Abstract
Purpose
The aim of this systematic review was to determine if adipose tissue-derived cell-based injectable therapies can induce disease-modifying effects in joints affected by osteoarthritis (OA).
Methods
A systematic review was performed on three electronic databases (PubMed, Web of Science, Embase) according to PRISMA guidelines. A synthesis of the results was performed investigating disease-modifying effects in preclinical studies comparing injectable adipose-derived products with OA controls or other products, different formulations or injection intervals, and the combination with other products. The risk of bias was assessed according to the SYRCLE’s tool.
Results
Seventy-one studies were included (2,086 animals) with an increasing publication trend over time. Expanded cells were used in 65 studies, 3 studies applied point of care products, and 3 studies investigated both approaches. Overall, 48 out of 51 studies (94%) reported better results with adipose-derived products compared to OA controls, with positive findings in 17 out of 20 studies (85%) in macroscopic, in 37 out of 40 studies (93%) in histological, and in 22 out of 23 studies (96%) in immunohistochemical evaluations. Clinical and biomarker evaluations showed positive results in 14 studies out of 18 (78%) and 12 studies out of 14 (86%), while only 9 studies out of 17 (53%) of the imaging evaluations were able to detect differences versus controls. The risk of bias was low in 38% of items, unclear in 51%, and high in (11%).
Conclusion
The current preclinical models document consistent evidence of disease-modifying effects of adipose-derived cell-based therapies for the treatment of OA. The high heterogeneity of the published studies highlights the need for further targeted research to provide recommendations on the optimal methodologies for a more effective application of these injective therapies for the treatment of OA in clinical practice.
Level of evidence
II.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoarthritis (OA) is the most frequent joint disease in the world population [1]. Highly disabling, OA is characterized by both inflammatory and degenerative events that culminate in progressive cartilage damage and exposure of subchondral bone [2], eventually leading to pain and disability [1]. To date, there is no conservative therapy capable of curing this pathology or arresting its progression, with current interventions providing only temporary symptom relief. Recently, autologous biological injective therapies with anti-inflammatory, restorative, and regenerative properties have been introduced as promising strategy to treat OA. These products, namely orthobiologics, are blood-derived or cell-derived products that can be prepared from patient’s tissues either at the point of care or in authorized facilities using more complex laboratory procedures [3].
The assumption underlying the use of cell-based orthobiologics is closely related to the properties and functions of the cell type used, which must have the ability to determine a specific action at the target site [4]. From this perspective, mesenchymal stromal cells (MSCs) are ideal candidates for the treatment of damaged tissues given their restorative and pro-regenerative properties [5]. These cells can be found in most of the vascularized tissues, being a subtype of pericytes with pro-regenerative properties, and in particular in adipose tissue, bone marrow, and foetal annexes [6,7,8]. The former is not only abundant and very easy to harvest through a simple liposuction procedure, but it also contains one of the best performing MSC populations [9]. The key therapeutic effector of adipose-based orthobiologic products is the stromal vascular fraction (SVF) that, together with precursor and mature endothelial cells, pericytes, lymphocytes, pre-adipocytes and mature adipocytes, also contains adipose-derived MSCs (ASCs) [9]. Abundant literature has demonstrated the anti-inflammatory and adaptive properties of ASCs based on environmental conditions, with anti-inflammatory effects on chondrocytes and synoviocytes as well as polarization of M0 macrophages and dendritic cells towards anti-inflammatory phenotypes [10,11,12]. Given these premises, from a clinical perspective, the autologous use of ASCs would be expected not only to counteract inflammatory processes, but also to promote regenerative processes in the joint synovium and the chondral surfaces [13], and therefore to exert disease-modifying effects in joints, as already demonstrated in blood-derived products in preclinical studies [14].
Therefore, the aim of this systematic review was to investigate in preclinical studies the presence of disease-modifying effects driven by cell-based products used for the injective treatment of OA. The usefulness of this systematic review relies on the possibility to get pivotal information on the disease-modifying effects of adipose-based orthobiologics for the treatment of OA which is otherwise impossible to collect in a clinical setting due to ethical and practical concerns. While additional publications by this group (The ESSKA Orthobiologic Initiative = ORBIT) will investigate other MSC sources, this article will focus on the effects of adipose tissue derived products. This systematic review will help to define the advantages of using orthobiologics for the treatment of OA, in order to achieve a consolidated background for using these therapies in the daily clinical practice in the near future.
Materials and methods
Search strategy and article selection
A systematic review of the literature was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines on the intra-articular use of MSCs to address joints affected by OA. The search was conducted on January 10, 2022 on three electronic databases (PubMed, Web of Science, and Embase) with no time limitation and without any filters, using the following string on titles and abstracts: (MSC OR mesenchymal cell OR stem cell OR stromal cell OR progenitor cell OR bone marrow concentrate OR bone marrow aspirate concentrate OR BMAC OR micro-fra* adipose tissue OR microfra* adipose tissue OR stromal vascular fraction OR SVF OR amniotic suspension allograft OR ASA OR placenta* OR umbilical cord OR amnio*) AND (osteoarthritis). The screening process and analysis were conducted by two independent authors (CP and YS) and any discrepancies between them were resolved by discussion and consensus with a third author (AB).
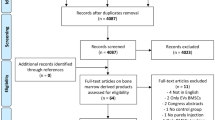
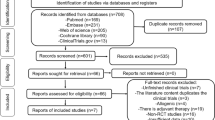
First, the reviewers screened the resulting records by title and abstract, then the full texts of selected manuscripts were entirely screened according to the following inclusion criteria: animal studies, written in English, on cell therapy as purely injective treatment for cartilage degeneration and OA. Exclusion criteria were: in vitro or clinical studies, congress abstracts, literature reviews, articles written in other languages, studies on joint diseases different from OA, studies analyzing combined surgery (e.g., scaffolding procedure, arthroscopy), studies on the use of secretome and extracellular vesicles from MSCs, and studies reporting the use of MSCs without a control group or the combined use of MSCs with another product without analyzing the specific contribution of MSCs treatment. In addition, the reference lists from the selected papers and previously published relevant reviews were also screened. The flowchart reported in Fig. 1 graphically describes the systematic review process. The current manuscript focuses on adipose-derived products, while additional publications by this group (The Orthobiologic Initiative = ORBIT) will investigate other MSC sources separately, which were therefore excluded for this analysis.
Data extraction and quality assessment
For the included studies, all relevant data were extracted and reviewed from article texts, tables, and figures, and then summarized and analyzed for the purpose of the present work. In particular, the following data were collected for each study: authors, journal and year of publication, number and type of evaluated animals, involved joint, OA model, type of treatments, follow-up length, results, and adipose-based orthobiologic products characteristics, including source, origin, dose, additional procedures, injective protocol, and processing modality (expanded versus “point of care”). A synthesis of the obtained results was performed analyzing the disease-modifying effects of intra-articular application of the different preparations, as assessed by objective evidence measures of effect (imaging, macroscopic, histological, or immunohistochemical) on OA processes going beyond the mere symptomatic improvement. The clinical outcome was reported as well. This was achieved by evaluating studies that compared animals treated with adipose-based orthobiologic products and OA controls (vehicle injection or no treatment). Moreover, other results were analyzed when available regarding the benefits provided by different doses or injection schedules, the effects versus other injectable treatments, and finally the effects derived from the combination of adipose-derived products with other products exploring potential synergistic effects.
The risk of bias of the included articles was assessed according to the Systematic Review Centre for Laboratory animal Experimentation (SYRCLE)’s tool [15]. This tool is an adapted version of the Cochrane Collaboration RoB Tool and contains 10 items related to the types of bias: selection bias, performance bias, detection bias, attrition bias, reporting bias, and “other” biases. All items could be judged as ‘yes’ (low risk of bias), ‘no’ (high risk of bias), and ‘unclear’ (unclear risk of bias). The assessment was independently performed by 2 authors (CP and YS), and any divergence was resolved through discussion and consensus with a third author (AB).
