin Invest 118:3537–3545
Alex P, Szodoray P, Knowlton N, Dozmorov IM, Turner M, Frank MB, Arthur RE, Willis L, Flinn D, Hynd RF, Carson C, Kumar A, El-Gabalawy HS, Centola M (2007) Multiplex serum cytokine monitoring as a prognostic tool in rheumatoid arthritis. Clin Exp Rheumatol 25:584–592
Chung SJ, Kwon YJ, Park MC, Park YB, Lee SK (2011) The correlation between increased serum concentrations of interleukin-6 family cytokines and disease activity in rheumatoid arthritis patients. Yonsei Med J 52:113–120
Le Goff B, Blanchard F, Berthelot JM, Heymann D, Maugars Y (2010) Role for interleukin-6 in structural joint damage and systemic bone loss in rheumatoid arthritis. Jt Bone Spine 77:201–205
Bakker AD, Silva VC, Krishnan R, Bacabac RG, Blaauboer ME, Lin YC, Marcantonio RA, Cirelli JA, Klein-Nulend J (2009) Tumor necrosis factor alpha and interleukin-1beta modulate calcium and nitric oxide signaling in mechanically stimulated osteocytes. Arthritis Rheum 60:3336–3345
Polzer K, Joosten L, Gasser J, Distler JH, Ruiz G, Baum W, Redlich K, Bobacz K, Smolen JS, van den Berg W, Schett G, Zwerina J (2010) Interleukin-1 is essential for systemic inflammatory bone loss. Ann Rheum Dis 69:284–290
Luyten FP, Lories RJ, Verschueren P, d Vlam K, Westhovens R (2006) Contemporary concepts of inflammation, damage and repair in rheumatic diseases. Best Pract Res Clin Rheumatol 20:829–848
Schett G, Gravallese E (2012) Bone erosion in rheumatoid arthritis: mechanisms, diagnosis and treatment. Nat Rev Rheumatol 8:656–664
Kulkarni RN, Bakker AD, Everts V, Klein-Nulend J (2012) Mechanical loading prevents the stimulating effect of IL-1beta on osteocyte-modulated osteoclastogenesis. Biochem Biophys Res Commun 420:11–16
Bakker AD, Kulkarni RN, Klein-Nulend J, Lems WF (2014) IL-6 alters osteocyte signaling toward osteoblasts but not osteoclasts. J Dent Res 93:394–399
De Benedetti F, Rucci N, Del Fattore A, Peruzzi B, Paro R, Longo M, Vivarelli M, Muratori F, Berni S, Ballanti P, Ferrari S, Teti A (2006) Impaired skeletal development in interleukin-6-transgenic mice: a model for the impact of chronic inflammation on the growing skeletal system. Arthritis Rheum 54:3551–3563
Klein-Nulend J, Sterck JGH, Semeins CM, Lips P, Joldersma M, Baart JA, Burger EH (2002) Donor age and mechanosensitivity of human bone cells. Osteoporos Int 13:137–146
Langan TJ, Chou RC (2011) Synchronization of mammalian cell cultures by sera deprivation. Methods Mol Biol 761:75–83
Lowry OH (1995) Micromethods for the asay of enzyme II specific procedure. Alkaline phosphatase. Meth Enzymol 4:371
Bloemen V, de Vries TJ, Schoenmaker T, Everts V (2009) Intercellular adhesion molecule-1 clusters during osteoclastogenesis. Biochem Biophys Res Commun 385:640–645
Vis M, Wolbink GJ, Lodder MC, Kostense PJ, van de Stadt RJ, de Koning MH, Dijkmans BA, Lems WF (2003) Early changes in bone metabolism in rheumatoid arthritis patients treated with infliximab. Arthritis Rheum 48:2996–2997
Caparbo VF, Prada F, Silva CA, Regio PL, Pereira RM (2009) Serum from children with polyarticular juvenile idiopathic arthritis (pJIA) inhibits differentiation, mineralization and may increase apoptosis of human osteoblasts “in vitro”. Clin Rheumatol 28:71–77
Wang SY, Liu YY, Ye H, Guo JP, Li R, Liu X, Li ZG (2011) Circulating Dickkopf-1 is correlated with bone erosion and inflammation in rheumatoid arthritis. J Rheumatol 38:821–827
