Abstract
Purpose
Human herpesviruses, particularly cytomegalovirus (CMV) and herpes simplex virus (HSV), frequently reactivate in critically ill patients, including those with acute respiratory distress syndrome (ARDS) related to coronavirus disease 2019 (COVID-19). The clinical interpretation of pulmonary herpesvirus reactivation is challenging and there is ongoing debate about its association with mortality and benefit of antiviral medication. We aimed to quantify the incidence and pathogenicity of pulmonary CMV and HSV reactivations in critically ill COVID-19 patients.
Methods
Mechanically ventilated COVID-19 patients seropositive for CMV or HSV were included in this observational cohort study. Diagnostic bronchoscopy with bronchoalveolar lavage was performed routinely and analyzed for alveolar viral loads and inflammatory biomarkers. Utilizing joint modeling, we explored the dynamic association between viral load trajectories over time and mortality. We explored alveolar inflammatory biomarker dynamics between reactivated and non-reactivated patients.
Results
Pulmonary reactivation (> 104 copies/ml) of CMV occurred in 6% of CMV-seropositive patients (9/156), and pulmonary reactivation of HSV in 37% of HSV-seropositive patients (63/172). HSV viral load dynamics prior to or without antiviral treatment were associated with increased 90-day mortality (hazard ratio [HR] 1.24, 95% confidence interval [CI] 1.04–1.47). The alveolar concentration of several inflammatory biomarkers increased with HSV reactivation, including interleukin (IL)-6, IL-1β, granulocyte colony stimulating factor (G-CSF), and tumor necrosis factor (TNF).
Conclusion
In mechanically ventilated COVID-19 patients, HSV reactivations are common, while CMV reactivations were rare. HSV viral load dynamics prior to or without antiviral treatment are associated with mortality. Alveolar inflammation is elevated after HSV reactivation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Our findings of prevalent herpes simplex virus (HSV) reactivations in mechanically ventilated patients, along with the observed association between viral loads and mortality, emphasize the potential impact of pulmonary HSV reactivation in non-resolving acute respiratory distress syndrome. The resolution of inflammation and injury may be hindered due to HSV reactivation, prompting speculation that antiviral treatment holds promise for improving clinical outcomes. |
Introduction
Human herpesviruses establish lifelong latency after infection, and commonly reactivate in critically ill patients admitted to the intensive care unit (ICU) [1]. Mechanical ventilation, sepsis, corticosteroid therapy, and prolonged hospitalization have been identified as risk factors for reactivation [1,2,3,4,5,6,7,8]. Pulmonary reactivations of cytomegalovirus (CMV) and herpes simplex virus (HSV) have been implicated in persistent lung injury resulting in non-resolving acute respiratory distress syndrome (ARDS) [9,10,11,12]. The coronavirus disease 2019 (COVID-19) pandemic has resulted in a surge of ARDS cases, often characterized by persistent lung injury. Critically ill patients with COVID-19 have biological and clinical characteristics of an acquired immunosuppressive state, and remarkably high cumulative risks for CMV and HSV reactivation have been identified in patients with COVID-19 [2, 13, 14].
Systemic reactivation of CMV and HSV has been associated with prolonged invasive mechanical ventilation and increased mortality in ARDS patients, whether due to COVID-19 [2, 13, 15] or other conditions [9, 10, 16, 17]. Lower respiratory tract HSV loads can increase exponentially during ICU admission [12]. However, there is conflicting evidence on the association between pulmonary CMV and HSV reactivation and mortality [5, 18, 19]. Studying pulmonary herpesvirus reactivation is challenging due to inconsistent alveolar sampling methods and timing, the frequent absence of viral load assays, unclear thresholds for defining clinically significant reactivation, and the risk of immortal time bias in statistical analysis [20, 21].
This knowledge gap is reflected by the persistent debate in critical care practice on the use of antiviral medication for pulmonary CMV or HSV reactivation. Pre-emptive aciclovir therapy at prophylactic doses suggested clinical benefits in invasively ventilated patients experiencing oropharyngeal HSV reactivation [22]. Moreover, a recent meta-analysis indicated a survival benefit from aciclovir in patients with pulmonary HSV reactivation, although this analysis was subject to a high risk for bias [23]. If CMV or HSV reactivation in the respiratory tract indeed contributes to persistent lung injury and prevents resolution of ARDS, it would be an important treatable trait in a patient population where alternative treatments are scarce [24].
In this study, we aimed to quantify the incidence, mortality impact and effect of antiviral treatment of CMV and HSV reactivation in the lower respiratory tract of critically ill COVID-19 patients. We hypothesized that CMV and HSV reactivations are common in mechanically ventilated COVID-19 patients, and that the CMV and HSV viral loads are associated with mortality. We also postulated that antiviral treatment reduces viral loads and that the association between load and mortality disappears after initiation of treatment. We further explored the association between viral reactivation and the alveolar inflammatory response.
Methods
Study cohort and clinical data collection
This observational cohort study was performed at Amsterdam University Medical Center, locations AMC and VUmc, Amsterdam, The Netherlands, and results on alveolar inflammatory response have been described before [25, 26]. The study included all mechanically ventilated patients with COVID-19 confirmed by polymerase chain reaction (PCR) admitted to the ICU, who underwent a diagnostic bronchoscopy with bronchoalveolar lavage (BAL) for COVID-19-related ARDS according to the Berlin criteria [27], and who were at risk for viral reactivation, indicated by seropositivity for immunoglobulin (Ig) G antibodies against HSV or CMV. This resulted in two study cohorts, one comprising CMV-seropositive patients and the other comprising HSV-seropositive patients. Clinical data were prospectively collected, with further information provided in the supplementary methods section.
Sampling and assays
Sampling
Routine diagnostic bronchoscopy with BAL was performed weekly in patients showing no respiratory improvement, as part of clinical practice according to standardized protocol. The decision to perform a BAL was made by joint expert opinion during multidisciplinary team meetings. During bronchoscopy, four times 20-ml 0.9% NaCl was inserted into single segments of the lungs and collected in fractions. The first, third, and fourth fractions were used for microbiological diagnostics, including CMV and HSV PCR assays as described below.