Results
Study selection and analysis
A total of 4087 potential articles were identified according to the search strategy, resulting in 71 studies included in the qualitative data synthesis (Fig. 1). Since the first report in 2007, the publication trend remarkably increased over the years, with over 50% of articles published since 2018 (Fig. 2). Fifty-one studies were on small animals (32 on rodents, 19 on rabbits) and 20 studies on large animals (9 on dogs, 6 on sheep, 4 on horses, 1 on goats), for a total of 2086 animals, of which 955 rodents, 654 rabbits, 224 dogs, 112 horses, 111 sheep and 30 goats. The treated joints were knees in 61 articles, hips in 4 articles, ankles in 2 articles, elbows and temporo-mandibular joints in 1 article each, and 2 articles focussed on multiple joints. The OA model was surgically induced in 44 studies (mostly through meniscectomy and/or ligament transection), chemically induced in 18 studies (through the injection of chondrotoxic or pro-inflammatory products such as collagenase, mono-iodoacetate, papain, streptozotocin), naturally occurring in eight studies (veterinary studies), and induced by joint immobilization in 1 study.
Treatments were reported as allogeneic in 30 studies, autologous in 17 studies, and xenogeneic in 18 studies, with 16 of them using human-derived ASCs. Moreover, one study used both autologous and xenogeneic (human) ASCs [16], one study used both allogeneic and xenogeneic (human) ASCs [17], while four studies did not specify the MSC origin. Expanded ASCs were used in 65 studies, 3 studies used adipose-derived cell-based products produced at the point of care, and 3 studies applied both approaches. Twenty-four studies evaluated adipose-based orthobiologic products with additional procedures including microencapsulation, 3D cell culture dishes with spheroids, induction of overexpressed transcription factors, preconditioning with vitamin E, or chondrogenic differentiation. ASC dose was described in 70 studies and ranged from 2.0 × 104 to 5.0 × 107, while the amount of the injected volume was reported in 64 studies and ranged from 6.0 μL to 2.0 mL in small animals and from 0.5 mL to 5.0 mL in large animals. The most common injection frequency protocol was a single injection administration (59 studies), while multiple-injection protocols were used in 12 studies, with injection intervals ranging from 3-day to 4-week intervals. The follow-up ranged from 1 week to 12 months. Further details are reported in the supplementary material.
Disease-modifying effects on OA joints
Fifty-one studies (42 in small animals and 9 in large animals) investigated the disease-modifying effects of intra-articular adipose-derived injections in comparison to OA controls (untreated joints or vehicle injections), of which 46 reported the effects on cartilage and 10 the effects on synovial membrane. Overall, 48 studies (94.1%) reported better results in animals treated with adipose-derived products compared to OA controls and 3 studies (5.9%) revealed no improvement following injection. Noteworthy, no studies showed detrimental effects. In detail, 17 out of 20 studies (85.0%) with macroscopic evaluations (gross morphological scores) reported overall better results, 37 out of 40 studies (92.5%) with histological evaluations, and 22 out of 23 studies (95.7%) with immunohistochemical evaluations. Similar results were observed comparing small and large animal OA models (95.2% and 88.9% overall positive disease-modifying effects, respectively). A more detailed analysis is reported in the following paragraphs and in Fig. 3.
ASCs effects on OA joints. The bar chart shows the percentage of studies that met the specific effects. Positive effects vs no effects in imaging findings (n = 17), clinical results (n = 18), biomarker evaluation (n = 14), macroscopic results (n = 20), histological results (n = 40) and immunohistochemical results (n = 23). ASCs, adipose-derived mesenchymal stromal cells; IHC, immunohistochemistry; OA, osteoarthritis
Disease-modifying effects at the cartilage level
Out of the 46 studies investigating the disease-modifying effects on cartilage tissue, 44 (95.7%) reported positive results as a treatment for OA affected joints. In particular, at macroscopic evaluation of damaged cartilage areas, appearance was milder compared to OA controls, reporting a smoother articular surface with less erosion, fibrillation, and osteophytes [α]. Histological analysis provided evidence on the protective role on cartilage, featuring improved tissue thickness and arrangement of chondrocytes, accompanied by decreased extracellular matrix loss and higher amount of cartilage specific extracellular matrix (proteoglycan and type II collagen) [β]. Moreover, less severe sclerosis and lower thickening of the subchondral bone plate was observed [18]. Immunohistochemical analysis also demonstrated the promotion of chondrogenic (type II collagen and aggrecan) and cell proliferation (proliferating cell nuclear antigen—PCNA) marker expression, while reducing the expression of fibro-cartilaginous (type I collagen), catabolic (matrix metalloproteinase and aggrecanase), apoptotic (caspase 3), and inflammatory (tumour necrosis factor-α—TNF-α, nuclear factor kappa-light-chain-enhancer of activated B cells—NF-κB) markers [γ].
Disease-modifying effects at the synovial membrane level
Only 10 studies investigated the disease-modifying effects of intra-articular adipose-derived products injections on the synovial membrane, with 6 of them (60%) reporting positive results and the others showing no differences compared to OA controls. In particular, adipose-derived products improved the synovitis status reducing the thickness of the lining layer of the synovial membrane and decreasing the infiltration of inflammatory cells (mononuclear cells and neutrophils) in the sub-synovium [δ]. Immunohistochemical analyses showed that ASCs treatment inhibited metalloproteinase-1 (MMP-1) and TNF-α expression, and reduced the ratio of inflammatory macrophages (iNOS positive-cells) in the synovial membrane [19, 20].
Effects on OA biomarker profile
Fourteen studies evaluated the disease-modifying effects through the measurement of synovial fluid (SF) or serum biomarkers related to cartilage metabolism or inflammation. Among these, 12 studies (86%) reported positive changes with a decrease in the SF concentration of lymphocytes and inflammatory biomarkers, such as interleukin-1β (IL1-β), IL-6, TNF-α, and prostaglandin E2 (PGE2) [ε]. Moreover, they provided a reduction of SF matrix-degrading enzymes (MMP-3 and MMP-13) and C-terminal telopeptide of type II collagen (CTX2) [21, 22], as well as the serum levels of inflammatory markers, such as IL-6, TNFα, S100 Calcium Binding Protein A9 (S100A9), and monocyte chemoattractant protein 1 (MCP-1), the serum levels of cartilage oligomeric matrix protein (COMP), and those of osteoprotegerin (OPG) [ζ].
Clinical effects
Eighteen studies quantitatively evaluated the clinical effects of adipose-based orthobiologic products: 14 studies (78%) reported better clinical results compared to OA controls in both small (6 out of 7) and large animals (8 out of 11), with increased passive range of motion and reduced lameness and pain on manipulation [η]. The injections also promoted paw-withdrawal latency and threshold, as well as better weight bearing [θ].
Imaging analysis
Seventeen studies evaluated the effects by imaging analysis: nine studies (52.9%) reported significant differences in favour of adipose-derived products, while eight studies observed no differences. In particular, micro-computed tomography (micro-CT) evaluation was performed in six studies, with positive effects in five studies in terms of prevention of cartilage and subchondral bone degeneration and improvement of bone thickness and volume [23,24,25,26,27]. Radiographic evaluation was performed in 6 studies with only one study showing a statistically significant difference in favour of adipose-derived products [28]. Magnetic resonance imaging (MRI) was used in 4 studies with positive results observed in 2 studies in terms of improvement of the cartilage repair process with significantly higher Magnetic Resonance Observation of Cartilage Repair Tissue (MOCART) scores than in the untreated one [29, 30]. Moreover, positron emission tomography (PET) analysis performed in one study reported a significant difference compared to untreated OA joints in enhancing cartilage regeneration [31]. Finally, one study used back scattered electron imaging to assess the morphology, porosity, and heterogeneity of mineralization within the subchondral bone without observing significant differences between ASC-treated and untreated groups [32]
Comparison of formulations and protocols
Twenty-nine studies compared injective protocols involving ASCs or adipose-derived cell-based products, differing in origin, preparation, doses, times and interval of administration, and additional cell procedures/pre-treatments. In particular, 11 studies evaluated low-dose versus high-dose ASCs, reporting controversial results. On one hand, five studies showed a dose-dependent effect of the treatment, with high doses resulting in better macroscopic and histological results [ι]. On the other hand, five studies demonstrated comparable effects among different doses [κ]. Another study reported better cartilage preservation in the intermediate dose group (0.6 × 107/100 μL) with respect to low (0.18 × 107/100 μL) and high dose (1.8 × 107/100 μL) groups in a goat OA model [33].
