Abstract
Pre-ignition is an undesired combustion event known to restrict kinetic modeling validation. Previous methane oxidation studies reported premature ignition as part of ignition delay time measurements in shock tubes. In this context, the effect on the pre-ignition propensity and auto-ignition behavior of stoichiometric methane mixtures at different dilution levels of \(\hbox {N}_2\), Ar, He, and \(\hbox {CO}_2\) was studied at 10 bar and 25 bar and temperatures between 1080 K and 1350 K. In addition to conventional sidewall pressure and endwall light emission measurements, a high-speed imaging setup was utilized to visualize the ignition process. Relevant physicochemical parameters to describe and predict the pre-ignition phenomenon were used. The results suggest that dilution levels up to \(80\%\) of bath gas are not successful in mitigating early ignition occurrence and its effects at moderate pressures. Replacing \(\hbox {N}_2\) by He was found to suppress early ignition at 10 bar, attributed to an enhanced dissipation of temperature inhomogeneities in the test gas section. The present findings demonstrate that \(\hbox {CO}_2\) has potential for pre-ignition heat release mitigation, while Ar was confirmed to promote premature ignition. To the best of our knowledge, we present the first detailed study on pre-ignition mitigation for methane mixtures in shock tubes, where further insights into its ignition non-idealities are given.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Gaseous fuels are of rising interest in transport and electricity generation. They can be synthetically produced via biomass gasification and, more recently, based on the use of renewable electricity [1]. The kinetic mechanism of the small hydrocarbons (C1–C4) lays the foundation for the development of mechanisms for these gaseous fuels, with the ultimate aim of achieving maximum operation efficiency and minimum pollutant emissions [2]. Ignition delay time (IDT) data have been used for many decades to validate these kinetic models. The shock tube is a standard facility to measure ignition delay times over a broad range of temperatures (600–3000 K) and pressures from sub-atmospheric regime to as high as 1000 atm [3].
Methane (\(\hbox {CH}_4\)) oxidation and ignition in shock tubes have been intensively investigated over the past six decades via species profiles and ignition delay time measurements using different thermal bath gases [4]. Nitrogen (\(\hbox {N}_2\)) [5,6,7,8,9,10,11] and argon (Ar) [12,13,14,15,16] were mainly used as diluent in \(\hbox {CH}_4\) kinetic modeling investigations. The effects of \(\hbox {CO}_2\) on the \(\hbox {CH}_4\) ignition behavior have been also studied recently in [17,18,19,20,21], concluding that, under certain conditions, kinetic models are able to predict the auto-ignition behavior of \(\hbox {CH}_4\) mixtures, despite the non-idealities that high-\(\hbox {CO}_2\) dilution levels generate, i.e., shock bifurcation [20] and amplified fluid motion [18].
The majority of prior \(\hbox {CH}_4\) oxidation research in shock tubes focused on kinetic modeling under the assumption of zero-dimensional transport-free auto-ignition behavior, i.e., as a constant-volume process with constant internal energy. Only a few works reported premature ignition as potentially affecting the ignition behavior of methane mixtures at low temperatures [2, 5, 9, 21, 22]. This non-ideal ignition mode, commonly known as pre-ignition, has been observed in shock tubes for the last 20 years [2, 21, 23,24,25,26,27,28] and extensively documented in gasoline engines and rapid compression machines (RCM) for many decades [29, 30].
Pre-ignition leads to an undesired localized non-homogeneous ignition [24]. It is often observed starting in the form of a flame kernel with a simultaneous non-ideal gradual increase in pressure, resulting in shorter measured IDTs [23]. Such phenomenon only occurs at extreme pressure and temperature conditions, and therefore, most ST measurements reported in the literature are otherwise valid for kinetic model development. Understanding the pre-ignition phenomenon is important to prevent erroneous assumptions when developing kinetic models.
Several studies focused on analyzing, understanding, and preventing pre-ignition in shock tubes for different fuels, with the help of innovative imaging techniques. Troutman et al. [31] investigated the inhomogeneous combustion of n-heptane mixtures using a high-repetition-rate imaging system and concluded that window recessions and the accumulation of particulates in the test gas section can expedite premature ignition. Barak et al. [32] observed the inhomogeneous ignition of oxy-syngas mixtures at low pressures (\({<2}\) atm) using a high-speed imaging endwall configuration and found that experiments showing mild ignition under high-diluted \(\hbox {CO}_2\) conditions were not well predicted by the kinetic models employed. Ninnemann et al. [28] observed premature inhomogeneous ignition events for hydrogen mixtures at low temperatures leading to discrepancies between experimental and predicted IDTs. This work also showed nearly identical ignition delay times from both clean and dirty shock tube facilities. All these works identified and visually characterized pre-ignition events, showing its impact on the auto-ignition behavior, using an endwall imaging setup.
Some studies used similar endwall imaging configurations to investigate how this non-ideal effect could be mitigated and how much this phenomenon is linked to certain physical properties of the mixtures. Figueroa et al. [33] studied the effects of pre-ignition on the auto-ignition characteristics of ethanol, providing high-speed \(\hbox {OH}^*\) chemiluminescence results. Thermal diffusivity (\(\alpha \)) and laminar flame thickness (\(t_{\textrm{L}}\)) of the mixture were found to have an influence on the likelihood and effects of pre-ignition. More recently, Figueroa et al. [34] showed that pre-ignition propensity can be evaluated using additional predictive criteria, i.e., the Sankaran number, the Damköhler number, and the laminar burning velocity (\(S_\textrm{L}\)). Tulgestke et al. [35] captured homogeneous and inhomogeneous ignition of n-heptane, iso-octane, and surrogate fuels using a high-speed camera. The fraction of particles that contribute to causing early ignition can be reduced by replacing Ar with He as diluent gas, due to its higher thermal diffusivity. Nativel et al. [36] studied ethanol–air mixtures containing different bath gases at moderate pressures (20 atm) and different dilution levels, providing \(\hbox {OH}^*\) chemiluminescence imaging results. Diluting the mixture and adding small amounts of He was shown to mitigate pre-ignition events.
All these works employed endwall imaging configurations to visualize the ignition process axially. More recently, however, this approach has been complemented with simultaneous lateral imaging. Figueroa et al. [37] utilized simultaneous endwall and lateral high-speed imaging to visualize the ignition on representative ethanol, methanol, and n-hexane experiments, showing that far-endwall ignition events cannot be identified by endwall diagnosis alone. This work was extended in [38], and the imaging results were complemented with pressure traces and IDT measurements. These diagnostics techniques allowed to characterize the ignition process and its influence on the auto-ignition behavior at low pressures (< 3 bar) more precisely. Ninnemann et al. [39] visualized mild ignition events of methane, n-hexane, and iso-octane mixtures at low pressures (2 bar) using simultaneous endwall-lateral imaging. They concluded that non-homogeneous ignition under heavy shock bifurcation conditions carries increased uncertainty due to the arisen gradients in temperature and species concentrations. Figueroa et al. [40] reported non-homogeneous ignition for n-hexane mixtures at 10, 15, and 20 bar using simultaneous endwall-sidewall imaging in a high-pressure shock tube. The inclusion of He as diluent was shown to increase the resistance to the formation of early ignition kernels but did not completely mitigate non-homogeneous ignition at the studied conditions.
