Abstract
Despite significant advancements in critical care medicine, limited attention has been given to sex and gender disparities in management and outcomes of patients admitted to the intensive care unit (ICU). While “sex” pertains to biological and physiological characteristics, such as reproductive organs, chromosomes and sex hormones, “gender” refers more to sociocultural roles and human behavior. Unfortunately, data on gender-related topics in the ICU are lacking. Consequently, data on sex and gender-related differences in admission to the ICU, clinical course, length of stay, mortality, and post-ICU burdens, are often inconsistent. Moreover, when examining specific diagnoses in the ICU, variations can be observed in epidemiology, pathophysiology, presentation, severity, and treatment response due to the distinct impact of sex hormones on the immune and cardiovascular systems. In this narrative review, we highlight the influence of sex and gender on the clinical course, management, and outcomes of the most encountered intensive care conditions, in addition to the potential co-existence of unconscious biases which may also impact critical illness. Diagnoses with a known sex predilection will be discussed within the context of underlying sex differences in physiology, anatomy, and pharmacology with the goal of identifying areas where clinical improvement is needed. To optimize patient care and outcomes, it is crucial to comprehend and address sex and gender differences in the ICU setting and personalize management accordingly to ensure equitable, patient-centered care. Future research should focus on elucidating the underlying mechanisms driving sex and gender disparities, as well as exploring targeted interventions to mitigate these disparities and improve outcomes for all critically ill patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
While there is a growing recognition of the influence of sex and gender differences on disease presentation in intensive care unit, current evidence points towards ongoing discrepancies in the delivery of intensive care treatment and clinical outcomes between sexes. In addition, the heterogeneity of critically ill patients presents a formidable challenge, underscoring the necessity for future investigations to incorporate a standardized approach toward integrating sex and gender considerations. |
Introduction
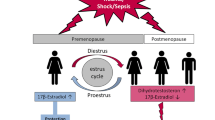
Over the last years, sex and gender differences in diagnosis, clinical care, and outcomes have become an increasing concern in medicine and critical care research [1, 2]. Inequalities in medical care were first described via several landmark publications in the early 1990s which revealed that women often received inequitable treatment compared to men, often resulting in worse outcomes. Bernadine Healy famously termed this phenomenon the “Yentl Syndrome” after the heroine of Isaac Bashevis Singer’s story, in which a young Jewish woman pretended to be a man, aiming to receive equal education as men [3]. Historically, research has widely disregarded sex differences, with women being underrepresented in clinical trials and experimental research, leading to a lack of understanding of their specific healthcare needs and differences in outcomes [4]. Addressing sex differences in critical care medicine is particularly challenging given the heterogeneity of patients and diagnoses in the intensive care unit (ICU) [1, 5]. Biological factors (sex) and sociocultural factors (gender) have been increasingly investigated and both have been held responsible for sex disparities in disease manifestation, provision of medical care, and outcomes [6, 7]. However, while biological sex comprises differences in hormonal status, sex chromosomes and physiology, gender refers to socially constructed norms, behaviors, expressions, and identities of women and men (Fig. 1). Regarding the specific nature of sex, many anatomical and physiological characteristics partly explain some of these differences (Fig. 2 and Table 1).
Over the last decade, advances have been made to better understand the complex interplay of sex and gender in diseases, to guide clinicians’ decision-making when incorporating sex and gender into patient-centered medicine, and to support a more systematic approach in the reporting of sex and gender across disciplines and in research by releasing the SAGER (Sex and Gender Equity in Research) guidelines [8, 9] (Fig. 3) and with the development of gender scores to make gender “measurable” [10, 11]. Current evidence indicates that gender-related variables allow for the characterization of individuals beyond biological sex and its impact on clinical outcomes. However, limitations remain given the multidimensional aspects and complexity of gender and the co-existence of unconscious biases.
In this narrative review, based on a comprehensive, critical, and objective analysis of the current literature, our aim is to summarize the current evidence of sex and gender differences in the provision of critical care, including treatment and outcomes with a focus on sex disparities in the most common critical illnesses.