Five studies compared disease-modifying effects of cultured ASCs vs freshly isolated cell-based products without finding consistent results. In fact, while two studies reported that cultured ASCs yielded better results in terms of MRI measures and macroscopic findings [30, 31], one study revealed that micro-fragmented adipose tissue (MFAT) provided better synovitis reduction and articular cartilage status compared to SVF and expanded ASCs [34], and another study demonstrated better results in terms of macroscopic findings and decrease of SF TNF-a and IL-6 in the enzymatic SVF group compared to expanded ASCs [22]. Another study did not find histological differences between expanded ASCs, SVF, and MFAT [35].
Three studies evaluated the injection of microencapsulated ASCs aggregated into spheroids, all showing improved chondroprotective effects and pain relief compared to ASCs alone [36,37,38].
Two studies compared products injected at different time points from OA induction, both observing a lower cartilage degeneration in the early injection groups compared to the late injection groups [39, 40].
Two studies investigated the role of the ASCs chondrogenic differentiation before injection: one study reported better histological results of pre-differentiated ASCs compared to undifferentiated ones in a rat OA model [41], while the other study did not show any difference in a rabbit OA model [42].
Tang et al. compared ASCs obtained from different adipose tissue sources (subcutaneous fat versus visceral fat) reporting higher chondroprotective effects in the subcutaneous ASCs group [43].
Finally, seven studies evaluated benefits of different additional procedures, reporting better results compared to ASCs alone in case of pre-treatments aimed to induce overexpression of Fos member of the AP-1 family (Fra-1) or bone morphogenetic protein‑9 (BMP-9), platelet-derived growth factor (PDGF) or PDGF receptor-β transduction, interferon-γ (IFN-γ) or signal transducer and activator of transcription 3 (STAT3). No advantages were observed when SOX transduction was induced [λ].
Comparison with other injectable products
Five studies compared ASCs versus expanded bone marrow MSCs (BMSCs): 4 studies did not show differences in terms of clinical improvement, macroscopic and histological findings, while one study reported better mechanical properties after BMSCs injection compared to ASCs [μ]. A further study compared enzymatic SVF with expanded BMSCs, reporting higher improvement in flexion and SF profile for the BMSC group [44]. Five studies compared adipose-based orthobiologic products and PRP, showing controversial findings. Two studies demonstrated better results for adipose cell-based products in terms of macroscopic and histological findings in a rat OA model and in terms of clinical improvement in a randomized controlled trial on dogs with naturally occurred OA [ν]. Conversely, three studies showed similar histological and immunohistochemical benefits between the two treatments in surgically induced OA models (mice, rats, and dogs) [ξ]. Two studies used viscosupplementation as treatment comparison. While one study did not demonstrate any difference in a rabbit knee OA model, one study in a temporo-mandibular rabbit OA model reported a higher cartilage protection in the cell therapy group, even though no significant differences were found in terms of cartilage thickness [32, 45]. One study compared ASCs (expanded or SVF) versus expanded amniotic epithelial stem cells (AECs) in a sheep OA model. Among the three treatments, SVF provided the best histological performance while ASCs resulted in the least improvement of the three [22]. Finally, one study compared ASCs with human umbilical cord Wharton’s jelly-derived MSCs (WJMSCs) in a rat OA model, reporting a higher type II collagen expression in the WJMSCs group, although no other differences were observed in terms of histological analysis, micro-computed tomography, and immunohistochemistry staining [24].
Effects of combined treatments
Five studies investigated the combined use of adipose-based orthobiologic products and hyaluronic acid to address OA models. All these studies reported a synergistic effect of this combined approach with higher inhibiting effects on cartilage degeneration progression compared to viscosupplementation alone [ο]. The combined use of expanded ASCs and PRP revealed a synergistic effect in two OA models with higher extracellular matrix synthesis, chondrocyte proliferation, and anti-inflammatory effects [46, 47]. Adipose-derived products also showed synergistic effects in addressing OA progression when combined with shockwave therapy [23, 24], chondral cell suspension [48], carboxymethyl chitosan [45], xanthan gum [49], thrombospondin 2 [28], or acupotomy [50]
Risk of bias assessment
There was a 79% agreement between the two authors involved in the evaluation of the risk of bias. Most items (51%) were rated as unclear, while low and high risk of bias were observed in 38% and 11%, respectively. The evaluation of risk of bias over time did not show a trend towards improving the quality of the included studies, with low-risk items reported in 37% vs 40% in more recent papers vs older ones. Details of the risk of bias assessment of all included studies are illustrated in Fig. 4.
Discussion
This systematic review supports that injectable adipose-derived cell-based therapies exert disease-modifying effects on joint tissues in both small and large animal OA models. Among these effects, they provide a structural/macroscopic improvement of both cartilage and synovial membrane properties compared to untreated OA joints as well as a decrease in serum and SF levels of both inflammatory and cartilage degradation markers. These effects were also confirmed by improvement in clinical measures.
The high number of studies underlines the growing interest on this type of conservative treatment option for OA, confirmed by the increasing trend in publications covering this topic in recent years. Advantages of using adipose tissue derived cells and/or products are widely described in the literature, being even more advantageous than BMSCs in some respects, including higher cellular yield and remarkable regenerative and immunomodulatory potential [51,52,53]. A recent meta-analysis on the safety and efficacy of adipose tissue derived cell-based therapies in humans for the treatment of knee OA [54] showed a reduction in pain and an improvement in knee function at least up to two years [55]. Nevertheless, these therapies are still rather immature in the clinical setting for various reasons, including regulatory restrictions, heterogeneity of the techniques available, cost-effectiveness, and the lack of objective data concerning their effectiveness [56]. In the latter regard, most of the information on the biological effects of these injective therapies for treating OA resides in the preclinical setting. This systematic review assessed the disease-modifying effects of these treatments in animal studies to provide useful information regarding the clinical effectiveness observed in humans and to promote a consolidated background supporting the use of these procedures in daily clinical practice. The administration of adipose tissue derived cell-based injective therapies demonstrated to exert a protective role on the cartilage tissue at both histological and immunohistochemical level. These findings are largely supported by previous in vitro studies in which the trophic effects of MSCs (derived from fat or bone marrow) co-cultured with chondrocytes enhance cartilage formation, matrix deposition, and proliferative activity in chondrocytes [57].
In addition, data from these studies suggest that the injection of these products also exerts a positive effect on the synovial membrane, improving the synovitis [δ]. When co-cultured in vitro with inflamed OA chondrocytes or synoviocytes, ASCs determined an anti-inflammatory action [10], reflected by a decrease in expression of IL1β, IL6, and CXCL8/IL-8. A similar effect was observed in synoviocytes after administration of ASC-derived extracellular vesicles, which are suggested to be the real effectors in cell-based therapies [58]: after 10 days inflammatory markers are significantly downregulated, with C-C motif chemokine ligand 2 (CCL2) and CCL5 returning to pre-inflammation basal levels.
The results collected in this systematic review about the effects on the inflammatory/catabolic status of the OA joint measured in the SF or serum reported a decrease in levels of lymphocytes and inflammatory biomarkers, indicating strong anti-inflammatory action of ASCs, and the reduction of MMP3, MMP13 and CTX2, which indicates a positive effect on matrix degradation [ε]. The interaction between ASCs with the degenerated/inflamed environment is a crucial factor in understanding their role. SF is a good indicator of the joint's general condition as the content of biomarkers correlates with the inflammatory/pathological state of cartilage and synovial membrane [59]. In a recent article, SF analysis of OA patients detected several cytokines and chemokines linked to inflammation, where IL-6 and IL-8 were the most abundant factors [60]. The secretory profile of ASCs treated with these SF samples revealed more than 50 factors in the cell secretome mainly involved in the organization and homeostasis regulation of the extracellular matrix, the interaction with cells of the immune system, and the regulation of cytokine production, including TNFα, IL-1β, and IL6 receptors [60]. Overall, these results suggest a possible molecular explanation for the effects observed in vivo in most of the studies included in the systematic review.
Results as objective as those reported in animals are difficult to reproduce in humans due to various ethical and practical concerns. Only a few clinical studies attempted to evaluate disease-modifying effects following injection of adipose-derived cell-based therapies in OA patients, including MFAT, SVF, and ASCs [61,62,63,64]. In this light, the analysis of the preclinical literature can help identify effects and mechanisms of action, as well as more promising strategies (e.g., number of cells, number of injections, preculturing, etc.). However, a critical issue is underlined by this systematic review. Almost all the preclinical studies (65 out of 71) focused on the use of culture-expanded ASCs, while only 6 studies reported the use of products prepared at the point of care (SVF or MFAT). Interestingly, this is in countertrend if compared to clinical trials where the majority reported results of products prepared at the point of care from autologous adipose tissue [65]. Orthobiologics prepared at the point of care by minimal manipulation are easier to use, more cost-effective than culture-expanded cells, and more accessible in the clinical routine from the regulatory perspective.