Premature ignition in shock tubes for methane–air mixtures at low temperatures (< 1250 K) and moderated pressures (15–40 bar) was previously reported in [2, 5, 9] using conventional diagnosis, i.e., pressure transducers and photodetectors. Similarly, Laich et al. [21] identified premature energy release, with the help of an \(\hbox {OH}^*\) emission detector, for non-diluted mixtures of methane in \(\hbox {N}_2\) and \(\hbox {CO}_2\) as bath gases at 15 bar and low temperature (1040–1160 K). In all these works, the impact of pre-ignition on the auto-ignition behavior of methane mixtures was clearly visible in the Arrhenius plots, but the pre-ignition phenomenon itself was not extensively addressed. The pre-ignition characterization of methane-air mixtures is still lacking and such an effort will enable us to conclusively answer some of the fundamental questions regarding inhomogeneous ignition in shock tubes. Therefore, it is the goal of this study to complement conventional diagnostics with high-speed endwall imaging to further understand the methane ignition process that has been shown to exhibit non-idealities in the post-reflected shock region.
Measurements of ignition delay times (IDTs) and endwall high-speed imaging in a high-pressure shock tube for stoichiometric methane/\(\hbox {O}_2\) mixtures compressed pressures of at 10 and 25 bar (\(P_5\)) and temperatures ranging between 1080 and 1350 K (\(T_5\)) were carried out for a range of mixture compositions, where the molar ratio of total diluent to \(\hbox {O}_2\), or dilution ratio, was modified by adding extra nitrogen (\(\hbox {N}_2\)) to the mixture and different bath gases were used: helium (He), argon (Ar), and carbon dioxide (\(\hbox {CO}_2\)). All experimental results were complemented by IDT simulations and 1-D flame calculations to explore relevant theoretical criteria that can be helpful to predict the occurrence and effects of the inhomogeneous ignition on methane mixtures.
2 Experiments
2.1 Ignition delay time setup
Ignition delay time measurements behind reflected shock waves are measured in the HGD kinetic shock tube (formerly PCFC-ST). Details of the apparatus can be found in [41]. The incident shock velocities were measured over the last 1.0 m of the driven section using seven PCB-113B22. To determine the gas conditions behind the reflected shock, an in-house code was used based on the “Shock and Detonation Toolbox” [42] developed for Cantera [43]. Thermochemical data for methane have been taken from the literature [44]. The experimental data discussed in this work are summarized in the Supplemental Information (SI).
The uncertainties of temperatures and pressures after the reflected shock wave were estimated based on the law of propagation of errors, as detailed in the SI. Uncertainties in the measurements of the initial temperature and pressure and the shock velocity lead to an uncertainty in \(P_5\) and \(T_5\) of 2.75% and 1.3% on average, respectively. This uncertainty can be translated into an expected scatter of IDT ranging from 10 to 25%, similar to the uncertainties derived in [45]. These values, represented as error bars, will be taken as maximum IDT deviations in the upcoming discussion.
Ignition delay times were defined based on the time between the pressure rise caused by the reflected shock and the steepest pressure rise resulting from the ignition. In this work, the IDTs are deduced from the sidewall pressure trace obtained from the silicon-coated pressure sensor located closest to the endwall (9 mm), as typically done in works where kinetic models are validated (e.g., [26, 41]). This method has also been used in works where the pre-ignition impact on the IDTs of different fuels is investigated (e.g., [26, 46]).
Additionally, a line-of-sight light emission diagnostic was implemented using the Hamamatsu H10721 photosensor module at the endwall of the shock tube. No bandpass filter was used; hence, the sensor indicates any chemiluminescence in its detection range of 185–700 nm. Similar to the IDT definition, the deflagration time (\(\Delta \tau \)) is defined as the time between the onset of the first flame kernel, which is defined based on the normalized emission signal exceeding 0.05 (\(5\%\)), also used in [31, 39], and the steepest pressure rise resulting from the ignition.
2.2 High-speed camera setup
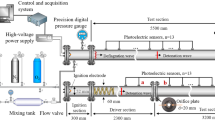
To support the interpretation of the pressure measurements, high-speed imaging has been used in this work to visualize the ignition process. A high-speed camera (Phantom v1612), coupled to a ZEISS Milvus \(2/100\,\hbox {M}\) focusing objective, was operated at 25 kHz to capture shock tube cross-sectional images, as depicted in Fig. 1. The transparent endwall consisted of a fused silica disk with 78 mm diameter and 45 mm thickness. The trigger signal used to start the recordings was a TTL signal obtained from a Stanford Research Systems DG645 delay generator connected to the pressure sensor closest to the shock tube endwall.
2.3 Mixture conditions
Experiments with stoichiometric fuel mixtures are targeted at multiple \(\hbox {O}_2\)/diluent mole ratios, where \(\hbox {N}_2\), He, Ar, and \(\hbox {CO}_2\) were used as diluents (see Table 1). Further insights into the mixture preparation can be found in [41]. In previous works, different diluent gases and dilution levels and bath gases have been investigated to understand the mechanisms behind pre-ignition in shock tubes. Highly diluted \(\hbox {N}_2\) mixtures were found to be more prone to ignite homogeneously than non-diluted mixtures [36]. Argon has been widely used rather than \(\hbox {N}_2\) as bath gas in pre-ignition studies [26, 28, 31, 35]. Previous works [35, 36, 40] reported that He can be useful to mitigate pre-ignition due to its high thermal diffusivity under certain conditions. Several studies have been conducted to investigate the effects of \(\hbox {CO}_2\) on the combustion process of \(\hbox {CH}_4\) [17,18,19,20,21]. To the best of our knowledge, however, this is the first study where \(\hbox {CO}_2\) is used as a potential diluent candidate for pre-ignition suppression in shock tubes. It is important to note that, according to constant-volume simulations, the variation of the bath gas has a negligible effect on kinetics (see SI).
3 Simulations
Ignition delay time simulations were performed with Cantera [42] using non-reactive (NR) effective volume traces to account for facility effects which cannot be captured by assuming a constant pressure rise, i.e., appreciable and non-uniform change in pressure and temperature behind the reflected shock due to shock attenuation and shock-contact surface interaction effects. In the non-reactive experiments, the oxygen fraction of the mixture was replaced by nitrogen to ensure similar boundary layer effects. These experiments were conducted at similar conditions as those reported in the reactive experiments (maximum deviation of 1% in \(T_5\) and 2% in \(P_5\)). Based on the pressure traces of the non-reactive experiments, effective volume traces were deduced assuming an isentropic change of conditions, similar to the procedure established for rapid compression machines [47]. The resulting volume traces are then used to prescribe a volume change in the homogenous reactor model to mimic in simulation the change in pressure and temperature induced by the gas-dynamic effects in the shock tube experiment. The non-reactive volume traces can be found in the SI. The time of ignition was defined as the time of maximum rate of pressure increase, similar to the experiments.
Additionally, freely propagating, adiabatic, 1-D flame simulations were conducted with Cantera to estimate relevant flame-related properties, including laminar flame thickness and burning velocity. All simulations have been conducted using a version of NUIGMech1.0 [44] reduced to species not larger than C3. The underlying chemistry relevant in the present study remains the same as in the most up-to-date version of NUI Galway: NUIGMech1.1 [48].
4 Discussion
4.1 Problem description
Ignition delay times were initially measured for mixture M1, for which the \(\hbox {N}_2/\hbox {O}_2\) ratio corresponds to that of air, at a nominal pressure of 25 bar. Our reference data are compared against previous experimental results [2, 9, 10], where shock tubes with different dimensions were used. As shown in Fig. 2, for the whole temperature range a reasonable agreement between literature data and the present experimental results has been obtained, especially in the regimes where non-homogeneous ignition is observed.