The second fraction, along with paired blood samples, was processed and stored for subsequent biomarker analysis when logistically feasible (see corresponding methods section and supplementary methods for details).
CMV and HSV serology
CMV and HSV IgG levels were assessed using a chemiluminescent microparticle immunoassay on the Liaison XL (DiaSorin, Saluggia, Italy) in either blood serum or ethylenediamine tetraacetic acid (EDTA) plasma. The tests were conducted in accordance with the manufacturer’s instructions. Results ≥ 14 U/ml for CMV and ≥ 1.1 index for HSV-1,2 IgG were considered positive. The blood samples were collected within 24 h of ICU admission or, if not feasible, from samples obtained before or during ICU stay.
CMV and HSV PCR in BAL
The full methods are described in the supplement. In short, CMV and HSV detection and quantification in BAL fluid were conducted using the LightCycler 480 Real-Time PCR System (LC480) (Roche Diagnostics, Penzberg, Germany). Viral DNA load estimates [in copies per ml (c/ml)] were calculated based on crossing point (Cp) values, employing calibration curves derived from known quantities of virus stock dilutions. Samples were classified as negative (Cp > 40), positive but below clinically relevant threshold (Cp ≤ 40 but load ≤ 104 c/ml), or clinically relevant reactivation (load > 104 c/ml). The determination of this threshold was based on local protocol, guided by expert opinion and extrapolation from studies conducted in different patient populations [11, 12, 18, 28,29,30,31,32], and prompted antiviral therapy initiation. Ganciclovir was the preferred treatment for CMV, and aciclovir for HSV. In cases where both viruses reactivated, the treatment of choice was ganciclovir. Loads ≤ 104 c/ml did not prompt treatment.
Biomarker assays
A comprehensive panel of 41 inflammatory biomarkers (Table S1) was measured in stored BAL and paired plasma samples by Luminex multiplex assay (R&D Systems, Abingdon, United Kingdom), using a Bio-Plex 200 System (Bio-Rad Laboratories, Hercules, CA, USA).
The supplementary methods section contains more detailed information on the abovementioned assays.
Statistical analysis
All analysis were performed separately for the CMV cohort (i.e., all CMV-seropositive patients) and the HSV cohort (i.e., all HSV-seropositive patients). In these analyses, CMV and HSV reactivations refer to clinically relevant reactivations (viral load > 104 c/ml).
The probability of developing clinically relevant CMV and HSV reactivations was visualized in probability plots using multistate modeling (msSurv package [33]) (Fig. S1). Differences in characteristics between patients with and without clinically relevant reactivation were evaluated using a Student’s t test, Mann–Whitney U test, or Chi-squared test, as appropriate.
The association between log-transformed viral load trajectories and mortality was modeled using a joint modeling approach (JM package) [34], which combines a linear mixed model with a Cox proportional hazards model. This method was selected for its accuracy in estimating time-related associations and handling informative censoring—relevant as deceased or extubated patients cannot undergo additional bronchoscopies. The initial analysis focused on examining the trajectories of viral loads prior to the initiation of, or without antiviral treatment, deliberately excluding the influence of antiviral treatment on the viral load dynamics. Subsequently, a specific analysis was conducted on patients receiving antiviral treatment, including data from its initiation. All joint models were adjusted for age and body mass index (BMI), factors associated with 90-day mortality in the studied cohort.
We conducted a cutoff analysis to retrospectively re-evaluate the relevance of the a priori defined cutoff of 104 c/ml viral load for a clinically relevant reactivation, as per the local clinical protocol, and to compare it with alternative cutoff values. In this analysis, we studied two aspects across various cutoffs: the maximum probability of transitioning from a non-reactivated state to a reactivated state, and the mortality risk. Next, employing the predefined clinical cutoff of > 104 c/ml, we evaluated the mortality risk in patients with and without reactivation using multistate modeling techniques (msSurv package).
To evaluate the association between alveolar inflammation and viral reactivation, we performed multiple analyses, addressing concerns related to sampling bias, selection bias, and immortal time bias in patients experiencing viral reactivation. The analyses included cross-sectional analyses (both matched and unmatched) between patients with and without HSV reactivation, and matched longitudinal analyses (pre- and post-reactivation, and pre- and post-treatment). Significant biomarkers identified in BAL were also analyzed in plasma to differentiate between alveolar and systemic responses.
The online supplement provides details on all analyses. Calculations were performed in R version 4.2.1 using the RStudio interface. p values < 0.05 were considered statistically significant.
Results
Patient population and incidence
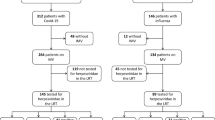
Between March 31th 2020 and June 4th 2021, 205 patients underwent bronchoscopy with BAL and were screened (Fig. 1). After excluding 11 ineligible patients, the cohort comprised 194 patients, of whom 80% were CMV seropositive, 89% were HSV seropositive, and 73% were seropositive for both CMV and HSV (Table S2). Patients not at risk for reactivation (i.e., seronegative patients) were excluded based on their particular virus serostatus, resulting in distinct cohorts. Specifically, CMV-seronegative patients were excluded, resulting in a CMV cohort of 156 CMV-seropositive patients with 254 consecutive BAL samples, while HSV-seronegative patients were similarly excluded, resulting in an HSV cohort of 172 HSV-seropositive patients with 391 consecutive BAL samples. Table 1 shows the demographics and clinical characteristics of both cohorts.