The current systematic review also highlights the effects of these products in relation to the protocol of application, dosage, processing methods (cultured ASCs or not) and in comparison to other injective therapies. While providing some interesting preliminary indications, this analysis did not result in sufficient information to propose strong recommendations, with the findings collected on these particular aspects scarce and often controversial. Similar to clinical trials [66], preclinical studies also suffer from inconsistencies in results due to excessive heterogeneity of methods relating to the use of both cultured ASCs and point of care products. In fact, no consensus regarding methods has been achieved so far, therefore no gold standard has been identified. However, from the aspects evaluated in this review, the augmentation of adipose-derived cell-based products with other therapies or by cellular pre-treatments provided more consistent results. A synergistic effect was observed in inhibiting cartilage degeneration and increasing the synthesis of extracellular matrix, the proliferation of chondrocytes, and the induction of anti-inflammatory effects when ASCs (both expanded and SVF) were associated with hyaluronic acid [ο] and when expanded ASCs were associated with PRP [46, 47], respectively. A few clinical studies showed the potential benefit of the combined use of SVF and PRP [67,68,69,70], so it would be interesting to conduct targeted pre-clinical and clinical studies investigating the combined use of cell-based products and PRP to further understand the possible synergistic effect of these orthobiologic agents.
The current systematic review has several limitations. The major one is the high heterogeneity found among the studies. In fact, different conditions related to the type of animal models, different follow-ups and measures, and different procedures are reported, which make the analysis of the outcomes more complex and the comparison of the effects of the therapies less reliable. The heterogeneity in methods, formulations, and results highlights the complexity of this area that should be also attributed to their recent introduction, and further highlights the need for achieving and setting clearer recommendations that are not yet available to date. Adhering to specific guidelines, for example using the SYRCLE’s tool to reduce the risk of bias when planning a study, would improve the quality and reliability of the results and increase the homogeneity of the studies, thus favouring more in-depth literature analyses. Another limitation of the study is that the pathophysiology of OA in animals and also the cartilage composition—especially in the smaller ones—as well as their response to treatment do not exactly represent the human equivalent conditions. Nevertheless, the biological effects of adipose tissue derived cell-based injective therapies seen in animal models may be considered as an indicator for potential processes and effects when orthobiologics are used in humans. Animal OA models could still be able to partially reproduce the effects of orthobiologics in human OA joints. Moreover, animal experiments also have the advantage when compared to human trials in terms of no placebo effect influence on the study findings, which makes the clinical improvement documented in this systematic review meaningful, with 78% of the clinical studies documenting a significant clinical improvement. Interestingly, imaging was the method which documented the lowest effect of adipose-derived cell-based therapy, suggesting that this approach is limited in its ability to detect disease-modifying effects in comparison to others utilised. MRI and radiographic evaluations are currently the most common option applied in the clinical setting and showed the lowest ability in detecting the effects of the applied treatments. This confirms the limits of the imaging evaluations already documented in the clinical practice, where there is a poor correlation between clinical outcome and imaging findings [71]. In this light, the limits of imaging techniques should be considered with caution to draw conclusions on treatment effectiveness in the clinical practice, as more disease-modifying effects have been found in the literature using other methods rather than MRI and radiographs, as underlined by the tissue and biomarker analysis in the animal models. These preclinical results could also serve as a useful comparison of different approaches to help identifying the most suitable cell-based strategy to be translated to humans for the treatment of OA.
Overall, this review provided a systematic view of the available literature about the disease-modifying effects of adipose-based orthobiologics in the treatment of OA in the preclinical setting, and therefore without being affected by the bias typical of the clinical literature. The information collected, in addition to contributing to the transition to the clinical setting of these therapeutic solutions, represents an important tool to understand their effects and to guide further research to optimize their use based on the clinical need.
Conclusions
Current preclinical models offer the opportunity to document consistent evidence of disease-modifying effects of adipose-derived cell-based therapies for the treatment of OA. Positive results have been observed at both the cartilage and synovial level, as well as in the analysis of biomarkers, clinical, and imaging results in most of the evaluated animals. The risk of bias and overall low quality of the published studies highlight the need for further targeted research to provide recommendations on the optimal methodologies required for a more effective application of adipose-derived cell-based therapies for the treatment of OA.
References
Hunter DJ, Bierma-Zeinstra S (2019) Osteoarthritis. Lancet Lond Engl 393:1745–1759
Katz JN, Arant KR, Loeser RF (2021) Diagnosis and treatment of hip and knee osteoarthritis: a review. JAMA 325:568–578. https://doi.org/10.1001/jama.2020.22171
Huebner K, Frank RM, Getgood A (2019) Ortho-biologics for osteoarthritis. Clin Sports Med 38:123–141. https://doi.org/10.1016/j.csm.2018.09.002
Dubey NK, Mishra VK, Dubey R, Syed-Abdul S, Wang JR, Wang PD, Deng W-P (2018) Combating osteoarthritis through stem cell therapies by rejuvenating cartilage: a review. Stem Cells Int 2018:5421019. https://doi.org/10.1155/2018/5421019
Muthu S, Jeyaraman M, Jain R, Gulati A, Jeyaraman N, Prajwal GS, Mishra PC (2021) Accentuating the sources of mesenchymal stem cells as cellular therapy for osteoarthritis knees-a panoramic review. Stem Cell Investig 8:13. https://doi.org/10.21037/sci-2020-055
Brown C, McKee C, Bakshi S, Walker K, Hakman E, Halassy S, Svinarich D, Dodds R, Govind CK, Chaudhry GR (2019) Mesenchymal stem cells: cell therapy and regeneration potential. J Tissue Eng Regen Med 13:1738–1755
Malekpour K, Hazrati A, Zahar M, Markov A, Zekiy AO, Navashenaq JG, Roshangar L, Ahmadi M (2021) The potential use of mesenchymal stem cells and their derived exosomes for orthopedic diseases treatment. Stem Cell Rev Rep 18(3):933–951
Maqsood M, Kang M, Wu X, Chen J, Teng L, Qiu L (2020) Adult mesenchymal stem cells and their exosomes: Sources, characteristics, and application in regenerative medicine. Life Sci 256:118002. https://doi.org/10.1016/j.lfs.2020.118002