Influence of sex and gender on ICU admission
Intensive care unit admission is provided less frequently to women compared to men [12] within most diagnostic groups. This flies in the face of data supporting that women outlive men everywhere in the world, and accordingly, a higher percentage of women should be represented particularly in the aging ICU population [13]. While a male predominance in the overall ICU population is seen worldwide, an exemption with a female preponderance is observed in metabolic disorders, deliberate self-poisoning, and non-traumatic subarachnoid hemorrhage [14, 15]. Besides, among the main comorbidities, there appears to be some differences between men and women at ICU admission. For instance, cardiovascular morbidity (dyslipidemia, coronary artery disease), chronic obstructive pulmonary disease (COPD), and acquired immunodeficiency syndrome (AIDS), tend to be less prevalent in women [5, 16].
There is an ongoing debate regarding the sex imbalance in the provision of intensive care. Most studies, including large meta-analyses, have demonstrated that the severity of illness on ICU admission is similar [16, 22] or higher in women [5, 23]. The latter was confirmed in a recent meta-analysis including 505,138 patients across all ICU admission diagnoses [5]. Similarly, Todorov et al. demonstrated in a nationwide database of 450,948 cardiovascular or neurovascular patients that the likelihood of women being admitted to ICU was significantly lower compared to men, despite higher estimates of illness severity [23]. Conversely, some studies report an overall lower severity of the disease in women, probably related to lower average age than men at admission to ICU [24, 25]. However, sex predilection toward certain diagnoses may also impact illness severity and outcomes: higher illness severity and mortality for women have been reported in diagnostic groups with a lower percentage of women and points toward the fact that sex distributions within diseases, diagnoses, and age groups should be considered [25].
Notably, illness assessment tools such as the Simplified Acute Physiology Score (SAPS) and Acute Physiology and Chronic Health Evaluation (APACHE) scoring systems were initially implemented when knowledge of sex disparities was lacking and neither biological sex nor sex-adjusted thresholds of physiological parameters were considered [26,27,28]. It is not surprising then, that risk-assessment scores perform differently in women and men [29]. Indeed, adding sex in risk-assessment scores can improve their performance [30] and may have important implications for admission strategies in ICU patients and outcomes.
Gender differences in symptom perception may further contribute to under-representation of women in the ICU. It is well known that women with acute myocardial infarction (AMI) often present with atypical chest pain and remain underdiagnosed [31]. In addition, sociocultural factors such as lower socioeconomic status, limited formal education, higher stress levels, and limited access to healthcare tend to occur more frequently in women [10]. Women are still more frequently involved in traditional social roles such as family responsibilities or household chores and may, therefore, defer their needs, presenting with a delay at an advanced stage of illness.
Finally, women may more often set limitations on life-sustaining therapies (advance directives), particularly in higher age groups or when divorced or widowed [32]. This may additionally impact ICU resource allocation or yield a bias where clinicians assume subsets of women may prefer less-aggressive care. Similarly, unconscious or implicit/explicit bias such as attitudes about race, ethnicity, abilities, age and gender may influence our decision-making in a discriminatory way, contributing to a delay in diagnosis and a delay or even denial of care [36]. Altogether, sociocultural rather than biological factors in addition to implicit and explicit bias seem to drive gender inequalities in the provision of intensive care (Fig. 4).
Influence of sex and gender on sepsis and septic shock
Male sex has been identified as a possible risk factor for sepsis. Indeed, epidemiological studies consistently report a higher prevalence of sepsis in men relative to women [37]. Men are more likely to develop infectious diseases than women, with a mean annual relative risk of 1.3 [37] and an almost 1.6 times higher risk of sepsis hospitalization [38]. Similarly, one meta-analysis reported a significant predominance of men (61%) among ICU septic shock patients [39]. This male overrepresentation is quite surprising since the sex ratio in the general population > 65 years old is around 0.8 male/female. Once in the surgical ICU, men also develop severe sepsis and septic shock more often than women. Although reports on the impact of sex on mortality from sepsis and septic shock have shown conflicting results, a recent analysis of a single-center large database study of more than 6000 patients admitted with sepsis reported an increased ICU length of stay (LOS) and mortality in men [38]. Similarly, men hospitalized for sepsis had a higher likelihood of death at 1 year, longer duration of hospital, and ICU stay. They were also more commonly readmitted to hospital within 90 days and 1 year after the incident sepsis hospitalization [38]. After admission, therapeutic strategies may be discrepant. For example, sex-based differences have been found in the emergency department management of critically ill patients with sepsis and septic shock, with men having 1-h sepsis bundles completed 38% more often than women, and with a 34% faster median time to antibiotic administration [40].