Flowchart of screening and inclusion process. Numbers of patients with numbers of BAL samples are displayed for the study period from March 31th, 2020, until June 4th, 2021. After excluding 11 ineligible patients, the cohort comprised 194 patients. Patients not at risk for reactivation (i.e., seronegative patients) were excluded based on their particular virus serostatus, resulting in distinct cohorts. Specifically, CMV-seronegative patients were excluded, resulting in a CMV cohort of 156 patients (all IgG positive for CMV), while HSV-seronegative patients were similarly excluded, resulting in an HSV cohort of 172 patients (all IgG positive for HSV). *A reactivation was considered ‘clinically relevant’ in case of a viral load of > 104 c/ml in the BAL fluid. BAL bronchoalveolar lavage, CMV cytomegalovirus, HSV herpes simplex virus, IgG immunoglobulin G, PCR polymerase chain reaction
Nine out of one hundred fifty-six patients (6%) within the seropositive CMV cohort developed a, what we a priori considered to be, clinically relevant CMV reactivation during ICU admission, whereas sixty-three out of one hundred seventy-two patients (37%) from the seropositive HSV cohort developed a clinically relevant HSV-1-reactivation. There were no clinically relevant HSV-2 reactivations in this cohort. Viral load distributions are shown in Fig. S2. The peak probability of CMV reactivation was 3%, at approximately 30 days from intubation. The median time to CMV reactivation was 28 days (Fig. S3-A). The peak probability of HSV reactivation was 22%, occurring at around 16 days post-intubation, and the median time to reactivation was 12 days (Fig. S3-B). Simultaneous occurrences of CMV and HSV reactivations were rare (n = 4), and no correlation was found between CMV and HSV viral loads (ρ = 0.072, 95% confidence interval [CI] [− 0.028, 0.169], p = 0.158), as illustrated in Fig. S4. Figure S5 provides a swimmer plot overview of BAL sampling moments and corresponding CMV- and HSV-reactivation results, and the administered antiviral treatment for all enrolled patients.
Patients with clinically relevant CMV or HSV reactivation (> 104 c/ml) had higher maximum temperatures at the time point of first positive BAL, than non-reactivated patients at a comparable time point (CMV: p = 0.044, HSV: p = 0.020). However, Sequential Organ Failure Assessment (SOFA) scores and routine clinical chemistry were not significantly different between the groups on the positive BAL sampling moment, nor on the day of ICU admission. HSV reactivations were more common in patients with diabetes mellitus (p = 0.003). Moreover, patients with pulmonary HSV reactivation exhibited a higher prevalence of positive BAL bacterial cultures (p = 0.020), along with prolonged ICU stays (p < 0.001) and mechanical ventilation (p < 0.001), and lower number of ventilator-free days on day 28 (p < 0.001) compared to patients without HSV reactivation (Table 1).
Viral loads and mortality
Joint model analysis evaluated the association between pulmonary reactivations and mortality (Table 2). When considering BAL samples taken before or without the initiation of antiviral treatment, alveolar CMV viral loads increased over time (β: 0.04 log10 copies/day, 95% CI 0.03–0.05), but this rise was not associated with higher mortality (hazard ratio [HR] 0.62, 95% CI 0.45–0.87). In contrast, HSV viral loads, in the absence of antiviral treatment, also increased during mechanical ventilation (β: 0.09 log10 copies/day, 95% CI 0.05–0.12) and were significantly associated with increased 90-day mortality (HR 1.24, 95% CI 1.04–1.47) when considering BAL samples taken before or without the initiation of antiviral treatment. Considering that only HSV reactivations were common and its viral loads were associated with higher mortality, subsequent analyses focused exclusively on HSV.
To evaluate the influence of antiviral treatment, we further analyzed only patients who received anti-HSV treatment (57/172; 33%), including data from its initiation. Within this subset, HSV viral loads significantly decreased over time after anti-HSV treatment initiation (β: − 0.04 log10 copies/day, 95% CI − 0.08 to − 0.01). The association between HSV viral loads and 90-day mortality was no longer statistically significant, although the confidence intervals were wide (HR 1.14, 95% CI 0.80–1.61).
Re-evaluation of clinically relevant cutoff
As our predefined, clinically used viral load cutoff of 104 c/ml was primarily based on expert opinion, we re-evaluated this cutoff compared to alternative cutoffs for viral load. Based on the maximum probability of transitioning from a non-reactivated state to HSV-reactivation, and risk of mortality, we assessed which viral load cutoff could be considered rational. Lower cutoffs did not result in a stronger association with mortality, and transition probability remained similar. Higher cutoffs reduced the probability of transitioning to reactivation, yet only worsened the predictive accuracy for mortality (Fig. S7).
Using the cutoff of > 104 c/ml, we evaluated the risk for mortality in patients with and without reactivation. Using multistate modeling, Figs. 2 and S7 show that patients with a clinically relevant pulmonary HSV reactivation had a higher likelihood of dying than patients without reactivation.
Aalen–Johansen plot for probability of state membership in the HSV cohort. A This figure illustrates the transition probabilities within a multistate modeling framework [20, 33]. All patients initially start in state 1 and could transition to the end states 3 (extubated) and 4 (deceased), either or not passing through the event state 2 (i.e., having a clinically relevant reactivation for CMV or HSV). B Aalen–Johansen plot illustrating the probability of state membership, with data presented up to day 90 and the last data point provided at 83 days. Left: probability of state membership at baseline; all patients start non-reactivated (blue). Blue = intubated, without clinically relevant HSV reactivation; orange = intubated, with clinically relevant HSV reactivation; green = no clinically relevant HSV-reactivation, extubated; red = no clinically relevant HSV reactivation, deceased. Right: probability of state membership at HSV reactivation; all patients start reactivated (orange). Orange = intubated, with clinically relevant HSV reactivation; green = HSV reactivated (> 104 c/ml), extubated; red = HSV reactivated (> 104 c/ml), deceased. Y-axis: probability of state membership. X-axis: days since intubation. Clinically relevant reactivation refers to a viral load of > 104 c/ml in the BAL fluid. CMV cytomegalovirus, HSV herpes simplex virus, BAL bronchoalveolar lavage
Alveolar inflammation
To mitigate potential sampling, selection and immortal time biases, we conducted three different analyses to evaluate the association between alveolar inflammation and clinically relevant HSV reactivation. These included cross-sectional, longitudinal pre- and post-reactivation, and longitudinal pre-and post-treatment analyses. The online supplement provides details on all analyses, including a flowchart displaying the patient selection and matching process for each analysis (Figs. S8, 9) and matching effectiveness plots (Figs. S10, S11, S13).