Bourin P, Bunnell BA, Casteilla L, Dominici M, Katz AJ, March KL, Redl H, Rubin JP, Yoshimura K, Gimble JM (2013) Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy 15:641–648
Manferdini C, Maumus M, Gabusi E, Piacentini A, Filardo G, Peyrafitte J-A, Jorgensen C, Bourin P, Fleury-Cappellesso S, Facchini A, Noël D, Lisignoli G (2013) Adipose-derived mesenchymal stem cells exert antiinflammatory effects on chondrocytes and synoviocytes from osteoarthritis patients through prostaglandin E2. Arthritis Rheum 65:1271–1281
Ortiz-Virumbrales M, Menta R, Pérez LM, Lucchesi O, Mancheño-Corvo P, Avivar-Valderas Á, Palacios I, Herrero-Mendez A, Dalemans W, de la Rosa O, Lombardo E (2020) Human adipose mesenchymal stem cells modulate myeloid cells toward an anti-inflammatory and reparative phenotype: role of IL-6 and PGE2. Stem Cell Res Ther 11:462. https://doi.org/10.1186/s13287-020-01975-2
Song W-J, Li Q, Ryu M-O, Ahn J-O, Ha Bhang D, Chan Jung Y, Youn H-Y (2017) TSG-6 secreted by human adipose tissue-derived mesenchymal stem cells ameliorates DSS-induced colitis by inducing M2 macrophage polarization in mice. Sci Rep 7:5187
Bora P, Majumdar AS (2017) Adipose tissue-derived stromal vascular fraction in regenerative medicine: a brief review on biology and translation. Stem Cell Res Ther 8:145
Boffa A, Salerno M, Merli G, De Girolamo L, Laver L, Magalon J, Sánchez M, Tischer T, Filardo G (2021) Platelet-rich plasma injections induce disease-modifying effects in the treatment of osteoarthritis in animal models. Knee Surg Sports Traumatol Arthrosc 29:4100–4121
Hooijmans CR, Rovers MM, de Vries RBM, Leenaars M, Ritskes-Hoitinga M, Langendam MW (2014) SYRCLE’s risk of bias tool for animal studies. BMC Med Res Methodol 14:43. https://doi.org/10.1186/1471-2288-14-43
Sun Q, Zhang L, Xu T, Ying J, Xia B, Jing H, Tong P (2018) Combined use of adipose derived stem cells and TGF-β3 microspheres promotes articular cartilage regeneration in vivo. Biotech Histochem 93:168–176. https://doi.org/10.1080/10520295.2017.1401663
van Dalen SCM, Blom AB, Walgreen B, Slöetjes AW, Helsen MMA, Geven EJW, ter Huurne M, Vogl T, Roth J, van de Loo FAJ, Koenders MI, Casteilla L, van der Kraan PM, van den Bosch MHJ, van Lent PLEM (2019) IL-1β-Mediated activation of adipose-derived mesenchymal stromal cells results in PMN reallocation and enhanced phagocytosis: a possible mechanism for the reduction of osteoarthritis pathology. Front Immunol 10:1075. https://doi.org/10.3389/fimmu.2019.01075
Delco ML, Goodale M, Talts JF, Pownder SL, Koff MF, Miller AD, Nixon B, Bonassar LJ, Lundgren-Åkerlund E, Fortier LA (2020) Integrin α10β1-selected mesenchymal stem cells mitigate the progression of osteoarthritis in an Equine Talar impact model. Am J Sports Med 48:612–623
Desando G, Cavallo C, Sartoni F, Martini L, Parrilli A, Veronesi F, Fini M, Giardino R, Facchini A, Grigolo B (2013) Intra-articular delivery of adipose derived stromal cells attenuates osteoarthritis progression in an experimental rabbit model. Arthritis Res Ther 15:R22. https://doi.org/10.1186/ar4156
Kamada K, Matsushita T, Yamashita T, Matsumoto T, Iwaguro H, Sobajima S, Kuroda R (2021) Attenuation of knee osteoarthritis progression in mice through polarization of M2 macrophages by intra-articular transplantation of non-cultured human adipose-derived regenerative cells. J Clin Med 10:4309
Mei L, Shen B, Ling P, Liu S, Xue J, Liu F, Shao H, Chen J, Ma A, Liu X (2017) Culture-expanded allogenic adipose tissue-derived stem cells attenuate cartilage degeneration in an experimental rat osteoarthritis model. PLoS ONE 12:e0176107
Veronesi F, Berni M, Marchiori G, Cassiolas G, Muttini A, Barboni B, Martini L, Fini M, Lopomo NF, Marcacci M, Kon E (2021) Evaluation of cartilage biomechanics and knee joint microenvironment after different cell-based treatments in a sheep model of early osteoarthritis. Int Orthop 45:427–435. https://doi.org/10.1007/s00264-020-04701-y
Cheng J-H, Yen K-T, Chou W-Y, Jhan S-W, Hsu S-L, Ko J-Y, Wang C-J, Kuo C-EA, Wu S-Y, Hsu T-C, Hsu C-C (2021) Autologous adipose-derived mesenchymal stem cells combined with shockwave therapy synergistically ameliorates the osteoarthritic pathological factors in knee joint. Pharm Basel Switz 14:318. https://doi.org/10.3390/ph14040318
Hsu C-C, Cheng J-H, Wang C-J, Ko J-Y, Hsu S-L, Hsu T-C (2020) Shockwave therapy combined with autologous adipose-derived mesenchymal stem cells is better than with human umbilical cord Wharton’s jelly-derived mesenchymal stem cells on knee osteoarthritis. Int J Mol Sci 21:1217. https://doi.org/10.3390/ijms21041217
Hsu GC, Cherief M, Sono T, Wang Y, Negri S, Xu J, Peault B, James AW (2021) Divergent effects of distinct perivascular cell subsets for intra-articular cell therapy in posttraumatic osteoarthritis. J Orthop Res 39:2388–2397. https://doi.org/10.1002/jor.24997
Lee S, Lee SH, Na HS, Kwon JY, Kim G-Y, Jung K, Cho K-H, Kim SA, Go EJ, Park M-J, Baek J-A, Choi SY, Jhun J, Park S-H, Kim SJ, Cho M-L (2018) The therapeutic effect of STAT3 signaling-suppressed MSC on pain and articular cartilage damage in a rat model of monosodium iodoacetate-induced osteoarthritis. Front Immunol 9:2881. https://doi.org/10.3389/fimmu.2018.02881
Parrilli A, Giavaresi G, Ferrari A, Salamanna F, Desando G, Grigolo B, Martini L, Fini M (2017) Subchondral bone response to injected adipose-derived stromal cells for treating osteoarthritis using an experimental rabbit model. Biotech Histochem 92:201–211. https://doi.org/10.1080/10520295.2017.1292366
Shin K, Cha Y, Ban Y-H, Seo DW, Choi E-K, Park D, Kang SK, Ra JC, Kim Y-B (2019) Anti-osteoarthritis effect of a combination treatment with human adipose tissue-derived mesenchymal stem cells and thrombospondin 2 in rabbits. World J Stem Cells 11:1115–1129
Feng C, Luo X, He N, Xia H, Lv X, Zhang X, Li D, Wang F, He J, Zhang L, Lin X, Lin L, Yin H, He J, Wang J, Cao W, Wang R, Zhou G, Wang W (2018) Efficacy and persistence of allogeneic adipose-derived mesenchymal stem cells combined with hyaluronic acid in osteoarthritis after intra-articular injection in a Sheep model. Tissue Eng Part A 24:219–233
Lv X, He J, Zhang X, Luo X, He N, Sun Z, Xia H, Liu V, Zhang L, Lin X, Lin L, Yin H, Jiang D, Cao W, Wang R, Zhou G, Wang W (2018) Comparative efficacy of autologous stromal vascular fraction and autologous adipose-derived mesenchymal stem cells combined with hyaluronic acid for the treatment of sheep osteoarthritis. Cell Transplant 27:1111–1125
Muñoz-Criado I, Meseguer-Ripolles J, Mellado-López M, Alastrue-Agudo A, Griffeth RJ, Forteza-Vila J, Cugat R, García M, Moreno-Manzano V (2017) Human suprapatellar fat pad-derived mesenchymal stem cells induce chondrogenesis and cartilage repair in a model of severe osteoarthritis. Stem Cells Int. https://doi.org/10.1155/2017/4758930
Köhnke R, Ahlers MO, Birkelbach MA, Ewald F, Krueger M, Fiedler I, Busse B, Heiland M, Vollkommer T, Gosau M, Smeets R, Rutkowski R (2021) Temporomandibular joint osteoarthritis: regenerative treatment by a stem cell containing advanced therapy medicinal product (ATMP)—An In Vivo Animal Trial. Int J Mol Sci 22:443. https://doi.org/10.3390/ijms22010443
Ko J-Y, Lee J, Lee J, Ryu YH, Im G-I (2019) SOX - 6, 9 -transfected adipose stem cells to treat surgically-induced osteoarthritis in goats. Tissue Eng Part A 25:990–1000. https://doi.org/10.1089/ten.TEA.2018.0189