Potential mechanisms underlying sex differences in sepsis are manifold. Men and women differ in their innate immune responses. Notably, sex differences begin at conception with women having two X chromosomes that harbor many genes that regulate immune function such as some interleukin and Toll-like receptors [17]. Although one X chromosome is randomly silenced in each cell, up to one-third of genes may escape silencing and are expressed at higher levels in women than in men [17]. In addition, by binding to specific receptors expressed in immune cells such as lymphocytes, macrophages, and dendritic cells, sex steroids can affect immune responses and impact bacterial metabolism, growth, and expression of virulence factors [17]. Estrogens, for example, can directly stimulate B lymphocytes immunoglobulin production, increasing the humoral immune response in women [41]. Anatomy and urinary microbiome also explain why women are more prone to genital and urinary tract infections, though these are typically less severe in women than in men [17].
Behavioral factors associated with gender may also alter the risk. Historically, smoking, alcohol, and substance misuse were more prevalent in men and associated with an increased risk of community-acquired pneumonia, particularly Streptococcus pneumoniae [42] and bloodstream infections [43]. More men were also traveling and more often exposed to Legionella pneumophila [44].
Influence of sex and gender on cardiac arrest
Cardiac arrest also occurs more often in men, with women accounting for 18–46% of patients in studies [45]. This seems to apply for both in-hospital cardiac arrests (IHCA) and out-of-hospital cardiac arrest (OHCA), though data are biased toward OHCA, which is reported more frequently [46]. Compared to men, women with OHCA are less likely to present with a shockable rhythm [47] and are often older with more comorbidities (e.g., hypertension, diabetes, obesity) [48]. More women suffer from cardiac arrest in their home and are less likely to be witnessed [45]. Women are further disadvantaged since those who suffer an OHCA are less likely to receive bystander cardiopulmonary resuscitation (CPR), even in public locations [49]. Further, layperson bystanders may be reluctant to perform CPR in women for fear of being accused of sexual misconduct. This concern was emphasized in an American public survey that highlighted concerns about inappropriate touching and allegations of sexual assault as potential barriers to performing CPR on women in public [50]. Following return of spontaneous circulation, women are less likely to receive in-hospital post-resuscitation therapies like percutaneous coronary intervention (PCI), coronary artery bypass grafting (CABG), mechanical circulatory support (MCS) [51], and targeted temperature management [45].
Despite these variations in presenting features and management, many studies and registries corroborate comparable mortality rates and neurological outcomes in men and women. This paradox that men may be physically stronger with fewer disabilities and/or comorbidities but have substantially higher mortality at all ages has been termed the “gender paradox” or “male–female health-survival paradox.” This remarkable discrepancy is visible where women experience comparable survival outcomes to men, despite exhibiting inferior prognostic factors. Once again, sexual differences could partly explain this paradox. Women have higher chest wall compliance than men, making CPR efforts easier and more effective [52]. Further, evidence from preclinical investigations suggests that estrogen may exhibit anti-inflammatory and antioxidant effects, mitigating ischemia–reperfusion-induced injury [53]. Additionally, estrogen may also enhance the stability of the mitochondrial membrane and prevent the intracellular calcium and potassium influx that contributes to intracellular death after cardiac arrest [54].
Influence of sex and gender on acute respiratory distress syndrome
Limited evidence exists regarding the impact of sex and gender on acute respiratory distress syndrome (ARDS). Recent randomized controlled trials (RCT) and registries report a marked prevalence of males among ARDS patients, with the proportion of females ranging from 28% to 38% [55]. In contrast, following a trauma, women were more likely than men to develop ARDS [56]. In this study, a pro-inflammatory sex hormone profile (low testosterone, high oestradiol) was associated with ARDS in both men and women [56]. Though expectedly occurring less often in men, this hormone profile was also associated with ARDS in men, suggesting that sex-specific hormonal differences may impact ARDS. Interestingly, in prepubertal children, when hormone levels are expected to be uniformly low in both sexes, an equivalence of ARDS caused by non-septic factors is reported [57]. However, ARDS secondary to sepsis appears to be more common in boys, suggesting a biological basis for sex disparities in the susceptibility to ARDS, despite comparable hormone levels at this age. Similarly, among patients > 50 years, there is no notable elevation in the ratio of women to men, suggesting that the ARDS incidence in women does not increase after the hormonal changes of menopause [56].