Chemical kinetic modeling results obtained with the NUIGMech1.0 mechanism were also in reasonable agreement with the reported IDTs at high temperatures (\(T_5 > 1250\) K) when taking facility effects into account. In this temperature range, an Arrhenius trend can be clearly recognized. This data range is characterized by strong and clear ignition (see Fig. 3), where the deflagration time is smaller than 0.2 ms (\(\Delta \tau<\) 0.2 ms) and ignition occurs near the endwall. All this is necessary for a meaningful IDT measurement [49].
Between 1100 and 1250 K, a remarkable pressure rise prior to the main ignition and a larger \(\Delta \tau \) (see 1197 K case in Fig. 3) is observed, revealing a significant energy release before the volumetric ignition. This effect on the test gas pressure and temperature is expected when pre-ignition and inhomogeneous ignition take place [26, 27]. The onset of the pre-ignition event is clearly identified by the light detector signal. The early energy release can be attributed to premature hot spot mild ignition leading to the formation of one or several flame kernels, as previously observed in the high-speed imaging studies [22, 28, 33,34,35,36]. The formation of these hot spots can occur due to inhomogeneities, especially in temperature or radical concentration, but also due to diaphragm fragments that travel toward the test gas section [23]. Imaging results are reported and discussed in Sect. 4.3.2 (see Fig. 10) to illustrate this phenomenon. Note that, due to mechanical strength limitations of the optical section, imaging results were only reported for tests performed at a lower reflected pressure of 10 bar. At even lower temperatures (\(T_5 < 1110~\textrm{K}\)), where the reactivity is rather low, the ignition proceeds via the so-called mild ignition scenario, where the majority of the combustible mixture is consumed via deflagration. This is indicated by a smoother pressure rise and a less pronounced light signal increase.
High-temperature levels are required to observe any reactivity for methane mixtures in a shock tube process time scale. Consequently, pre-ignition occurs at higher temperature levels compared to other fuels, e.g., ethanol. Nativel et al. [36] reported homogeneous ignition for experiments of ethanol at 20 bar and stoichiometric conditions, at temperatures around 950 K. At such low-temperature conditions, no ignition could be observed for the investigated methane mixtures.
The reactivity of those experiments exhibiting non-homogeneous ignition is underpredicted by conventional kinetic models, as many works have shown (e.g., [26, 33, 34, 36]). In this work, the negative deviations between experiments undergoing premature ignition and model predictions are systematically higher than the uncertainty limits, and of the same order of magnitude as the pre-ignition data reported in [26, 34], which also shows the impact of pre-ignition on the present experiments.
Those experiments undergoing premature ignition are also prone to auto-ignite further from the endwall [46, 50], especially those undergoing strong shock bifurcation effects [51]. The assumption of a constant-internal-energy process becomes invalid due to the resulting large gradients of temperature and density far from the endwall of the shock tube [36]. Using a large number of pressure transducers located along the test section of the shock tube, over the last 1 m, the development of the ignition blast wave can be described by means of x–t diagrams [26]. This method does not provide the exact location of the first flame kernel formation but at least a first estimation of the main ignition location. Most main ignition events reported in this work occurred between the first and the second sensors, i.e., between 9 and 149 mm from the endwall. It was also found that the distance between the endwall and the position of the origin of the blast wave increases with decreasing temperature. A more detailed description of how the x–t information was derived is given in the SI.
Ignition far from the endwall has been recently corrected in [52] based on a 1-D temperature-gradient model [53]. This model assumes that ignition is initiated spontaneously and not from a singular ignition point. For the investigations in [52], this assumption seems to be valid as no significant pressure rise before the main ignition induced by a deflagration process could be observed. In the present study, however, such a pressure rise is visible in pre-ignition experiments (see Fig. 3), indicating the presence of a substantial deflagrative process. Besides, in the imaging results discussed in Sect. 4.3.2 (see Fig. 10) a clear deflagration process starting in the form of a flame kernel is visualized. All this indicates that the pre-ignition events reported in this work are induced by local flame kernels, and therefore, the measured IDTs cannot be corrected using the model from [53].
4.1.1 Pre-ignition phenomenon
To further discuss the pre-ignition phenomenon, three different aspects need to be considered. First, the origin of inhomogeneities that eventually lead to pre-ignition. Second, the transition from an inhomogeneity to a first flame kernel. Third, the propagation of the flame originated from this flame kernel.
Aspect \(\#1\): It is known that the presence of reacting diaphragm particles in the test section and the temperature stratification induced by shock bifurcation can lead to inhomogeneous ignition of the mixture [35, 51]. On the one hand, the influence of reacting particles might be reduced by frequently cleaning the test gas section, but this is hard to quantify precisely. On the other hand, shock bifurcation occurs when the boundary layer lacks the momentum needed to pass through the normal portion of the shock wave. Previous studies have emphasized that the boundary layer interaction effects can be attenuated by using monoatomic gases such as Ar and He, due to their decreased molecular degrees of freedom. However, the bifurcation Damköhler number calculations provided in the SI, defined in [51], indicate that the shock bifurcation is likely to happen for all experimental cases studied in this work, which suggests that this phenomenon can be an important root cause of temperature inhomogeneities under the investigated conditions. The use of different bath gases and dilution ratios, however, was found insufficient to avoid the formation of shock bifurcation inhomogeneities. This can be attributed to the high post-reflected shock temperatures and consequently high Mach numbers leading to large bifurcation shock heights.
Aspect \(\#2\): The early formation of a flame kernel is governed by the thermal diffusivity and laminar flame thickness of the mixture, as discussed in [33, 34]. If local inhomogeneities exist, a flame kernel might not form if either the temperature or radical inhomogeneities are dissipated at high rates into the surrounding gas (high thermal and mass diffusivity), or the originating hot spots are not larger than the laminar flame thickness.
Aspect \(\#3\): Once the flame has already been established, the deflagration front expands leading to a compression of the unburnt mixture. This additional compression of the reactive mixture eventually leads to early auto-ignition. Nativel et al. [36] argued that the deflagration process can be influenced by the heat release rate, as illustrated in [34] by laminar burning velocity calculations. The expression “heat release” refers to the overall macroscopic energy release that results from the balance between the chemical reactions’ energy release and the energy that is being stored in the gas, defined by the heat capacities (\(C_p\)) of all components. Even if local flame kernels are formed, the resulting pressure and temperature increase in the unburnt mixture might be too low to induce a significant acceleration of the auto-ignition process, provided the flame propagation is slow.
In the next sections, relevant physicochemical parameters of the mixtures will be evaluated to predict the pre-ignition occurrence and understand how \(\hbox {N}_2\) dilution and different bath gases influence the occurrence (Aspect \(\#2\)) and effects (Aspect \(\#3\)) of pre-ignition in methane mixtures. The numerical values of these relevant pre-ignition parameters are listed in Table 1. Before addressing this topic, a well-known predictive criterion, the Sankaran number, was calculated to complement the evaluation of the pre-ignition propensity of mixture M1 (see Sect. 4.1).
4.1.2 Sankaran criterion
Sankaran et al. [54] defined predictive criteria, based on the definition of the speed of a spontaneous ignition front given by Zeldovich in [55], to identify the two distinct ignition regimes in HCCI engines: the spontaneous propagation, or strong ignition, and the deflagration regime, typically attributed to pre- and mild ignition modes.