Filardo G, Tschon M, Perdisa F, Brogini S, Cavallo C, Desando G, Giavaresi G, Grigolo B, Martini L, Nicoli Aldini N, Roffi A, Fini M, Kon E (2021) Micro-fragmentation is a valid alternative to cell expansion and enzymatic digestion of adipose tissue for the treatment of knee osteoarthritis: a comparative preclinical study. Knee Surg Sports Traumatol Arthrosc 30(3):773–781
Desando G, Bartolotti I, Martini L, Giavaresi G, Nicoli Aldini N, Fini M, Roffi A, Perdisa F, Filardo G, Kon E, Grigolo B (2019) Regenerative features of adipose tissue for osteoarthritis treatment in a rabbit model: enzymatic digestion versus mechanical disruption. Int J Mol Sci 20:2636. https://doi.org/10.3390/ijms20112636
Choi S, Kim J-H, Ha J, Jeong B-I, Jung YC, Lee G-S, Woo H-M, Kang B-J (2018) Intra-articular injection of alginate-microencapsulated adipose tissue-derived mesenchymal stem cells for the treatment of osteoarthritis in rabbits. Stem Cells Int 2018:1–10. https://doi.org/10.1155/2018/2791632
Ko J-Y, Park J-W, Kim J, Im G-I (2021) Characterization of adipose-derived stromal/stem cell spheroids versus single-cell suspension in cell survival and arrest of osteoarthritis progression. J Biomed Mater Res A 109:869–878
Song SY, Hong J, Go S, Lim S, Sohn HS, Kang M, Jung G, Yoon J, Kang ML, Im G, Kim B (2020) Interleukin-4 gene transfection and spheroid formation potentiate therapeutic efficacy of mesenchymal stem cells for osteoarthritis. Adv Healthc Mater 9:1901612
ter Huurne M, Schelbergen R, Blattes R, Blom A, de Munter W, Grevers LC, Jeanson J, Noël D, Casteilla L, Jorgensen C, van den Berg W, van Lent PLEM (2012) Anti-inflammatory and chondroprotective effects of intraarticular injection of adipose-derived stem cells in experimental osteoarthritis. Arthritis Rheum 64:3604–3613
Sakamoto T, Miyazaki T, Watanabe S, Takahashi A, Honjoh K, Nakajima H, Oki H, Kokubo Y, Matsumine A (2019) Intraarticular injection of processed lipoaspirate cells has anti-inflammatory and analgesic effects but does not improve degenerative changes in murine monoiodoacetate-induced osteoarthritis. BMC Musculoskelet Disord 20:335. https://doi.org/10.1186/s12891-019-2710-1
Latief N, Raza FA, Bhatti F-U-R, Tarar MN, Khan SN, Riazuddin S (2016) Adipose stem cells differentiated chondrocytes regenerate damaged cartilage in rat model of osteoarthritis: ADSCs differentiated chondrocytes repair cartilage. Cell Biol Int 40:579–588
Hermeto LC, DeRossi R, Oliveira RJ, Pesarini JR, Antoniolli-Silva ACMB, Jardim PHA, Santana ÁE, Deffune E, Rinaldi JC, Justulin LA (2016) Effects of intra-articular injection of mesenchymal stem cells associated with platelet-rich plasma in a rabbit model of osteoarthritis. Genet Mol Res. https://doi.org/10.4238/gmr.15038569
Tang Y, Pan Z, Zou Y, He Y, Yang P, Tang Q, Yin F (2017) A comparative assessment of adipose-derived stem cells from subcutaneous and visceral fat as a potential cell source for knee osteoarthritis treatment. J Cell Mol Med 21:2153–2162
Frisbie DD, Kisiday JD, Kawcak CE, Werpy NM, McIlwraith CW (2009) Evaluation of adipose-derived stromal vascular fraction or bone marrow-derived mesenchymal stem cells for treatment of osteoarthritis: Mesenchymal stem cells for osteoarthritis. J Orthop Res 27:1675–1680
Kim J-H, Yun S, Seo M-S, Bae S, Jang M, Ku S-K, Kwon Y-S, Lee HB (2020) Synergistic effect of carboxymethyl chitosan and adipose-derived mesenchymal stem cells on osteoarthritis model in rabbits. J Vet Clin 37:261–269. https://doi.org/10.17555/jvc.2020.10.37.5.261
Ahmad MR, Badar W, Ullah Khan MA, Mahmood A, Latif N, Iqbal T, Khan Assir MZ, Sleem MA (2020) Combination of preconditioned adipose-derived mesenchymal stem cells and platelet-rich plasma improves the repair of osteoarthritis in rat. Regen Med 15:2285–2295
Yun S, Ku S-K, Kwon Y-S (2016) Adipose-derived mesenchymal stem cells and platelet-rich plasma synergistically ameliorate the surgical-induced osteoarthritis in Beagle dogs. J Orthop Surg 11:9
Jacer S, Shafaei H, Soleimani Rad J (2018) An investigation on the regenerative effects of intra articular injection of co-cultured adipose derived stem cells with chondron for treatment of induced osteoarthritis. Adv Pharm Bull 8:297–306
Mei L, Shen B, Xue J, Liu S, Ma A, Liu F, Shao H, Chen J, Chen Q, Liu F, Ying Y, Ling P (2017) Adipose tissue–derived stem cells in combination with xanthan gum attenuate osteoarthritis progression in an experimental rat model. Biochem Biophys Res Commun 494:285–291
An X, Wang T, Zhang W, Yu H, Chunhua Zhao R, Guo Y, Wang C, Qin L, Guo C (2020) Chondroprotective effects of combination therapy of acupotomy and human adipose mesenchymal stem cells in knee osteoarthritis rabbits via the GSK3β-Cyclin D1-CDK4/CDK6 signaling pathway. Aging Dis 11:1116. https://doi.org/10.14336/AD.2019.1104
El-Badawy A, Amer M, Abdelbaset R, Sherif SN, Abo-Elela M, Ghallab YH, Abdelhamid H, Ismail Y, El-Badri N (2016) Adipose stem cells display higher regenerative capacities and more adaptable electro-kinetic properties compared to bone marrow-derived mesenchymal stromal cells. Sci Rep 6:37801
Mazini L, Rochette L, Amine M, Malka G (2019) Regenerative capacity of adipose derived stem cells (ADSCs), comparison with mesenchymal stem cells (MSCs). Int J Mol Sci 20:E2523. https://doi.org/10.3390/ijms20102523
Strioga M, Viswanathan S, Darinskas A, Slaby O, Michalek J (2012) Same or not the same? Comparison of adipose tissue-derived versus bone marrow-derived mesenchymal stem and stromal cells. Stem Cells Dev 21:2724–2752
Agarwal N, Mak C, Bojanic C, To K, Khan W (2021) Meta-analysis of adipose tissue derived cell-based therapy for the treatment of knee osteoarthritis. Cells 10:1365. https://doi.org/10.3390/cells10061365
Jo CH, Chai JW, Jeong EC, Oh S, Shin JS, Shim H, Yoon KS (2017) Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: a 2-year follow-up study. Am J Sports Med 45:2774–2783
Lavagnolo U, Veronese S, Negri S, Magnan B, Sbarbati A (2021) Lipoaspirate processing for the treatment of knee osteoarthritis: a review of clinical evidences. Biomed Pharmacother 142:111997
Pleumeekers MM, Nimeskern L, Koevoet JLM, Karperien M, Stok KS, van Osch GJVM (2018) Trophic effects of adipose-tissue-derived and bone-marrow-derived mesenchymal stem cells enhance cartilage generation by chondrocytes in co-culture. PLoS ONE 13:e0190744
Ragni E, Perucca Orfei C, De Luca P, Lugano G, Viganò M, Colombini A, Valli F, Zacchetti D, Bollati V, de Girolamo L (2019) Interaction with hyaluronan matrix and miRNA cargo as contributors for in vitro potential of mesenchymal stem cell-derived extracellular vesicles in a model of human osteoarthritic synoviocytes. Stem Cell Res Ther 10:109. https://doi.org/10.1186/s13287-019-1215-z
Boffa A, Merli G, Andriolo L, Lattermann C, Salzmann GM, Filardo G (2021) Synovial fluid biomarkers in knee osteoarthritis: a systematic review and quantitative evaluation using BIPEDs criteria. Cartilage 13:82S-103S
Ragni E, Colombini A, Viganò M, Libonati F, Perucca Orfei C, Zagra L, de Girolamo L (2021) Cartilage protective and immunomodulatory features of osteoarthritis synovial fluid-treated adipose-derived mesenchymal stem cells secreted factors and extracellular vesicles-embedded miRNAs. Cells 10:1072. https://doi.org/10.3390/cells10051072