Cross-sectional comparison revealed no differences in alveolar inflammatory response biomarkers, also after matching (Tables S3-5). In a matched longitudinal analysis pre- and post-reactivation (n = 20), HSV-reactivated patients showed a significant increase in biomarker concentrations compared to the non-reactivated patients for the following biomarkers: granulocyte colony stimulating factor (G-CSF), interleukin (IL)-6, IL-1β, C-X-C motif chemokine ligand 2 (CXCL2), tumor necrosis factor (TNF), CD40 Ligand, C-C motif ligand 11 (CCL11), IL-2, IL-12p70, IL-33, and C-X3-C motif ligand 1 (CX3CL1) (Table S1 for biomarker abbreviations; Fig. 3A, Table S6-left column). Figure S12 illustrates the limited role of CMV loads in this analysis, indicated by the relatively stable CMV loads following HSV reactivation, compared to the expected increase in HSV loads. In another matched analysis pre- and post-treatment (n = 22), chemokine C–C motif ligand 18 (CCL18) significantly decreased for HSV-reactivated patients who were treated, with no further increase observed in previously elevated biomarkers after reactivation (Fig. 3B, Table S6-right column). Similar trends were absent in plasma (Fig. S14).
Alveolar biomarker dynamics for HSV-reactivated and non-reactivated patients, pre- and post-reactivation and pre-and post-treatment initiation. This figure displays the longitudinal profiles of four selected biologically relevant biomarkers that show a significant difference in reactivation biomarker dynamics, compared to the non-reactivated group. HSV reactivation refers to a clinically relevant reactivation (viral load of > 104 c/ml in the BAL fluid). A Sampling time points before and after reactivation, for HSV-reactivated patients (n = 10), and matched controls without HSV reactivation (n = 10). B Sampling time points before and after treatment, for HSV-reactivated patients who received antiviral treatment (n = 11), and matched controls without HSV reactivation who did not receive antiviral treatment (n = 11). Patient selection is shown in Fig. S9. Orange and blue lines indicate the trajectories of alveolar biomarker concentrations for HSV-reactivated versus non-HSV-reactivated patients, respectively. Dotted line indicates the 95% confidence intervals. Linear mixed-effects models were used to provide beta and 95% confidence intervals. G-CSF granulocyte colony stimulating factor, IL interleukin, TNF tumor necrosis factor, CI confidence interval
Discussion
The key findings of this study are that pulmonary HSV reactivations are common in mechanically ventilated COVID-19 patients, whereas CMV reactivations were relatively rare. HSV viral load dynamics prior to or without antiviral treatment were associated with mortality. We also found that alveolar hyper-inflammation occurred in patients who experienced pulmonary HSV reactivation, relative to patients without HSV reactivation, and stabilized after the start of antiviral treatment.
On the surface, the here reported incidence of reactivation of HSV and CMV in the lower respiratory tract seems lower than previously reported numbers, ranging between 16 and 35% for CMV [5, 6, 10, 35, 36], and 19–64% for HSV [5, 10, 11, 35, 37]. Incidence is influenced by variation in patient selection, sampling of upper or lower respiratory tract, analytical techniques for detecting the virus, and the definition of what constitutes a clinically relevant reactivation [36]. In most other studies, viral reactivation was defined based on PCR positivity without a specific threshold for the diagnosis, while we used > 104 c/ml for the viral load to define clinically relevant viral reactivation. Other studies that reported CMV and HSV reactivations in critically ill COVID-19 patients using a more conservative load threshold yielded numbers reminiscent of ours [38, 39]. It has been postulated that viral reactivations are more frequent in patients with non-resolving ARDS caused by COVID-19, and that this could be due to the immunological background including the immunosuppressive character of the virus [40]. However, the limited implementation of regular and protocolized bronchoscopies prior to the pandemic may have contributed to an underestimation of the previously reported numbers. In fact, the incidence of PCR positivity in our study was quite similar to those of the COVID-19 ARDS population [2, 41, 42]. Based on the collective findings of previous literature and our own findings, it can be stated that HSV reactivations are more frequent than CMV reactivations, that HSV reactivations occur earlier compared to CMV reactivations and that there is no meaningful between-virus interplay [35, 39, 41, 43]. However, clinical studies in critically ill patients predominantly focused on pulmonary CMV rather than HSV reactivations. Therefore, we advocate for an increased emphasis on HSV reactivations.
We identified an association between higher pre-treatment HSV loads in BAL and increased mortality. Inconsistency in results from existing literature could be the result of evaluating non-quantitative results, non-standardized estimations of viral loads and varying cutoffs, as well as the insufficient addressing of time-dependent biases [5, 15, 18,19,20]. In contrast, our study endeavors to incorporate these aspects to the best of our ability and supports the notion that HSV reactivation may impede resolution of ARDS and therefore recovery of the patient. However, concerning CMV, the late occurrence of reactivation may introduce immortal time bias, impacting the interpretation of our findings [20]. This, together with the unexpected protective effect contradicting our initial hypotheses, leads us to cautiously suggest the association is not harmful, based on our analysis. One key challenge in making practical strides in studying viral reactivations remains the identification of a viral load threshold that identifies such ‘clinically relevant’ reactivation, as many patients experience some viral replication during critical illness. A lung biopsy might offer insights into defining and classifying such reactivation, yet its feasibility in ICU patients is constrained. Consequently, we utilized the best available analytical techniques to re-evaluate the clinical cutoff. The predefined arbitrary clinical cutoff of 104 c/ml seemed rational in our dataset, with lower cutoffs not exhibiting a stronger association with mortality yet showing stable probabilities of reactivation, and higher cutoffs showing lower probabilities of reactivation but at the cost of diminishing predictive accuracy for mortality. Yet, caution is warranted given the observational nature of our study and assay variability between laboratories, limiting causal inferences.