The definition of this transition parameter applied to either shock tubes and RCMs [34, 40, 56,57,58] reads: \(\hbox {S}\mathrm {_a}=\beta S_\textrm{L} |\frac{\partial \tau _\textrm{g}}{\partial T_5} |\frac{ T_\textrm{f}}{l_\textrm{c} \mathrm {Re_T}^{-0.5}}\), where \(\beta \) is an empirical constant, which is chosen as 0.5 in [40, 54, 57, 58], indicating a certain delay in developing a deflagration from the ignition kernel, whereas in [34] this parameter is not considered. The derivative of the IDT with respect to the test temperature, \(\frac{\partial \tau _\textrm{g} }{\partial T}\), is estimated based on kinetic model predictions, as previously done in [34, 40, 58]; \(S_\textrm{L}\) is the laminar flame speed of the bulk gas after the reflected shock, \(\mathrm {Re_T}\) is the turbulent Reynolds number, roughly estimated in [57] as \(l\mathrm {_c} T_\textrm{f} / \alpha \), where \(l_\textrm{c}\) and \(T_\textrm{f}\) are the characteristic length scale and the RMS fluctuation of temperature of the core gas, respectively, and \(\alpha \) is the thermal diffusivity. In shock tube studies, \(l_\textrm{c}\) is typically chosen as 1 mm [34, 40, 58] and \(T_\textrm{f}\) is chosen as 2 K in [40, 58] or 1 K in [34]. Despite the differences in the mentioned parameters, the product \(\beta T_\textrm{f}\) is identical in all cited works.
This definition can be interpreted as follows: If S\(\mathrm {_a > 1}\), meaning the laminar flame velocity is more than two times higher than the speed of the spontaneous ignition front (\(\beta = 0.5\)), the overall speed of reactions is much slower than the rate of development of a deflagration within the bulk gas, resulting in mild ignition or premature energy release prior to the main ignition onset. If \(\hbox {S}\mathrm {_a} < 1\), the gas inhomogeneities will not have enough time to develop a flame before the main ignition takes place, and therefore the bulk gas will ignite homogeneously.
Selecting the values of the empirical parameters suggested in the literature, i.e., 0.5 for \(\beta \), 2 K for \(T_\textrm{f}\), and 1 mm for \(l_\textrm{c}\), the Sankaran criterion for mixture M1 at 25 bar can be estimated. Figure 4 shows that the transition number is below 1 for all the conditions studied (solid line), which indicates that all the experiments in this study should undergo strong ignition. However, as is evident from the conventional diagnostics (see Fig. 3), the pressure traces and light sensor results show that at 1250 K, and especially below, the ignition mode transitions into deflagration. This brings us to the conclusion that the empirical parameters taken from the literature are not appropriate for the investigated conditions.
Sankaran criterion for mixture M1 at 25 bar. The horizontal line defines the transition from strong ignition to deflagration according to the Sankaran criterion. Vertical lines define the ignition modes observed in the experiments taken from Fig. 2
The assumption of constant \(T_\textrm{f}\) conflicts with previous research [31, 39], where this parameter was found to be strongly dependent on the test temperature. In previous works, \(T_\textrm{f}\) was determined based on the Arrhenius equation. In this work, a similar approach using a three-parameter fit [59] has been utilized to describe the non-Arrhenius temperature dependence of the predicted IDTs: \(\frac{1}{\tau }=\frac{1}{\tau _{(\textrm{A})}}+\frac{1}{2\tau _{(\textrm{nA})}}\). Here, \(\tau _{(\textrm{A})}\) and \(\tau _{(\textrm{nA})}\) are the characteristic times of those model predictions following or not an Arrhenius trend, respectively. These quantities are calculated using the Arrhenius equation, \(\tau =A \textrm{e}^{\frac{E_\textrm{a}}{RT_5}}\), where R is the gas constant, \(E_\textrm{a}\) is the activation energy of the studied mixture under the investigated conditions, and A is the pre-exponential factor. As is evident from Fig. 2, the resulting correlation reasonably reproduces the auto-ignition behavior of the studied mixture over the whole investigated temperature range.
The temperature gradients (\(T_\textrm{f}\)) in the core gas region are estimated by using the derivative expression of the fitting equation proposed (\(\frac{\partial \tau }{\partial T_5}\)) and the deflagration time (\(\Delta \tau \)), as  . This expression describes how much a flame kernel could potentially impact the temperature distribution in the core gas. The estimated temperature fluctuations in the core gas for the studied mixtures are presented in Fig. 5 (top). As one can infer from the M1 data at 25 bar, the temperature fluctuations in the core gas region increase as the reflected shock temperature decreases, from values of 2–5 K for those experiments undergoing strong ignition up to values of 35 K for those showing mild and premature ignition. This tendency is in agreement with previous works [23, 39]. Additionally, Fig. 5 (top) shows that the temperature fluctuations are also highly dependent on the mixture composition and test pressure. Due to the fact that this parameter can drastically change over the studied conditions, it needs to be determined for every experiment before the ignition transition parameter is calculated.
. This expression describes how much a flame kernel could potentially impact the temperature distribution in the core gas. The estimated temperature fluctuations in the core gas for the studied mixtures are presented in Fig. 5 (top). As one can infer from the M1 data at 25 bar, the temperature fluctuations in the core gas region increase as the reflected shock temperature decreases, from values of 2–5 K for those experiments undergoing strong ignition up to values of 35 K for those showing mild and premature ignition. This tendency is in agreement with previous works [23, 39]. Additionally, Fig. 5 (top) shows that the temperature fluctuations are also highly dependent on the mixture composition and test pressure. Due to the fact that this parameter can drastically change over the studied conditions, it needs to be determined for every experiment before the ignition transition parameter is calculated.
Using the new definition of \(T_\textrm{f}\), the Sankaran number describes in a more reliable way the transition from strong to pre-ignition for mixture M1 at 25 bar, compared to the approximation where a constant-temperature fluctuation value was used, as shown in Fig. 4. According to this new estimation, the corresponding transition is situated at 1290 K. This behavior only differs slightly from what is observed in the experiments, where the transition from strong to pre-ignition occurs at around 1250 K. Nevertheless, a marginal energy release prior to the main ignition is already observed at 1270 K. The estimation of the transition from strong ignition to deflagration would be in better agreement with the experiments by increasing the characteristic length scale of the temperature fluctuation in the core gas, \(l_\textrm{c}\), from 1 mm to 6 mm. The ignition mode for all the other conditions studied in this work is also well described by the Sankaran criterion (see Fig. 5, bottom). Further evaluation and more data points would be required to find the transition from strong to mild ignition for those mixtures where the bath gas and/or dilution ratio are varied.
This evaluation highlights the importance of estimating the temperature fluctuations in the core gas for every condition studied, rather than assuming a constant value over the entire experimental batch. This finding conflicts with one of the assumptions of Susa’s model [53], where the axial temperature gradient is presumed constant, regardless of the test temperature. Overall, the Sankaran parameter cannot be used for ignition regime predictions at those conditions where the temperature gradients are highly sensitive to the test conditions. Deriving a reliable predictive pre-ignition criteria would require a much wider experimental database, and therefore, it is beyond the scope of this study.
4.2 Effect of \(\hbox {N}_2\) dilution
Ignition delay time experiments at different dilution levels (3.76, 4.69, and 5.51) were conducted at 25 ± 0.3 bar and temperatures near 1170, 1200, and 1230 K (± 5 K), which is the temperature region where mixture M1 exhibits strong pre-ignition events. The objective here was to investigate the influence of dilution on the ignition behavior of methane mixtures.