Garza JR, Campbell RE, Tjoumakaris FP, Freedman KB, Miller LS, Santa Maria D, Tucker BS (2020) Clinical efficacy of intra-articular mesenchymal stromal cells for the treatment of knee osteoarthritis: a double-blinded prospective randomized controlled clinical trial. Am J Sports Med 48:588–598
Hudetz D, Borić I, Rod E, Jeleč Ž, Radić A, Vrdoljak T, Skelin A, Lauc G, Trbojević-Akmačić I, Plečko M, Polašek O, Primorac D (2017) The effect of intra-articular injection of autologous microfragmented fat tissue on proteoglycan synthesis in patients with knee osteoarthritis. Genes 8:E270. https://doi.org/10.3390/genes8100270
Louis ML, Dumonceau RG, Jouve E, Cohen M, Djouri R, Richardet N, Jourdan E, Giraudo L, Dumoulin C, Grimaud F, George FD, Veran J, Sabatier F, Magalon J (2021) Intra-articular injection of autologous microfat and platelet-rich plasma in the treatment of knee osteoarthritis: a double-blind randomized comparative study. Arthroscopy 37:3125-3137.e3
Lu L, Dai C, Zhang Z, Du H, Li S, Ye P, Fu Q, Zhang L, Wu X, Dong Y, Song Y, Zhao D, Pang Y, Bao C (2019) Treatment of knee osteoarthritis with intra-articular injection of autologous adipose-derived mesenchymal progenitor cells: a prospective, randomized, double-blind, active-controlled, phase IIb clinical trial. Stem Cell Res Ther 10:143
Di Matteo B, Vandenbulcke F, Vitale ND, Iacono F, Ashmore K, Marcacci M, Kon E (2019) Minimally manipulated mesenchymal stem cells for the treatment of knee osteoarthritis: a systematic review of clinical evidence. Stem Cells Int 2019:1735242. https://doi.org/10.1155/2019/1735242
Lopa S, Colombini A, Moretti M, de Girolamo L (2019) Injective mesenchymal stem cell-based treatments for knee osteoarthritis: from mechanisms of action to current clinical evidences. Knee Surg Sports Traumatol Arthrosc 27:2003–2020
Koh Y-G, Choi Y-J, Kwon S-K, Kim Y-S, Yeo J-E (2015) Clinical results and second-look arthroscopic findings after treatment with adipose-derived stem cells for knee osteoarthritis. Knee Surg Sports Traumatol Arthrosc 23:1308–1316
Mehranfar S, Abdi Rad I, Mostafav E, Akbarzadeh A (2019) The use of stromal vascular fraction (SVF), platelet-rich plasma (PRP) and stem cells in the treatment of osteoarthritis: an overview of clinical trials. Artif Cells Nanomedicine Biotechnol 47:882–890. https://doi.org/10.1080/21691401.2019.1576710
Pak J, Chang J-J, Lee JH, Lee SH (2013) Safety reporting on implantation of autologous adipose tissue-derived stem cells with platelet-rich plasma into human articular joints. BMC Musculoskelet Disord 14:337. https://doi.org/10.1186/1471-2474-14-337
Van Pham P, Bui KH-T, Duong TD, Nguyen NT, Nguyen TD, Le VT, Mai VT, Phan NL-C, Le DM, Ngoc NK (2014) Symptomatic knee osteoarthritis treatment using autologous adipose derived stem cells and platelet-rich plasma: a clinical study. Biomed Res Ther 1:2
Vasiliadis AV, Galanis N (2020) Effectiveness of AD-MSCs injections for the treatment of knee osteoarthritis: analysis of the current literature. J Stem Cells Regen Med 16:3–9
Kuroda Y, Matsumoto T, Hayashi S, Hashimoto S, Takayama K, Kirizuki S, Tsubosaka M, Kamenaga T, Takashima Y, Matsushita T, Niikura T, Kuroda R (2019) Intra-articular autologous uncultured adipose-derived stromal cell transplantation inhibited the progression of cartilage degeneration. J Orthop Res 37:1376–1386
Lee JC (2018) Microarray analysis after adipose derived mesenchymal stem cells injection in monosodium iodoacetate-induced osteoarthritis rats. Genes Genomics 40:25–37
Dubey NK, Wei H-J, Yu S-H, Williams DF, Wang JR, Deng Y-H, Tsai F-C, Wang PD, Deng W-P (2019) Adipose-derived stem cells attenuates diabetic osteoarthritis via inhibition of glycation-mediated inflammatory cascade. Aging Dis 10:483. https://doi.org/10.14336/AD.2018.0616
Li M, Luo X, Lv X, Liu V, Zhao G, Zhang X, Cao W, Wang R, Wang W (2016) In vivo human adipose-derived mesenchymal stem cell tracking after intra-articular delivery in a rat osteoarthritis model. Stem Cell Res Ther 7:160. https://doi.org/10.3892/etm.2018.6754
Zhang R, Meng F, Zhang Q, Zou Z, Xiao K, Zhu T, Li H, Zhang W, Ma J, Ma J (2021) Allogeneic adipose-derived mesenchymal stem cells promote the expression of chondrocyte redifferentiation markers and retard the progression of knee osteoarthritis in rabbits. Am J Transl Res 13:632–645
Takagi T, Kabata T, Hayashi K, Fang X, Kajino Y, Inoue D, Ohmori T, Ueno T, Yoshitani J, Ueoka K, Yamamuro Y, Tsuchiya H (2020) Periodic injections of adipose-derived stem cell sheets attenuate osteoarthritis progression in an experimental rabbit model. BMC Musculoskelet Disord 21:691. https://doi.org/10.1186/s12891-020-03718-z
Zhou J, Wang Y, Liu Y, Zeng H, Xu H, Lian F (2019) Adipose derived mesenchymal stem cells alleviated osteoarthritis and chondrocyte apoptosis through autophagy inducing. J Cell Biochem 120:2198–2212
Maumus M, Roussignol G, Toupet K, Penarier G, Bentz I, Teixeira S, Oustric D, Jung M, Lepage O, Steinberg R, Jorgensen C, Noel D (2016) Utility of a mouse model of osteoarthritis to demonstrate cartilage protection by ifnγ-primed equine mesenchymal stem cells. Front Immunol 7:392. https://doi.org/10.3389/fimmu.2016.00392
Toupet K, Maumus M, Luz-Crawford P, Lombardo E, Lopez-Belmonte J, van Lent P, Garin MI, van den Berg W, Dalemans W, Jorgensen C, Noël D (2015) Survival and biodistribution of xenogenic adipose mesenchymal stem cells is not affected by the degree of inflammation in arthritis. PLoS ONE 10:e0114962
Black LL, Gaynor J, Gahring D, Adams C, Aron D, Harman S, Gingerich DA, Harman R (2007) Effect of adipose-derived mesenchymal stem and regenerative cells on lameness in dogs with chronic osteoarthritis of the coxofemoral joints: a randomized, double-blinded, multicenter, controlled trial. Vet Ther Res Appl Vet Med 8:272–284
Maki CB, Beck A, Wallis C-BCC, Choo J, Ramos T, Tong R, Borjesson DL, Izadyar F (2020) Intra-articular administration of allogeneic adipose derived MSCs reduces pain and lameness in dogs with hip osteoarthritis: a double blinded, randomized, placebo controlled pilot study. Front Vet Sci 7:570. https://doi.org/10.3389/fvets.2020.00570
Mariñas-Pardo L, García-Castro J, Rodríguez-Hurtado I, Rodríguez-García MI, Núñez-Naveira L, Hermida-Prieto M (2018) Allogeneic adipose-derived mesenchymal stem cells (Horse Allo 20) for the treatment of osteoarthritis-associated lameness in horses: characterization, safety, and efficacy of intra-articular treatment. Stem Cells Dev 27:1147–1160. https://doi.org/10.1089/scd.2018.0074
Wits MI, Tobin GC, Silveira MD, Baja KG, Braga LMM, Sesterheim P, Camassola M, Nardi NB (2020) Combining canine mesenchymal stromal cells and hyaluronic acid for cartilage repair. Genet Mol Biol 43:e20190275
Riester SM, Denbeigh JM, Lin Y, Jones DL, de Mooij T, Lewallen EA, Nie H, Paradise CR, Radel DJ, Dudakovic A, Camilleri ET, Larson DR, Qu W, Krych AJ, Frick MA, Im H-J, Dietz AB, Smith J, van Wijnen AJ (2017) Safety studies for use of adipose tissue-derived mesenchymal stromal/stem cells in a rabbit model for osteoarthritis to support a Phase I clinical trial: stem cell safety in osteoarthritis. Stem Cells Transl Med 6:910–922. https://doi.org/10.5966/sctm.2016-0097