The results of this study, therefore, highlight the need for a targeted intervention study on antiviral treatment for pulmonary reactivation of HSV in patients with non-resolving ARDS. This question was not addressed by the sole randomized controlled trial (RCT) focusing on oropharyngeal sampling—which may not be representative for viral reactivation in the lower respiratory tract—and did not demonstrate a ventilator-free day benefit with pre-emptive antiviral treatment [22]. Another RCT indicated a potential decrease in HSV reactivation frequency after treatment with prophylactic aciclovir in ARDS patients, yet outcomes did not improve [44]. This study, however, conducted in 1987, relied on virus culture rather than PCR and included seronegative patients, making quantitative comparisons challenging. A recent meta-analysis suggested a benefit of treatment of viral reactivations in the respiratory tract [23]. The present study provides reasonable clinical and microbiological data to inform such an intervention trial.
In the course of HSV reactivation, we observed an elevated trajectory of the concentrations of IL-6, TNF, and IL-1β, along with other inflammatory cytokines in the alveolar space, compared to patients with non-resolving ARDS without HSV reactivation. This aligns with previous literature linking herpesviruses to systemic inflammatory responses, including elevated IL-6, TNF, and IL-1β [45, 46]. Although the inflammation did not escalate further after treatment, we did not measure a substantial reduction in inflammatory biomarkers, except for CCL18. The lack of significant reduction in biomarkers is consistent with a few trials conducted in critically ill patients with CMV infection, albeit with systemic measurements [47, 48]. Valuable data specifically encompassing alveolar biomarkers related to HSV reactivation are notably lacking, with existing studies often focusing on alternative contexts such as blood biomarkers or other viral infections like CMV. It is important to highlight that systemic (plasma) cytokines might not necessarily reflect local (alveolar) inflammation [49, 50]. Indeed, our analysis of plasma biomarkers did not reveal comparable trends, underscoring the need to assess the alveolar inflammatory response, given the pulmonary manifestation of the virus in this context.
To our knowledge, this is the most extensive cohort study with longitudinal pulmonary viral load data for CMV and HSV in critically ill patients prone to viral reactivations. However, our findings’ generalizability may be limited due to several aspects—first, due to the focus on COVID-19 patients. Further research is needed to ascertain if similar associations extend to other causes of ARDS, as we propose these findings have relevance beyond the COVID-19-related ARDS population. Second, the limited number of patients for matching in each analysis may impact the generalizability of our results due to potential information loss. Third, our single-center study design could further affect generalizability due to potential variance in clinical protocols, including the systematic use of antibiotics under the local Selective Digestive Decontamination protocol—which is relatively less utilized globally—and the BAL procedure. However, this design facilitated rigorous standardization of procedures, thereby minimizing within-study variability and enhancing the reliability of our results. Moreover, given ethical considerations, the design of such a study inherently adopts a clinical practice-based approach. Notable limitations arising from this include potential selection bias, variability in sampling dilution, a lack of subsequent sampling in patients who either decease or clinically improve, and the absence of an untreated comparison group. Furthermore, it is essential to recognize the potential for residual confounding in time-series analyses, given the inherent complexities of distinguishing confounders from colliders and mediators [51]. Despite the aforementioned limitations, our study employs a robust methodology, including protocolized indications for bronchoscopy to limit selection bias, standardized volume instillation during BAL procedures to limit sampling dilution, and advanced statistical approaches like joint modeling and multistate modeling to limit time-dependent bias and informative censoring influences. These measures contribute to mitigating methodological issues and ensure a reliable understanding of the role of viral reactivations in critically ill patients, offering valuable insights compared to existing studies.
Conclusion
Pulmonary HSV reactivations occur frequently in patients with non-resolving ARDS due to COVID-19, with the probability peaking around 2 weeks after intubation. HSV viral load dynamics prior to or without antiviral treatment were associated with mortality. Alveolar inflammation increased after HSV reactivation and stabilized upon antiviral treatment. Altogether, these findings suggest that pulmonary HSV reactivation in mechanically ventilated COVID-19 patients may limit resolution of inflammation and injury. We suggest that quantification of pulmonary HSV reactivation using alveolar sampling is appropriate in patients with non-resolving ARDS within this specific context. Moreover, we speculate that antiviral treatment could potentially enhance clinical outcomes, although further evidence to support this and to confirm the ideal cutoff is required.
Data availability
De-identified participant data with data dictionary can be shared after approval of a proposal with a signed data access agreement and always in collaboration with the study group.
References
Ong DSY, Bonten MJM, Spitoni C, Verduyn Lunel FM, Frencken JF, Horn J et al (2017) Epidemiology of multiple herpes viremia in previously immunocompetent patients with septic shock. Clin Infect Dis 64(9):1204–1210. https://doi.org/10.1093/cid/cix120
Shafiee A, Teymouri Athar MM, Amini MJ, Hajishah H, Siahvoshi S, Jalali M et al (2023) Reactivation of herpesviruses during COVID-19: a systematic review and meta-analysis. Rev Med Virol. https://doi.org/10.1002/rmv.2437