Measured pressure traces and IDT measurements for the diluted and non-diluted mixtures are presented in Figs. 6 and 7, respectively. As one can infer from Fig. 6, the slope of the gradual pressure rise and the maximum pressure prior to the main ignition decrease as the dilution ratio increases. Diluting the mixture also delays the onset of the pre-ignition event. However, highly diluted methane mixtures still show a remarkable pre-ignition energy release. The impact of the undesired early energy release on the ignition delay time deviation is also observed in Fig. 7. On average, differences between simulated and measured IDTs are lower for the highly diluted mixture (\(\sim 17\%\)) than for the non-diluted case (\(\sim 22\%\)), given the weaker pre-ignition pressure rise observed in the highly diluted experiments. However, the deviation to predicted IDTs is still larger than the average uncertainty.
The observed deviations in ignition behavior can be explained due to differences in the likelihood of developing a flame (Aspect \(\#2\)) and the flame front development (Aspect \(\#\)3). As the dilution rate increases, the adiabatic flame temperature decreases, as well as the laminar burning velocity [60]. This results in a reduction of the pre-ignition heat release rate (Aspect \(\#\)3). One way to describe this effect is via 1-D, freely propagating flame, laminar burning velocity calculations. Increasing the dilution ratio of the methane/\(\hbox {O}_2\) mixture with \(\hbox {N}_2\) from 3.76 to 5.51 reduces the laminar burning velocity by around \(45\%\). This overall hinders the deflagration front development within the mixture upon ignition [54].
The laminar flame thickness, also known as critical thickness, and thermal diffusivity of the mixture can have a significant influence on the early flame kernel formation [33, 36] (Aspect \(\#\hbox {2}\)). Diluting the mixture with \(\hbox {N}_2\) does not influence the thermal diffusivity of the mixture under the investigated conditions. To estimate the critical thickness of the unburnt mixture, the direct method suggested by Figueroa et al. [34] is proposed. Increasing the dilution rate of the mixture from 3.76 (M1) to 5.51 (M3) enlarges the critical thickness by more than \(50\%\). Consequently, local hot spots need more time to grow before they can trigger the first flame kernel formation so that the onset of pre-ignition should be delayed at high dilution levels. This positive trend, however, is reduced by the fact that at constant temperature also the auto-ignition is delayed at higher levels of dilution. This provides more time for flame kernels to form so that even at the highest investigated dilution level pre-ignition influences the auto-ignition behavior.
4.3 Effect of different bath gases
4.3.1 25 bar: \(\hbox {N}_2\) vs. Ar vs. He
Ignition experiments were performed using Ar or He as diluents at 25 ± 0.5 bar and temperatures near 1170, 1200, and 1230 K (± 12 K). Argon or He was added to the methane “in air” mixture (M1) so that the volume fraction of the diluent was \(25\%\), resulting in mixture A3 and H3, respectively. These were compared to experiments with nitrogen dilution at the same dilution level (M3) to study the effect of the carrier gas. This allows us to decouple the effect of dilution from the ignition behavior evaluation.
Measured pressure traces and IDT measurements for the studied mixtures are presented in Figs. 8 and 9, respectively. As is evident from Fig. 8, the pressure rise prior to the main ignition becomes more pronounced when replacing \(\hbox {N}_2\) by Ar, as a result of a higher pre-ignition energy release. This leads to even larger differences between experiments and simulations for the highly diluted case, as shown in Fig. 9. Under these conditions, replacing a fraction of \(\hbox {N}_2\) by He (H3) does not have a strong impact on the pre-ignition energy release, i.e., both pressure traces show a similar pressure rise shape before the main ignition. This correlates well with the fact that both mixtures show nearly the same deviations between measured and calculated IDTs (see Fig. 9).
As described in Sect. 4.2, some physicochemical parameters can be evaluated to account for the different ignition behaviors. Both Ar and He mixtures exhibit laminar burning velocities 25 and \(50\%\) higher than the \(\hbox {N}_2\)-containing mixture, respectively, as expected according to the lower heat capacities of these gases. This effect expedites the deflagration front development within the mixture upon ignition (Aspect \(\#\hbox {3}\)). The difference between Ar and He lies in the thermal diffusivity and critical thickness. On the one hand, mixture A3 exhibits almost no difference in thermal diffusivity and a \(15\%\) lower critical thickness compared to M3, which eases the formation of early flame kernels (Aspect \(\#2\)). On the other hand, mixture H3 shows an increase of \(60\%\) in thermal diffusivity, while the critical thickness is similar to the \(\hbox {N}_2\) case. This overall helps to dissipate temperature inhomogeneities, which are the main cause of early flame kernel formation (Aspect \(\#2\)).
This evaluation provides insights into the distinct ignition phenomena for the mixtures studied. The experimental results suggest that Ar-containing mixtures are more prone to ignite inhomogeneously compared to \(\hbox {N}_2\)-containing mixtures under these conditions. This is in line with the above relevant parameters evaluation and previous findings [33, 36]. Argon was chosen because it is frequently used in IDT studies at high dilution levels [26, 28, 33, 36]. Helium does not reduce the pre-ignition propensity of the mixture and behaves similarly to \(\hbox {N}_2\) at these conditions, as experimental data and simulations revealed. This can be attributed to the competition between higher thermal diffusivity, which would mainly help avoid pre-ignition, and higher heat release [36], which would exacerbate the effect of pre-ignition, resulting in no significant difference on the pre-ignition behavior. To conclude, pre-ignition cannot always be avoided, but its effect can be minimized by adding components with higher heat capacity. This will be investigated in the following section by the use of \(\hbox {CO}_2\).
4.3.2 10 bar: \(\hbox {N}_2\) vs. \(\hbox {CO}_2\) vs. He
To further investigate the impact of different bath gases on the pre-ignition phenomenon, high-speed chemiluminescence imaging was performed for non-diluted methane mixtures containing \(\hbox {N}_2\), He, and \(\hbox {CO}_2\). Due to mechanical limitations of the optical window, IDT experiments at 10 ± 0.2 bar and temperatures near 1285 K (\(\pm 13\) K) were conducted at a fixed dilution level, replacing a fraction of \(\hbox {N}_2\) by \(\hbox {CO}_2\) or He, resulting in mixtures C1 and H1, respectively. As in the previous section, the ignition behavior of these mixtures was compared to a \(\hbox {N}_2\)-dilution inhomogeneous ignition case at the same dilution level (M1). The goal here was to investigate the influence and possible suppression of pre-ignition pressure rise by using components with higher heat capacity (\(\hbox {CO}_2\)) or higher thermal diffusivity (He). Experiments were repeated three times and complemented with an additional high-speed imaging measurement to make more meaningful comparisons.
As is evident from the image sequences in Fig. 10, localized/inhomogeneous ignition was observed for the \(\hbox {N}_2\)-containing mixture (M1). When the pressure trace exhibits a pressure increase due to heat release (see Fig. 11), the high-speed images depict a bright spot that grows as a single kernel toward the wall of the shock tube cross section until the main ignition event occurs after around 0.44 ms. This deflagration process was also observed in previous works where the shock bifurcation is described as a potential pre-ignition precursor [28, 31, 34, 35]. Overall, the smooth pressure increase prior to the main ignition leads to deviations between experiments and simulations close to the average uncertainty, as shown in Fig. 10.