Despite all these differences and conflicting data in presentation and management, most studies and registries report similar mortality rates in non-coronavirus disease 2019 (COVID-19) ARDS in men and women [58]. This is in the context of unique risk factors, where women with ARDS are more likely to be immunocompromised, and men with ARDS are more likely to have trauma, COPD, and chronic renal failure [56]. Regarding COVID-19 ARDS specifically, the latest data report a male bias in mortality [11]. However, despite intense research efforts, there is currently no consensus about the precise mechanisms by which female sex might protect against COVID-19 ARDS [59].
ARDS management may also differ between men and women. A study by Han et al. revealed that patients of shorter stature suffering from severe sepsis-related acute lung injury were less likely to receive lung-protective ventilation [60]. This is crucial as women with ARDS tend to be shorter and with a higher body mass index [61]. This concern has been validated in an analysis of ARDS network trials [62] and also in an ancillary analysis of the LUNG SAFE study, which showed that a higher proportion of women received non-protective lung ventilation. This resulted in higher plateau and driving pressures compared with men [61]. Indeed, height is often estimated incorrectly, especially in patients with shorter body length [63], further increasing the risk of women not receiving adequate lung-protective ventilation (Fig. 2). In contrast, more women than men received adjunctive measures, such as veno-venous extracorporeal membrane oxygenation and inhaled vasodilators [61].
Influence of sex and gender on cardiogenic shock
Given the association of cardiogenic shock (CS) with a high risk of morbidity and mortality, the impact of sex and gender on treatment and outcomes is poorly understood. In most recent studies on cardiogenic shock, women are consistently less represented than men, with a prevalence of about 33–40%. However, among patients with CS, women are more likely to present with non-ischemic (NI) CS than with AMI complicated by cardiogenic shock (AMI-CS) [64]. This could be partly explained by the fact that etiologies of NI-CS such as Takotsubo syndrome have a higher prevalence among women [65].
About 5–10% of AMI patients will develop AMI-CS [66] which is associated with in-hospital mortality rates ranging from 40% to 60% [67]. Post-menopause, decreasing oestrogen levels, and reduced vascular protective effects are associated with the development of microvascular damage after menopause [68], and may contribute biologically to an increased risk of developing more frequent complications after AMI [69].
Women who suffer from AMI frequently display an atypical clinical presentation and tend to delay seeking medical attention for their symptoms, leading to a longer total ischemia time and an increased risk of developing CS [70]. Despite sociocultural factors, the evidence from both RCT’s and registries consistently indicates that women who experience CS are typically older and have a greater incidence of comorbidities, such as hypertension and diabetes than men [69]. Unfortunately, after presentation for AMI-CS, women again receive less-frequent guideline-recommended care including coronary angiography, PCI, CABG, and MCS [51, 71] compared with men. Similarly, in NI-CS, a recent study showed the same trend of lower MCS use among women [72]. Women are also less likely to receive guideline-recommended pharmacological therapies at discharge in AMI-CS [73]. Nonetheless, despite compelling differences in the features and management of CS, there seems to be an absence of sex disparities in long-term outcomes [69].
Influence of sex and gender on acute kidney injury
Although experimental models of ischemic acute kidney injury (AKI) have consistently demonstrated that female sex protects against the development of renal damage, the consensus view is that female sex is an independent risk factor for AKI in humans. There is growing evidence that significant sex differences exist in the response of the kidney to injury [74]. They may be mediated by the effects of sex hormones on cellular processes instrumental in the pathogenesis of AKI. Differences also exist in the generation of nitric oxide, vascular response to endothelin-1, and in the renal hemodynamic response to angiotensin II [75].