Walton AH, Muenzer JT, Rasche D, Boomer JS, Sato B, Brownstein BH et al (2014) Reactivation of multiple viruses in patients with sepsis. PLoS One 9(2):e98819. https://doi.org/10.1371/journal.pone.0098819
Osawa R, Singh N (2009) Cytomegalovirus infection in critically ill patients: a systematic review. Crit Care 13(3):R68. https://doi.org/10.1186/cc7875
Coisel Y, Bousbia S, Forel JM, Hraiech S, Lascola B, Roch A et al (2012) Cytomegalovirus and herpes simplex virus effect on the prognosis of mechanically ventilated patients suspected to have ventilator-associated pneumonia. PLoS One 7(12):e51340. https://doi.org/10.1371/journal.pone.0051340
Chiche L, Forel JM, Roch A, Guervilly C, Pauly V, Allardet-Servent J et al (2009) Active cytomegalovirus infection is common in mechanically ventilated medical intensive care unit patients. Crit Care Med 37(6):1850–1857. https://doi.org/10.1097/CCM.0b013e31819ffea6
Lepiller Q, Sueur C, Solis M, Barth H, Glady L, Lefebvre F et al (2015) Clinical relevance of herpes simplex virus viremia in Intensive Care Unit patients. J Infect 71(1):93–100. https://doi.org/10.1016/j.jinf.2015.02.013
Jaber S, Chanques G, Borry J, Souche B, Verdier R, Perrigault PF, Eledjam JJ (2005) Cytomegalovirus infection in critically ill patients: associated factors and consequences. Chest 127(1):233–241. https://doi.org/10.1378/chest.127.1.233
Ong DSY, Spitoni C, Klein Klouwenberg PMC, Verduyn Lunel FM, Frencken JF, Schultz MJ et al (2016) Cytomegalovirus reactivation and mortality in patients with acute respiratory distress syndrome. Intensive Care Med 42(3):333–341. https://doi.org/10.1007/s00134-015-4071-z
Hraiech S, Bonnardel E, Guervilly C, Fabre C, Loundou A, Forel JM et al (2019) Herpes simplex virus and cytomegalovirus reactivation among severe ARDS patients under veno-venous ECMO. Ann Intensive Care 9(1):142. https://doi.org/10.1186/s13613-019-0616-6
Luyt CE, Combes A, Deback C, Aubriot-Lorton MH, Nieszkowska A, Trouillet JL et al (2007) Herpes simplex virus lung infection in patients undergoing prolonged mechanical ventilation. Am J Respir Crit Care Med 175(9):935–942. https://doi.org/10.1164/rccm.200609-1322OC
De Vos N, Van Hoovels L, Vankeerberghen A, Van Vaerenbergh K, Boel A, Demeyer I et al (2009) Monitoring of herpes simplex virus in the lower respiratory tract of critically ill patients using real-time PCR: a prospective study. Clin Microbiol Infect 15(4):358–363. https://doi.org/10.1111/j.1469-0691.2009.02704.x
Le Balc’h P, Pinceaux K, Pronier C, Seguin P, Tadie JM, Reizine F (2020) Herpes simplex virus and cytomegalovirus reactivations among severe COVID-19 patients. Crit Care 24(1):530. https://doi.org/10.1186/s13054-020-03252-3
Katz J, Yue S, Xue W (2022) Herpes simplex and herpes zoster viruses in COVID-19 patients. Ir J Med Sci 191(3):1093–1097. https://doi.org/10.1007/s11845-021-02714-z
Meyer A, Buetti N, Houhou-Fidouh N, Patrier J, Abdel-Nabey M, Jaquet P et al (2021) HSV-1 reactivation is associated with an increased risk of mortality and pneumonia in critically ill COVID-19 patients. Crit Care 25(1):417. https://doi.org/10.1186/s13054-021-03843-8
Kalil AC, Florescu DF (2009) Prevalence and mortality associated with cytomegalovirus infection in nonimmunosuppressed patients in the intensive care unit. Crit Care Med 37(8):2350–2358. https://doi.org/10.1097/CCM.0b013e3181a3aa43
Lachance P, Chen J, Featherstone R, Sligl WI (2017) Association between cytomegalovirus reactivation and clinical outcomes in immunocompetent critically ill patients: a systematic review and meta-analysis. Open Forum Infect Dis 4(2):ofx029. https://doi.org/10.1093/ofid/ofx029
Linssen CF, Jacobs JA, Stelma FF, van Mook WN, Terporten P, Vink C et al (2008) Herpes simplex virus load in bronchoalveolar lavage fluid is related to poor outcome in critically ill patients. Intensive Care Med 34(12):2202–2209. https://doi.org/10.1007/s00134-008-1231-4
Gangneux JP, Dannaoui E, Fekkar A, Luyt CE, Botterel F, De Prost N et al (2022) Fungal infections in mechanically ventilated patients with COVID-19 during the first wave: the French multicentre MYCOVID study. Lancet Respir Med 10(2):180–190. https://doi.org/10.1016/S2213-2600(21)00442-2
de Brabander J, Boers LS, Kullberg RFJ, Duitman JW, Bos LDJ (2022) Time-dependent bias when analysing COVID-19-associated pulmonary aspergillosis. Lancet Respir Med 10(3):e25–e26. https://doi.org/10.1016/S2213-2600(21)00582-8
Gangneux JP, Morcet J, Laviolle B, Dannaoui E, Timsit JF, Ruckly S et al (2022) Time-dependent bias when analysing COVID-19-associated pulmonary aspergillosis-Authors’ reply. Lancet Respir Med 10(3):e27. https://doi.org/10.1016/S2213-2600(22)00048-0
Luyt CE, Forel JM, Hajage D, Jaber S, Cayot-Constantin S, Rimmele T et al (2020) Acyclovir for mechanically ventilated patients with herpes simplex virus oropharyngeal reactivation: a randomized clinical trial. JAMA Intern Med 180(2):263–272. https://doi.org/10.1001/jamainternmed.2019.5713
Hagel S, Scherag A, Schuierer L, Hoffmann R, Luyt CE, Pletz MW et al (2020) Effect of antiviral therapy on the outcomes of mechanically ventilated patients with herpes simplex virus detected in the respiratory tract: a systematic review and meta-analysis. Crit Care 24(1):584. https://doi.org/10.1186/s13054-020-03296-5
Papazian L, Calfee CS, Chiumello D, Luyt CE, Meyer NJ, Sekiguchi H et al (2016) Diagnostic workup for ARDS patients. Intensive Care Med 42(5):674–685. https://doi.org/10.1007/s00134-016-4324-5
de Brabander J, Boers LS, Kullberg RFJ, Zhang S, Nossent EJ, Heunks LMA et al (2023) Persistent alveolar inflammatory response in critically ill patients with COVID-19 is associated with mortality. Thorax 78(9):912–921. https://doi.org/10.1136/thorax-2023-219989
Kullberg RFJ, de Brabander J, Boers LS, Biemond JJ, Nossent EJ, Heunks LMA et al (2022) Lung microbiota of critically ill patients with COVID-19 are associated with nonresolving acute respiratory distress syndrome. Am J Respir Crit Care Med 206(7):846–856. https://doi.org/10.1164/rccm.202202-0274OC