A similar ignition mode is observed from the camera recordings for the \(\hbox {CO}_2\)-containing mixture (C1). In this case, a flame kernel is formed at approximately the same time as in the \(\hbox {N}_2\) case. This suggests that a larger critical thickness (17% difference) is compensated by a smaller thermal diffusivity (5% difference), indicating the greater importance of the latter criterion. Besides, the flame front propagates in a slower manner as the pure \(\hbox {N}_2\) flame does (\(\#3\)), which correlates with the \(30\%\) lower laminar burning velocity of the \(\hbox {CO}_2\)-containing mixture. This effect is attributed to the higher heat capacity of \(\hbox {CO}_2\) compared to \(\hbox {N}_2\), resulting in lower pre-ignition heat release. As suggested by the pressure traces shown in Fig. 11, the pressure increase right after the light emission onset takes place more closely to the main ignition, compared to the \(\hbox {N}_2\)-containing pressure traces. This means that the unburnt mixture is at elevated pressure and temperature for a shorter time and consequently the auto-ignition is less influenced by the pre-ignition event compared to the \(\hbox {N}_2\) case. Such differences lead to consistently lower IDT for the simulations compared to experiments (see Fig. 10, bar chart).
Only the He-containing mixture (H1) shows homogeneous ignition at these conditions, as indicated on the camera recordings. Around 0.16 ms after the initial emission observation, flame kernels appear across the shock tube cross section in a faster and uniform manner, burning the complete mixture homogeneously. This leads to a stronger ignition which is identified as a steep and pronounced rise in the pressure, leading to a smaller difference between experiments and simulations. In this case, the pre-ignition suppression is attributed to a significant increase in thermal diffusivity of \(60\%\) due to He addition, accompanied by a similar critical thickness (larger by \(5\%\)), which overall leads to an inhibition of early flame kernels (Aspect \(\#2\)). This effect compensates for the higher heat release characteristic of He-containing mixtures (Aspect \(\#3\)), illustrated by an increase of \(30\%\) in the laminar burning velocity compared to the \(\hbox {N}_2\)-containing mixture. As can be inferred from the 10 and 25 bar results (see Sect. 4.3.1), pre-ignition suppression by high thermal diffusivity is more effective at lower pressures.
These results suggest that pre-ignition inhibition in methane mixtures should be investigated from two perspectives. First, by reducing the temperature inhomogeneities, which are the main cause of early flame kernels formation (Aspect #2). Helium was used as bath gas for that purpose, due to its high thermal diffusivity. The second is once the flame is developed, by hindering the deflagration front propagation (Aspect \(\#3\)). This effect has been obtained via the addition of small amounts of \(\hbox {CO}_2\), resulting in lower pre-ignition heat release. However, higher \(\hbox {CO}_2\) concentrations can induce additional undesired non-idealities in the test section [18]. Even if pre-ignition heat release is not completely suppressed by \(\hbox {CO}_2\) addition, its influence on the pressure trace becomes evident closer to the main ignition, thus preventing large deviations from the ideal ignition behavior.
5 Conclusion
Ignition delay time experiments were conducted for stoichiometric methane “in air” at 25 bar and temperatures between 1080 K and 1350 K. Experiments below 1250 K exhibited early light emission and strong pressure rise prior to the main ignition, producing a large deviation from the IDTs predicted by a validated kinetic model. This behavior could only be described by the Sankaran criterion if it is estimated based on empirical parameters taken from the experiments, meaning that it cannot be used as a predictive criterion at the test conditions.
The effect of dilution and alternative bath gases was investigated to suppress premature ignition and their impact on the auto-ignition behavior. Nitrogen dilution was found to help reduce the pre-ignition energy release but did not completely mitigate the effects of this non-ideality on the auto-ignition behavior at the studied conditions. The use of Ar as bath gas at high dilution levels was found to be inadequate due to the cumulative effects of low thermal diffusivity, smaller critical thickness, and higher heat release rate. The increased thermal diffusivity of He was found to be insufficient to inhibit strong premature ignition at moderate pressures.
The influence of He and \(\hbox {CO}_2\) was further studied at lower pressures using high-speed camera visualization through the shock tube endwall. This technique showed flame kernel formation prior to the volumetric ignition for \(\hbox {N}_2\) and \(\hbox {CO}_2\)-containing mixtures. However, the pre-ignition heat release affected the auto-ignition behavior of the \(\hbox {CO}_2\)-containing mixture at a lower level due to the higher heat capacity of this diluent. Camera recordings showed homogeneous ignition for the He-containing mixture. At low pressures, the thermal diffusivity increase was found to be sufficient to compensate for the higher heat release rate expected from He-containing mixtures.
This work underlines the usefulness of He in suppressing pre-ignition events in methane mixtures. To our knowledge, this is the first study where \(\hbox {CO}_2\) has been considered as a potential pre-ignition mitigating diluent candidate. Carbon dioxide was found to hinder strong pre-ignition energy releases at a certain level, thus resulting in more ideal auto-ignition behavior. This investigation provides critical insights into pre-ignition inhibition in shock tube measurements for methane mixtures. Further research is needed to characterize the pre-ignition behavior of methane mixtures to a greater extent.
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files (SI).
References
Biswas, S., Kulkarni, A.P., Giddey, S., Bhattacharya, S.: A review on synthesis of methane as a pathway for renewable energy storage with a focus on solid oxide electrolytic cell-based processes. Front. Energy Res. 8, 570112 (2020). https://doi.org/10.3389/fenrg.2020.570112
Heufer, K.A., Olivier, H.: Determination of ignition delay times of different hydrocarbons in a new high pressure shock tube. Shock Waves 20, 74–80 (2010). https://doi.org/10.1007/s00193-010-0262-2
Hanson, R.K., Davidson, D.F.: Recent advances in laser absorption and shock tube methods for studies of combustion chemistry. Prog. Energy Combust. Sci. 44, 103–114 (2014). https://doi.org/10.1016/j.pecs.2014.05.001
Spadaccini, L.J., Colket, M.B., III.: Ignition delay characteristics of methane fuels. Prog. Energy Combust. Sci. 20, 431–460 (1994). https://doi.org/10.1016/0360-1285(94)90011-6