A meta-analysis of 83 AKI studies from 1978 to 2018, including 6,758,124 patients, showed that the risk of developing hospital-associated AKI was significantly greater in men [odds ratio, OR, 1.23 (1.11, 1.36)] [76]. Furthermore, the association of male sex with AKI was strongest in patients undergoing non-cardiac surgery and critically ill patients in the ICU. In contrast, cardiac-surgery-associated AKI and radiocontrast-induced AKI showed no sexual dimorphism, suggesting that sex differences impact the pathogenesis of AKI. However, there are many limitations to untangling the relationship between sex and AKI. Importantly, differences exist in the rate of creatinine generation, (given the usually greater muscle mass, creatinine levels are higher in men), the elimination of creatinine, and in its volume of distribution. These features directly impact the diagnosis of AKI [28]. Further, sociocultural factors play a role, and processes of care may differ, including the use of nephroprotective medications or exposure to nephrotoxic factors. Finally, clinical AKI studies rarely report the menopausal status or the use of oral contraceptive or hormone therapy among participants and have not discerned how puberty, menopause, and transgender impact the susceptibility to AKI and risk of progression.
Influence of sex and gender on ICU delirium
Delirium is defined as a form of acute brain dysfunction with disturbance in awareness, attention, and cognition. It has been associated with worse outcomes such as higher mortality, longer LOS in ICU, post-ICU cognitive decline, and functional impairment after discharge [77]. Gender has not been consistently shown as a risk factor for ICU delirium, but a study by Mehta et al. reported that delirious patients were more likely to be men (61.1% vs 46.6%; p = 0.005) [78]. Data regarding the influence of sex on ICU delirium duration are conflicting, with a study by Liu et al. demonstrating no difference [79]. Similarly, delirium severity in acutely ill hospitalized patients was similar between sexes [80]. Sex differences in delirium subtype (hypoactive, hyperactive, or mixed) are also unclear. Current literature reports a higher likelihood in women to suffer from hypoactive delirium as compared to men in acute medicine background [80], while other works have shown no sex differences in ICU delirium subtype [81]. Documented agitation and hyperactive delirium are reported to occur more frequently in men than in women, with a higher likelihood of men being initiated on antipsychotic medications due to higher visibility of hyperactive symptoms and safety issues associated with agitation [82]. Figure 5 demonstrates the impact of sex and gender on most common ICU diagnoses.
Influence of sex and gender on medical treatment
Evolving evidence has supported that women are undertreated in the ICU, despite higher illness severity. This comprises the application of organ support measures, including renal replacement therapy (RRT), mechanical ventilation, invasive procedures, and early goal directed therapies [1, 23, 40]. By neglecting sex-specific thresholds of creatinine or urea, a systematic bias in clinical routine and risk prediction/survival scores may contribute to an underestimation of renal impairment and thus, lower assignment to RRT, and less-frequent transition to permanent dialysis access or organ transplantation in women [83,84,85]. As mentioned above, advanced directives are more prevalent in women and may confound treatment assignments.
While women less frequently receive mechanical organ support measures, medication-based treatments are primarily applied in equal measure to critically ill men and women. However, sex differences in the pharmacokinetics and pharmacodynamics of drugs can lead to overdosing and more adverse side effects in women (Table 1). For instance, women are more prone to develop Torsade de Pointes arrhythmias with QT-prolonging drugs (e.g., amiodarone) due to the effect of estrogens on lengthening the cardiac repolarization time [86]. Furthermore, women experience more side effects with loop diuretics based on 30–40% higher maximal serum concentrations than men, and experience more bleeding complications with unfractionated heparin [87]. Sex-associated factors, such as lower weight, higher average body fat, and lower plasma volume and blood flow in women may lead to higher maximum concentrations and thus, to the excess bleeding risk observed. Finally, treatment delays are more common in women, too. A gender-linked delay in presentation is seen in women presenting with an acute abdomen of non-obstetric cause, often primarily referred for a gynecology opinion, but also in women with chest pain, stroke, and cancer. Socio-economic status, responsibility for family duties, and sex differences in pain perception may contribute to these differences [88].
General outcomes in the ICU
Influence of sex and gender on ICU LOS and short-term mortality
Data on ICU LOS and ICU mortality are inconsistent. Shorter ICU LOS has been reported in both women [22] and men [89]. In a pooled, adjusted (40,494 patients) analysis, a shorter ICU LOS was found in women, while no sex differences in hospital LOS were observed in a separate pooled dataset including 7 studies and 57,292 patients [1]. These disparate results may be related to differences in age/sex predominance (e.g., a higher percentage of elderly women), higher female mortality, and higher incidence of treatment limitations upon ICU admission, as previously discussed [90] (Fig. 4).