Force ADT, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E et al (2012) Acute respiratory distress syndrome: the Berlin definition. JAMA 307(23):2526–2533. https://doi.org/10.1001/jama.2012.5669
Lodding IP, Schultz HH, Jensen JU, Kirkby N, Perch M, Andersen C et al (2018) Cytomegalovirus viral load in bronchoalveolar lavage to diagnose lung transplant associated CMV pneumonia. Transplantation 102(2):326–332. https://doi.org/10.1097/TP.0000000000001927
Beam E, Germer JJ, Lahr B, Yao JDC, Limper AH, Binnicker MJ, Razonable RR (2018) Cytomegalovirus (CMV) DNA quantification in bronchoalveolar lavage fluid of immunocompromised patients with CMV pneumonia. Clin Transplant. https://doi.org/10.1111/ctr.13149
Lee HY, Rhee CK, Choi JY, Lee HY, Lee JW, Lee DG (2017) Diagnosis of cytomegalovirus pneumonia by quantitative polymerase chain reaction using bronchial washing fluid from patients with hematologic malignancies. Oncotarget 8(24):39736–39745. https://doi.org/10.18632/oncotarget.14504
Iglesias L, Perera MM, Torres-Minana L, Pena-Lopez MJ (2017) CMV viral load in bronchoalveolar lavage for diagnosis of pneumonia in allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant 52(6):895–897. https://doi.org/10.1038/bmt.2017.11
Boeckh M, Stevens-Ayers T, Travi G, Huang ML, Cheng GS, Xie H et al (2017) Cytomegalovirus (CMV) DNA quantitation in bronchoalveolar lavage fluid from hematopoietic stem cell transplant recipients with CMV pneumonia. J Infect Dis 215(10):1514–1522. https://doi.org/10.1093/infdis/jix048
Ferguson N, Datta S, Brock G (2012) msSurv: an R package for nonparametric estimation of multistate models. J Stat Softw 50(14):1–24
Rizopoulos DJM, An R (2010) Package for the joint modelling of longitudinal and time-to-event data. J Stat Softw 35(9):1–33
Heininger A, Haeberle H, Fischer I, Beck R, Riessen R, Rohde F et al (2011) Cytomegalovirus reactivation and associated outcome of critically ill patients with severe sepsis. Crit Care 15(2):R77. https://doi.org/10.1186/cc10069
Papazian L, Hraiech S, Lehingue S, Roch A, Chiche L, Wiramus S, Forel JM (2016) Cytomegalovirus reactivation in ICU patients. Intensive Care Med 42(1):28–37. https://doi.org/10.1007/s00134-015-4066-9
Bruynseels P, Jorens PG, Demey HE, Goossens H, Pattyn SR, Elseviers MM et al (2003) Herpes simplex virus in the respiratory tract of critical care patients: a prospective study. Lancet 362(9395):1536–1541. https://doi.org/10.1016/S0140-6736(03)14740-X
Giacobbe DR, Di Bella S, Dettori S, Brucci G, Zerbato V, Pol R et al (2022) Reactivation of herpes simplex virus type 1 (HSV-1) detected on bronchoalveolar lavage fluid (BALF) samples in critically ill COVID-19 patients undergoing invasive mechanical ventilation: preliminary results from two Italian centers. Microorganisms. https://doi.org/10.3390/microorganisms10020362
Franceschini E, Cozzi-Lepri A, Santoro A, Bacca E, Lancellotti G, Menozzi M et al (2021) Herpes simplex virus re-activation in patients with SARS-CoV-2 pneumonia: a prospective, Observational Study. Microorganisms. https://doi.org/10.3390/microorganisms9091896
Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y et al (2020) Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 395(10223):507–513. https://doi.org/10.1016/S0140-6736(20)30211-7
Luyt CE, Burrel S, Mokrani D, de Chambrun MP, Luyt D, Chommeloux J et al (2022) Herpesviridae lung reactivation and infection in patients with severe COVID-19 or influenza virus pneumonia: a comparative study. Ann Intensive Care 12(1):87. https://doi.org/10.1186/s13613-022-01062-0
Giacobbe DR, Di Bella S, Lovecchio A, Ball L, De Maria A, Vena A et al (2022) Herpes simplex virus 1 (HSV-1) reactivation in critically ill COVID-19 patients: a brief narrative review. Infect Dis Ther 11(5):1779–1791. https://doi.org/10.1007/s40121-022-00674-0
Fuest KE, Erber J, Berg-Johnson W, Heim M, Hoffmann D, Kapfer B et al (2022) Risk factors for Herpes simplex virus (HSV) and Cytomegalovirus (CMV) infections in critically-ill COVID-19 patients. Multidiscip Respir Med 17(1):815. https://doi.org/10.4081/mrm.2022.815
Tuxen DV, Wilson JW, Cade JF (1987) Prevention of lower respiratory herpes simplex virus infection with acyclovir in patients with the adult respiratory distress syndrome. Am Rev Respir Dis 136(2):402–405. https://doi.org/10.1164/ajrccm/136.2.402
Mallet F, Diouf L, Meunier B, Perret M, Reynier F, Leissner P et al (2021) Herpes DNAemia and TTV viraemia in intensive care unit critically ill patients: a single-centre prospective longitudinal study. Front Immunol 12:698808. https://doi.org/10.3389/fimmu.2021.698808
Imlay H, Limaye AP (2020) Current understanding of cytomegalovirus reactivation in critical illness. J Infect Dis 221(Suppl 1):S94–S102. https://doi.org/10.1093/infdis/jiz638
Cowley NJ, Owen A, Shiels SC, Millar J, Woolley R, Ives N et al (2017) Safety and efficacy of antiviral therapy for prevention of cytomegalovirus reactivation in immunocompetent critically ill patients: a randomized clinical trial. JAMA Intern Med 177(6):774–783. https://doi.org/10.1001/jamainternmed.2017.0895
Limaye AP, Stapleton RD, Peng L, Gunn SR, Kimball LE, Hyzy R et al (2017) Effect of ganciclovir on IL-6 levels among cytomegalovirus-seropositive adults with critical illness: a randomized clinical trial. JAMA 318(8):731–740. https://doi.org/10.1001/jama.2017.10569
de Brabander J, Boers LS, Kullberg RFJ, Zhang S, Nossent EJ, Heunks LMA et al (2023) Persistent alveolar inflammatory response in critically ill patients with COVID-19 is associated with mortality. Thorax. https://doi.org/10.1136/thorax-2023-219989
Morrell ED, Radella F 2nd, Manicone AM, Mikacenic C, Stapleton RD, Gharib SA, Wurfel MM (2018) Peripheral and alveolar cell transcriptional programs are distinct in acute respiratory distress syndrome. Am J Respir Crit Care Med 197(4):528–532. https://doi.org/10.1164/rccm.201703-0614LE
Hernán; MA, Robins JM. Causal Inference—What if. 2020.