Petersen, E.L., Davidson, D.F., Hanson, R.K.: Kinetics modeling of shock-induced ignition in low-dilution \({{\rm CH}_4}\)/\({{\rm O}_2}\) mixtures at high pressures and intermediate temperatures. Combust. Flame 117(1–2), 272–290 (1999). https://doi.org/10.1016/S0010-2180(98)00111-4
Petersen, E.L., Davidson, D.F., Hanson, R.K.: Ignition delay times of ram accelerator \({{\rm CH}_4}\)/\({{\rm O}_2}\)/diluent mixtures. J. Propul. Power 15(1), 82–91 (1999). https://doi.org/10.2514/2.5394
Petersen, E.L., Hall, J.M., Smith, S.D., de Vries, J., Amadio, A.R., Crofton, M.W.: Ignition of lean methane-based fuel blends at gas turbine pressures. J. Eng. Gas Turbines Power 129(4), 937–944 (2007). https://doi.org/10.1115/1.2720543
Zhukov, V.P., Sechenov, V.A., Starikovskii, A.Y.: Spontaneous ignition of methane-air mixtures in a wide range of pressures. Combust. Explos. Shock Waves 39(5), 487–495 (2003). https://doi.org/10.1023/A:1026186231905
Huang, J., Hill, P.G., Bushe, W.K., Munshi, S.R.: Shock-tube study of methane ignition under engine-relevant conditions: experiments and modeling. Combust. Flame 136(1–2), 25–42 (2004). https://doi.org/10.1016/j.combustflame.2003.09.002
Burke, U., Somers, K.P., O’Toole, P., Zinner, C.M., Marquet, N., Bourque, G., Petersen, E.L., Metcalfe, W.K., Serinyel, Z., Curran, H.J.: An ignition delay and kinetic modeling study of methane, dimethyl ether, and their mixtures at high pressures. Combust. Flame 162(2), 315–330 (2014). https://doi.org/10.1016/j.combustflame.2014.08.014
Hu, E., Li, X., Meng, X., Chen, Y., Cheng, Y., Xie, Y., Huang, Z.: Laminar flame speeds and ignition delay times of methane–air mixtures at elevated temperatures and pressures. Fuel 158, 1–10 (2015). https://doi.org/10.1016/j.fuel.2015.05.010
Petersen, E.L., Röhrig, M., Davidson, D.F., Hanson, R.K., Bowman, C.T.: High-pressure methane oxidation behind reflected shock waves. Symp. (Int.) Combust. 26(1), 799–806 (1996). https://doi.org/10.1016/S0082-0784(96)80289-X
Hidaka, Y., Sato, K., Henmi, Y., Tanaka, H., Inami, K.: Shock-tube and modeling study of methane pyrolysis and oxidation. Combust. Flame 118, 340–358 (1999). https://doi.org/10.1016/S0010-2180(99)00010-3
Jouzdani, S., Zheng, X., Coombs, D.M., Akih-Kumgeh, B.: Methane and methyl propanoate high-temperature kinetics. Energy Fuels 32(11), 11864–11875 (2018). https://doi.org/10.1021/acs.energyfuels.8b02277
Shao, J., Davidson, D.F., Hanson, R.K.: A shock tube study of ignition delay times in diluted methane, ethylene, propene and their blends at elevated pressures. Fuel 225(1), 370–380 (2018). https://doi.org/10.1016/j.fuel.2018.03.146
Shao, J., Ferris, A.M., Choudhary, R., Cassady, S.J., Davidson, D.F., Hanson, R.K.: Shock-induced ignition and pyrolysis of high-pressure methane and natural gas mixtures. Combust. Flame 221, 364–370 (2020). https://doi.org/10.1016/j.combustflame.2020.08.010
Zeng, W., Ma, H., Liang, Y., Hu, E.: Experimental and modeling study on effects of \({{\rm N}_2}\) and \({{\rm CO}_2}\) on ignition characteristics of methane/air mixture. J. Adv. Res. 6(2), 189–201 (2015). https://doi.org/10.1016/j.jare.2014.01.003
Hargis, J.W., Petersen, E.L.: Methane ignition in a shock tube with high levels of \({{\rm CO}_2}\) dilution: consideration of the reflected-shock bifurcation. Energy Fuels 29(11), 7712–7726 (2015). https://doi.org/10.1021/acs.energyfuels.5b01760
Koroglu, B., Pryor, O.M., Lopez, J., Nash, L., Vasu, S.S.: Shock tube ignition delay times and methane time-histories measurements during excess \({{\rm CO}_2}\) diluted oxy-methane combustion. Combust. Flame 164, 152–163 (2016). https://doi.org/10.1016/j.combustflame.2015.11.011
Pryor, O., Barak, S., Lopez, J., Ninnemann, E., Koroglu, B., Nash, L., Vasu, S.: High pressure shock tube ignition delay time measurements during oxy-methane combustion with high levels of \({{\rm CO}_2}\) dilution. J. Energy Resour. Technol. 139(4), 042208 (2017). https://doi.org/10.1115/1.4036254
Laich, A.R., Baker, J., Ninnemann, E., Sigler, C., Naumann, C., Braun-Unkhoff, M., Vasu, S.S.: Effects of high fuel loading and \({{\rm CO}_2}\) dilution on oxy-methane ignition inside a shock tube at high pressure. J. Energy Resour. Technol. 149(10), 102103 (2020). https://doi.org/10.1115/1.4047023
Merkel, A.C., Ciccarelli, G.: Visualization of lean methane–air ignition behind a reflected shock wave. Fuel 271, 117617 (2020). https://doi.org/10.1016/j.fuel.2020.117617
Fieweger, K., Blumenthal, R., Adomeit, G.: Self-ignition of S.I. engine model fuels: a shock tube investigation at high pressure. Combust. Flame 109(4), 599–619 (1997). https://doi.org/10.1016/S0010-2180(97)00049-7
Chaos, M., Dryer, F.L.: Chemical-kinetic modeling of ignition delay: considerations in interpreting shock tube data. Int. J. Chem. Kinet. 42(3), 143–150 (2010). https://doi.org/10.1002/kin.20471
Lee, C., Stijn, V., Heufer, K.A., Khomik, S.V., Uygun, O.H.Y., Fernandez, R.X.: On the chemical kinetics of ethanol oxidation: shock tube, rapid compression machine and detailed modeling study. Zeitschrift für Physikalische Chemie 226(1), 1–28 (2012). https://doi.org/10.1524/zpch.2012.0185
Uygun, Y., Ishihara, S., Olivier, H.: A high pressure ignition delay time study of 2-methylfuran and tetrahydrofuran in shock tubes. Combust. Flame 161(10), 2519–2530 (2014). https://doi.org/10.1016/j.combustflame.2014.04.004
Javed, T., Badra, J., Jaasim, M., Es-Sebbar, E., Labastida, M.F., Chung, S.H., Im, H.G., Farooq, A.: Shock tube ignition delay data affected by localized ignition phenomena. Combust. Sci. Technol. 189(7), 1138–1161 (2017). https://doi.org/10.1080/00102202.2016.1272599
Ninnemann, E., Koroglu, B., Pryor, O., Barakl, S., Nash, L., Loparo, Z., Sosa, J., Ahmed, K., Vasu, S.: New insights into the shock tube ignition of \({{\rm H}_2}\)/\({{\rm O}_2}\) at low to moderate temperatures using high-speed end-wall imaging. Combust. Flame 187, 11–21 (2018). https://doi.org/10.1016/j.combustflame.2017.08.021
Chapman, E.M., Costanzo, V.S.: A literature review of abnormal ignition by fuel and lubricant derivatives. SAE Int. J. Engines 9(1), 107–142 (2016). https://doi.org/10.4271/2015-01-1869
Corrigan, D.J., Fontanesi, S.: Knock: a century of research. SAE Int. J. Engines 15(1), 57–127 (2022). https://doi.org/10.4271/03-15-01-0004
Troutman, V.A., Strand, C.L., Campbell, M.F., Tulgestke, A.M., Miller, V.A., Davidson, D.F., Hanson, R.K.: High-speed OH* chemiluminescence imaging of ignition through a shock tube end-wall. Appl. Phys. B 122, 56 (2016). https://doi.org/10.1007/s00340-016-6326-y
Barak, S., Pryor, O., Lopez, J., Ninnemann, E., Vasu, S., Koroglu, B.: High-speed imaging and measurements of ignition delay times in oxy-syngas mixtures with high \({{\rm CO}_2}\) dilution in a shock tube. J. Eng. Gas Turbines Power 139(12), 121503 (2017). https://doi.org/10.1115/1.4037458
Figueroa-Labastida, M., Badra, J., Elbaz, A.M., Farooq, A.: Shock tube studies of ethanol preignition. Combust. Flame 198(1), 176–185 (2018). https://doi.org/10.1016/j.combustflame.2018.09.011