Matzelle MM, Gallant MA, Condon KW, Walsh NC, Manning CA, Stein GS, Lian JB, Burr DB, Gravallese EM (2012) Resolution of inflammation induces osteoblast function and regulates the Wnt signaling pathway. Arthritis Rheum 64:1540–1550
Abbas S, Zhang Y-H, Clohisy JC, Abu-Amer Y (2003) Tumor necrosis factor-alpha inhibits pre-osteoblast differentiation through its type-1 receptor. Cytokine 22:33–41
Hughes FJ, Howells GL (1993) Interleukin-6 inhibits bone formation in vitro. Bone Miner 21:21–28
Chaudhary LR, Spelsberg TC, Riggs BL (1992) Production of various cytokines by normal human osteoblast-like cells in response to interleukin-1 beta and tumor necrosis factor-alpha: lack of regulation by 17 beta-estradiol. Endocrinology 130:2528–2534
Vis M, Havaardsholm EA, Haugeberg G, Uhlig T, Voskuyl AE, van de Stadt RJ, Dijkmans BA, Woolf AD, Kvien TK, Lems WF (2006) Evaluation of bone mineral density, bone metabolism, osteoprotegerin and receptor activator of the NFkappaB ligand serum levels during treatment with infliximab in patients with rheumatoid arthritis. Ann Rheum Dis 65:1495–1499
Rufo A, Del Fattore A, Capulli M, Carvello F, De Pasquale L, Ferrari S, Pierroz D, Morandi L, De Simone M, Rucci N, Bertini E, Bianchi ML, De Benedetti F, Teti A (2011) Mechanisms inducing low bone density in Duchenne muscular dystrophy in mice and humans. J Bone Miner Res 26:1891–1903
Xiong J, Onal M, Jilka RL, Weinstein RS, Manolagas SC, O’Brien CA (2011) Matrix-embedded cells control osteoclast formation. Nat Med 17:1235–1241
Taguchi Y, Yamamoto M, Yamate T, Lin SC, Mocharla H, DeTogni P, Nakayama N, Boyce BF, Abe E, Manolagas SC (1998) Interleukin-6-type cytokines stimulate mesenchymal progenitor differentiation toward the osteoblastic lineage. Proc Assoc Am Physicians 110:559–574
King TJ, Georgiou KR, Cool JC, Scherer MA, Ang ES, Foster BK, Xu J, Xian CJ (2012) Methotrexate chemotherapy promotes osteoclast formation in the long bone of rats via increased pro-inflammatory cytokines and enhanced NF-κB activation. Am J Pathol 181:121–129
Lisbona MP, Maymo J, Perich J, Almirall M, Carbonell J (2010) Rapid reduction in tenosynovitis of the wrist and fingers evaluated by MRI in patients with rheumatoid arthritis after treatment with etanercept. Ann Rheum Dis 69:1117–1122
Verschueren P, Esselens G, Westhovens R (2008) Daily practice effectiveness of a step-down treatment in comparison with a tight step-up for early rheumatoid arthritis. Rheumatology (Oxford) 47:59–64
Acknowledgments
We thank Dr. Veerle Stouten for collecting RA serum, Dr. Teun J. de Vries and Ton Schoenmaker for technical support regarding the osteoclastogenesis assay, and Dr. Behrouz Zandieh Doulabi for primer design and technical support. This research was funded by the European Commission through MOVE-AGE, an Erasmus Mundus Joint Doctorate program (2011–2015).
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
J. Klein-Nulend and A.D. Bakker shared last authorship and contributed equally to this manuscript.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Table S1
(DOCX 19 kb)
Rights and permissions
About this article
Cite this article
Pathak, J.L., Bravenboer, N., Verschueren, P. et al. Inflammatory factors in the circulation of patients with active rheumatoid arthritis stimulate osteoclastogenesis via endogenous cytokine production by osteoblasts. Osteoporos Int 25, 2453–2463 (2014). https://doi.org/10.1007/s00198-014-2779-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-014-2779-1