Sinha P, Calfee CS, Beitler JR, Soni N, Ho K, Matthay MA, Kallet RH (2019) Physiologic analysis and clinical performance of the ventilatory ratio in acute respiratory distress syndrome. Am J Respir Crit Care Med 199(3):333–341. https://doi.org/10.1164/rccm.201804-0692OC
Yehya N, Harhay MO, Curley MAQ, Schoenfeld DA, Reeder RW (2019) Reappraisal of ventilator-free days in critical care research. Am J Respir Crit Care Med 200(7):828–836. https://doi.org/10.1164/rccm.201810-2050CP
Acknowledgements
The members are on behalf of the OPPORTUNE consortium, the Amsterdam UMC COVID study group and the ArtDECO consortium. The OPPORTUNE consortium: Paul E. Verweij, Simone J.C.F.M. Moorlag, Frank L. van de Veerdonk (Radboud University Medical Center, Nijmegen, The Netherlands); Lieuwe D.J. Bos, Leonoor S. Boers, Janke Schinkel, Frank van Someren Grevé (Amsterdam UMC location University of Amsterdam, Amsterdam, The Netherlands); Jeroen J.A. van Kampen (Erasmus MC, Rotterdam, The Netherlands); Joost Wauters, Katrien Lagrou, Simon Feys, Jannes Heylen (KU Leuven, Leuven, Belgium). The Amsterdam UMC COVID study group (Amsterdam UMC location University of Amsterdam and location Vrije Universiteit Amsterdam, Amsterdam, the Netherlands): Michiel van Agtmael, Anne Geke Algera, Brent Appelman, Floor van Baarle, Diederik van de Beek, Martijn Beudel, Harm Jan Bogaard, Lieuwe Bos, Michela Botta, Justin de Brabander, Godelieve de Bree, Matthijs C. Brouwer, Sanne de Bruin, Marianna Bugiani, Esther Bulle, David T.P. Buis, Osoul Chouchane, Alex Cloherty, Mirjam Dijkstra, Dave A. Dongelmans, Romein W.G. Dujardin, Paul Elbers, Lucas Fleuren, Suzanne Geerlings, Theo Geijtenbeek, Armand Girbes, Bram Goorhuis, Martin P. Grobusch, Laura Hagens, Jorg Hamann, Vanessa Harris, Robert Hemke, Sabine M. Hermans, Leo Heunks, Markus Hollmann, Janneke Horn, Joppe W. Hovius, Menno D. de Jong, Rutger Koning, Endry H.T. Lim, Niels van Mourik, Jeaninne Nellen, Esther J. Nossent, Frederique Paulus, Edgar Peters, Dan A.I. Piña-Fuentes, Tom van der Poll, Bennedikt Preckel, Jorinde Raasveld, Tom Reijnders, Maurits C.F.J. de Rotte, Michiel Schinkel, Marcus J. Schultz, Femke A.P. Schrauwen, Alex Schuurman, Jaap Schuurmans, Kim Sigaloff, Marleen A. Slim, Patrick Smeele, Marry Smit, Cornelis S. Stijnis, Willemke Stilma, Charlotte Teunissen, Patrick Thoral, Anissa M. Tsonas, Pieter R. Tuinman, Marc van der Valk, Denise Veelo, Alexander P.J. Vlaar, Carolien Volleman, Heder de Vries, Lonneke A. Vught, Michèle van Vugt, W. Joost Wiersinga, Dorien Wouters, A. H. (Koos) Zwinderman, Matthijs C. Brouwer. The ArtDECO consortium (Amsterdam UMC location University of Amsterdam and location Vrije Universiteit Amsterdam, Amsterdam, the Netherlands): E.J. Nossent, J.W. Duitman, A. Saris, H. de Vries, L.J. Meijboom, L.D.J. Bos, S.G. Blok, A.R. Schuurman, T.D.Y. Reijnders, J.J. Garcia Vallejo, H. Bontkes, A.P.J. Vlaar, W.J. Wiersinga, R. Lutter, T. van der Poll, H.J. Bogaard, L. Heunks, S. Zhang, R.F.J. Kullberg, J. de Brabander, and L.S. Boers.
Funding
This research was funded by an Amsterdam UMC fellowship to LDJB in 2020 (no award/grant number). Additional support was received from ZonMw under project number 10430102110011.
Author information
Authors and Affiliations
Consortia
Contributions
LSB, FvSG, JMvH, JdB, MdJ, TvdP, JD, JS, and LDJB contributed to the study concept and design. LSB, FvSG, JMvH, JdB, TZ, HvW, and MC were involved in the data collection. LSB and LDJB had access to the raw data, did the analyses, and drafted the manuscript. All authors commented on previous versions of the manuscript. All authors approved the final manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Consent
Informed consent was deferred until ICU discharge. Patients were prospectively included if they provided written informed consent or if the patient or relatives did not use the opt-out form.
Ethics approval
The study procedure was approved by the Review Committee of the Amsterdam UMC Biobank. The ethical boards of the participating hospitals approved the collection of data for the study purposes, ID AUMC 2020_065. The study was performed in accordance with the declaration of Helsinki and adheres to Dutch regulations.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Members of the OPPORTUNE consortium, the Amsterdam UMC COVID study, and the ArtDECO consortium are listed in Acknowledgements.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Boers, L.S., van Someren Gréve, F., van Hattem, J.M. et al. Pulmonary herpes simplex virus and cytomegalovirus in patients with acute respiratory distress syndrome related to COVID-19. Intensive Care Med (2024). https://doi.org/10.1007/s00134-024-07529-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00134-024-07529-x