Figueroa-Labastida, M., Luong, M.B., Badra, J., Im, H.G., Farooq, A.: Experimental and computational studies of methanol and ethanol preignition behind reflected shock waves. Combust. Flame 234, 111621 (2021). https://doi.org/10.1016/j.combustflame.2021.111621
Tulgestke, A.M., Johnson, S.E., Davidson, D.F., Hanson, R.K.: High-speed imaging of inhomogeneous ignition in a shock tube. Shock Waves 28(2), 1089–1095 (2018). https://doi.org/10.1007/s00193-018-0824-2
Nativel, D., Niegemann, P., Herzler, J., Fikri, M., Schulz, C.: Ethanol ignition in a high-pressure shock tube: ignition delay time and high-repetition-rate imaging measurements. Proc. Combust. Inst. 38(1), 901–909 (2021). https://doi.org/10.1016/j.proci.2020.07.021
Figueroa-Labastida, M., Farooq, A.: Simultaneous lateral and endwall high-speed visualization of ignition in a circular shock tube. Combust. Flame 214, 263–265 (2020). https://doi.org/10.1016/j.combustflame.2019.12.033
Figueroa-Labastida, M., Badra, J., Farooq, A.: Dual-camera high-speed imaging of the ignition modes of ethanol, methanol and n-hexane in a shock tube. Combust. Flame 224, 33–42 (2021). https://doi.org/10.1016/j.combustflame.2020.07.002
Ninnemann, E., Pryor, O., Barak, S., Neupane, S., Loparo, Z., Laich, A., Vasu, S.S.: Reflected shock-initiated ignition probed via simultaneous lateral and endwall high-speed imaging with a transparent, cylindrical test-section. Combust. Flame 224, 43–53 (2021). https://doi.org/10.1016/j.combustflame.2020.08.017
Figueroa-Labastida, M., Kashif, T.A., Farooq, A.: Dual-camera high-speed imaging of n-hexane oxidation in a high-pressure shock tube. Combust. Flame 248, 112586 (2023). https://doi.org/10.1016/j.combustflame.2022.112586
Minwegen, H., Burke, U., Heufer, K.A.: An experimental and theoretical comparison of C3–C5 linear ketones. Proc. Combust. Inst. 36(1), 561–568 (2017). https://doi.org/10.1016/j.proci.2016.05.050
Shock and Detonation Toolbox, available at https://shepherd.caltech.edu/EDL/ PublicResources/sdt/. Accessed 25 Aug 2021
Goodwin, D.G., Speth, R.L., Moffat, H.K., Weber, B.W.: Cantera: An Object-Oriented Software Toolkit for Chemical Kinetics, Thermodynamics, and Transport Processes (2021). https://www.cantera.org. Version 2.5.1
Baigmohammadi, M., Patel, V., Nagaraja, S.: Comprehensive experimental and simulation study of the ignition delay time characteristics of binary blended methane, ethane, and ethylene over a wide range of temperature, pressure, equivalence ratio, and dilution. Energy Fuels 34, 8808–8823 (2020). https://doi.org/10.1021/acs.energyfuels.0c00960
Zander, L., Vinkeloe, J., Djordjevic, N.: Ignition delay and chemical-kinetic modeling of undiluted mixtures in a high-pressure shock tube: nonideal effects and comparative uncertainty analysis. Int. J. Chem. Kinet. 53, 611–637 (2021). https://doi.org/10.1002/kin.21469
Figueroa-Labastida, M., Badra, J., Farooq, A.: Dual-camera high-speed imaging of the ignition modes of ethanol, methanol and n-hexane in a shock tube. Combust. Flame 224, 33–42 (2021). https://doi.org/10.1016/j.combustflame.2020.07.002
Sung, C.J., Curran, H.J.: Using rapid compression machines for chemical kinetics studies. Prog. Energy Combust. Sci. 44, 1–18 (2014). https://doi.org/10.1016/j.pecs.2014.04.001
Abd El-Sabor Mohamed, A., Panigrahy, S., Sahu, A.B., Bourque, G., Curran, H.J.: An experimental and modeling study of the auto-ignition of natural gas blends containing c1–c7 n-alkanes. Proc. Combust. Inst. 38, 365–373 (2021). https://doi.org/10.1016/j.proci.2020.06.015
Lipkowicz, J.T., Wlokas, I., Kempf, A.M.: Analysis of mild ignition in a shock tube using a highly resolved 3D-LES and high-order shock-capturing schemes. Shock Waves 29, 511–521 (2019). https://doi.org/10.1007/s00193-018-0867-4
Figueroa-Labastida, M., Badra, J., Farooq, A.: Simultaneous lateral and endwall high-speed visualization of ignition in a circular shock tube. Combust. Flame 214, 263–265 (2020). https://doi.org/10.1016/j.combustflame.2019.12.033
Grogan, K.P., Ihme, M.: Regimes describing shock boundary layer interaction and ignition in shock tubes. Proc. Combust. Inst. 36(2), 2927–2935 (2017). https://doi.org/10.1016/j.proci.2016.06.078
Susa, A.J., Hanson, R.K.: Simultaneous side-wall-schlieren and -emission imaging of autoignition phenomena in conventional and constrained-reaction-volume shock-tube experiments. Proc. Combust. Inst. (2022). https://doi.org/10.1016/j.proci.2022.08.063
Susa, A.J.: High-speed imaging studies of flames in a shock tube: refined techniques and new applications. Ph.D. Thesis, Stanford University (2022)
Sankaran, R., Im, H.G., Hawkes, E.R., Chen, J.H.: The effects of non-uniform temperature distribution on the ignition of a lean homogeneous hydrogen–air mixture. Proc. Combust. Inst. 30(1), 875–882 (2005). https://doi.org/10.1016/j.proci.2004.08.176
Zeldovich, Y.B.: Regime classification of an exothermic reaction with nonuniform initial conditions. Combust. Flame 39(2), 211–214 (1980). https://doi.org/10.1016/0010-2180(80)90017-6
Mansfield, A.B., Wooldridge, M.S.: High-pressure low-temperature ignition behavior of syngas mixtures. Combust. Flame 161(9), 2242–2251 (2014). https://doi.org/10.1016/j.combustflame.2014.03.001
Im, H.G., Pal, P., Wooldridge, M.S., Mansfield, A.B.: A regime diagram for autoignition of homogeneous reactant mixtures with turbulent velocity and temperature fluctuations. Combust. Sci. Technol. 187(8), 1263–1275 (2015). https://doi.org/10.1080/00102202.2015.1034355
Luong, M.B., Im, H.: Prediction of Ignition Modes in Shock Tubes Relevant to Engine Conditions. In: Kalghatgi, G., Agarwal, A.K., Leach, F., Senecal, K. (eds.) Engines and Fuels for Future Transport, pp. 10–13. Springer, Singapore (2022). https://doi.org/10.1007/978-981-16-8717-4_15
Weisser, G.A.: Modelling of combustion and nitric oxide formation for medium speed DI diesel engines: a comparative evaluation of zero- and three-dimensional approaches. Dissertation, Swiss Federal Institute of Technology in Zurich (ETHZ) (2001). https://doi.org/10.3929/ethz-a-004317089
Chong, C.T., Ng, J.: Chapter 4 - Combustion performance of biojet fuels. In: Biojet Fuel in Aviation Applications, pp. 175–230. Elsevier, Amsterdam (2021). https://doi.org/10.1016/B978-0-12-822854-8.00002-0
Acknowledgements
The authors would like to recognize the funding support from the German Research Foundation (Deutsche Forschungsgemeinschaft, DFG) through the project number—667296 (HE7599/2-2).
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by G. Ciccarelli.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Caravaca-Vilchez, J., Heufer, K.A. New insights into the pre-ignition behavior of methane behind reflected shock waves. Shock Waves 33, 315–328 (2023). https://doi.org/10.1007/s00193-023-01130-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00193-023-01130-9