Lee J-M, Im G-I (2012) SOX trio-co-transduced adipose stem cells in fibrin gel to enhance cartilage repair and delay the progression of osteoarthritis in the rat. Biomaterials 33:2016–2024
Liu X, Du M, Wang Y, Liu S, Liu X (2018) BMP9 overexpressing adipose-derived mesenchymal stem cells promote cartilage repair in osteoarthritis-affected knee joint via the Notch1/Jagged1 signaling pathway. Exp Ther Med 16(6):4623–4631
Oh J, Son YS, Kim WH, Kwon O-K, Kang B-J (2021) Mesenchymal stem cells genetically engineered to express platelet-derived growth factor and heme oxygenase-1 ameliorate osteoarthritis in a canine model. J Orthop Surg 16:43. https://doi.org/10.1186/s13018-020-02178-4
Schwabe K, Garcia M, Ubieta K, Hannemann N, Herbort B, Luther J, Noël D, Jorgensen C, Casteilla L, David J-P, Stock M, Herrmann M, Schett G, Bozec A (2016) Inhibition of osteoarthritis by adipose-derived stromal cells overexpressing Fra-1 in Mice: Fra-1-transgenic mouse ADSCs protect against OA. Arthritis Rheumatol 68:138–151. https://doi.org/10.1002/art.39425
Ude CC, Ng MH, Chen CH, Htwe O, Amaramalar NS, Hassan S, Djordjevic I, Rani RA, Ahmad J, Yahya NM, Saim AB, Hj Idrus RB (2015) Improved functional assessment of osteoarthritic knee joint after chondrogenically induced cell treatment. Osteoarthritis Cartilage 23:1294–1306
Ude CC, Shamsul BS, Ng MH, Chen HC, Ohnmar H, Amaramalar SN, Rizal AR, Johan A, Norhamdan MY, Azizi M, Aminuddin BS, Ruszymah BHI (2018) Long-term evaluation of osteoarthritis sheep knee, treated with TGF-β3 and BMP-6 induced multipotent stem cells. Exp Gerontol 104:43–51. https://doi.org/10.1016/j.exger.2018.01.020
Ude CC, Sulaiman SB, Min-Hwei N, Hui-Cheng C, Ahmad J, Yahaya NM, Saim AB, Idrus RBH (2014) Cartilage regeneration by chondrogenic induced adult stem cells in osteoarthritic sheep model. PLoS ONE 9:e98770
Cuervo B, Rubio M, Sopena J, Dominguez J, Vilar J, Morales M, Cugat R, Carrillo J (2014) Hip osteoarthritis in dogs: a randomized study using mesenchymal stem cells from adipose tissue and plasma rich in growth factors. Int J Mol Sci 15:13437–13460. https://doi.org/10.3390/ijms150813437
Stancker TG, Vieira SS, Serra AJ, do NascimentoLima R, dos SantosFeliciano R, Silva JA, dos Santos SA, dos SantosVieira MA, Simões MCB, Leal-Junior EC, de deTarsoCamilloCarvalho P (2018) Can photobiomodulation associated with implantation of mesenchymal adipose-derived stem cells attenuate the expression of MMPs and decrease degradation of type II collagen in an experimental model of osteoarthritis? Lasers Med Sci 33:1073–1084
Keller LE, Fortier LA, Delco ML, Okudaira M, Becktell L, Cercone M (2021) High-Plex RNA expression profiling of formalin-fixed paraffin-embedded synovial membrane indicates potential mechanism of mesenchymal stromal cells in the mitigation of posttraumatic osteoarthritis. Cartilage. https://doi.org/10.1177/1947603521993521
Lee J, Choe S (2020) Gene alterations after human adipose-derived stem cell-derived exosome injection in monosodium iodoacetate-induced osteoarthritis rats by microarray analysis. J Anat Soc India 69:37
Wang Z, Zhu H, Dai S, Liu K, Ge C (2020) Alleviation of medial meniscal transection-induced osteoarthritis pain in rats by human adipose derived mesenchymal stem cells. Stem Cell Investig 7:10–10
Li J, Zhu X, Shao Q, Xu F, Sun G (2019) Allogeneic adipose-derived stem cell transplantation on knee osteoarthritis rats and its effect on MMP-13 and DDR2. Exp Ther Med 18:99–104
Xie M, Luo S, Li Y, Lu L, Deng C, Cheng Y, Yin F (2019) Intra-articular tracking of adipose-derived stem cells by chitosan-conjugated iron oxide nanoparticles in a rat osteoarthritis model. RSC Adv 9:12010–12019
Harman R, Carlson K, Gaynor J, Gustafson S, Dhupa S, Clement K, Hoelzler M, McCarthy T, Schwartz P, Adams C (2016) A Prospective, randomized, masked, and placebo-controlled efficacy study of intraarticular allogeneic adipose stem cells for the treatment of osteoarthritis in dogs. Front Vet Sci 3:81. https://doi.org/10.3389/fvets.2016.00081
Mohoric L, Zorko B, Ceh K, Majdic G (2016) Blinded placebo study of bilateral osteoarthritis treatment using adipose derived mesenchymal stem cells. Slov Vet Res 53(3):167–174
Kuroda K, Kabata T, Hayashi K, Maeda T, Kajino Y, Iwai S, Fujita K, Hasegawa K, Inoue D, Sugimoto N, Tsuchiya H (2015) The paracrine effect of adipose-derived stem cells inhibits osteoarthritis progression. BMC Musculoskelet Disord 16:236. https://doi.org/10.1186/s12891-015-0701-4
Wang W, He N, Feng C, Liu V, Zhang L, Wang F, He J, Zhu T, Wang S, Qiao W, Li S, Zhou G, Zhang L, Dai C, Cao W (2015) Human adipose-derived mesenchymal progenitor cells engraft into rabbit articular cartilage. Int J Mol Sci 16:12076–12091. https://doi.org/10.3390/ijms160612076
Nicpon J, Marycz K, Grzesiak J, SMIESZEK A, Toker NY (2014) The advantages of Autologus Adipose Derived Mesenchymal Stem Cells (AdMSCs) over the Non-steroidal Anti-inflammatory Drugs (NSAIDs) application for degenerative elbow joint disease treatment in dogs—twelve cases. Kafkas Universitesi Veteriner Fakultesi Dergisi. https://doi.org/10.9775/kvfd.2013.10105
Schelbergen RF, van Dalen S, ter Huurne M, Roth J, Vogl T, Noël D, Jorgensen C, van den Berg WB, van de Loo FA, Blom AB, van Lent PLEM (2014) Treatment efficacy of adipose-derived stem cells in experimental osteoarthritis is driven by high synovial activation and reflected by S100A8/A9 serum levels. Osteoarthritis Cartilage 22:1158–1166
Toghraie F, Razmkhah M, Gholipour MA, Faghih Z, Chenari N, Torabi Nezhad S, Nazhvani Dehghani S, Ghaderi A (2012) Scaffold-free adipose-derived stem cells (ASCs) improve experimentally induced osteoarthritis in rabbits. Arch Iran Med 15:495–499
Toghraie FS, Chenari N, Gholipour MA, Faghih Z, Torabinejad S, Dehghani S, Ghaderi A (2011) Treatment of osteoarthritis with infrapatellar fat pad derived mesenchymal stem cells in Rabbit. Knee 18:71–75
Acknowledgements
Authors GF, LDG, LL, TT, JM and MS are members of the Orthobiologics Initiative (ORBIT) by the European Society of Sports Traumatology, Knee Surgery & Arthroscopy (ESSKA).
Funding
The work reported in this publication was funded by the Italian Ministry of Health, RCR-2021-23671217 project, under the “The Italian Musculoskeletal Apparatus Network RAMS”.
Author information
Authors and Affiliations
Contributions
Conceptualization, GF, LDG, and LL; methodology and data curation, AB, CPO, and YS; writing—original draft preparation, CPO and AB; writing—review, editing, and supervision, GF, LDG, LL, JM, MS, TT. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
JM is a co-founder of Remedex and received honoraria from Fidia Pharmaceuticals, Horiba, Arthrex, and Macopharma. LDG is scientific consultant for Lipogems S.p.A.. The manufactures had no role in the redaction of this manuscript of its decision for publication. The other authors declare no conflict of interest.
Ethical approval
Not applicable.
Informed consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Perucca Orfei, C., Boffa, A., Sourugeon, Y. et al. Cell-based therapies have disease-modifying effects on osteoarthritis in animal models. A systematic review by the ESSKA Orthobiologic Initiative. Part 1: adipose tissue-derived cell-based injectable therapies. Knee Surg Sports Traumatol Arthrosc 31, 641–655 (2023). https://doi.org/10.1007/s00167-022-07063-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-022-07063-7