Accordingly, data on ICU and short-time mortality show conflicting results. Some studies have found a higher risk-adjusted ICU mortality in women [22, 23], while others have refuted this association [24]. In a recent meta-analysis, sex differences in illness severity and mortality in 505,138 adult ICU patients (43.1% women) from 3 different continents were assessed. They found a significantly higher risk-adjusted ICU and 1-year mortality in women, while hospital and 30-day mortality did not differ between sexes [5]. However, when studies at high risk of bias were excluded, there were no statistically significant differences at any time point. Illness severity score discrepancies, mortality time-point differences, unique diagnostic groupings, and unequal sex representation therein all make data interpretation challenging.
An approach to overcome some of these limitations used a sex-balanced based analysis considering the sex distribution within each diagnostic subgroup of patients admitted to the ICU. In diagnostic groups with a lower female percentage (e.g., cardiovascular diseases, cardiac surgery), women were admitted with higher illness severity and were more likely to die. The same results were found in diagnostic groups with fewer men (e.g., metabolic disorders) [25]. Apart from illness-specific biological differences in each sex, a delay in recognition of low-probability conditions may contribute to the worse outcomes observed due to unconscious and preconceived judgment (Figs. 2 and 5). Furthermore, sociocultural factors (lifestyle/risk behavior, physical environment, access to and the use of available healthcare systems, gender roles and relations, socioeconomic status) are increasingly recognized as modifiers of diseases and outcomes, mostly studied in women with cardiovascular diseases [7, 10] (Fig. 4).
Influence of sex and gender on outcomes after critical illness
Outcomes after critical illness usually referred as the post-intensive care syndrome (PICS) [91] encompass a broad spectrum of issues, some of which are associated with sex differences in the frequency and presentation. ICU-acquired weakness (encompassing myosin-depletion myopathy and/or axonopathy) is nearly ubiquitous in long-stay ICU patients and female sex has consistently been identified as an independent risk factor in multiple international studies and meta-analyses [92]. While the underlying mechanism is still unclear, this diagnosis has significant consequences for women’s ability to regain functional independence, navigate care transitions, return to work, and reintegrate into the community [93].
Cognitive dysfunction is also highly prevalent after critical illness but has not been shown to have a consistent sex predilection across multiple international cohort studies. As demonstrated in the BRAIN-ICU study, its main determinants are the incidence and duration of delirium [94, 95].
Post-traumatic stress disorder (PTSD) occurs frequently in PICS, with a prevalence between 17 and 44% [96]. Sex differences in PTSD are debated; meta-analyses do not support differences (13 over 18 studies showed no association) [96]. Sex-related differences in ICU treatment may be a confounding factor in understanding this relationship. As one example, traumatic memories, a known PTSD risk factor, may increase with increasing doses of epinephrine in the cardiothoracic intensive care unit (CTICU), but only in men [97]. In contrast, metoprolol in the CTICU may decrease traumatic memory formation and later PTSD symptoms, but only in women [97]. Broadening from PTSD to all mental health diagnoses, women are more likely to have higher PTSD screening scores and are more at risk of new mental health diagnoses after critical care [93]. There may be an overarching bias in follow-up that fundamentally influences this literature and may be influenced by opportunity to participate based on access and responsibility for family caregiving and other restrictive social roles.
Summary and implications
Sex and gender differences impact the full spectrum of intensive care medicine, from critical care access to the severity of presentation, initial and ongoing management, and to short- and long-term outcomes. Biological differences in sex hormone profiles and immune responses seem to modify the trajectory of critical illness in common ICU diagnoses like sepsis or ARDS. However, evidence remains equivocal at this time, given the under-representation of women and the lack of information regarding hormonal status, hormone intake, and cyclic changes in many studies. Despite delayed (sepsis bundle) or inappropriate (ARDS lung-protective ventilation) treatments, short-term outcomes seem not to be impacted in female patients. Undertreatment remains not to be limited to these diagnoses and affects the general female population, starting from less-frequent ICU admissions to less organ support measures—despite being equally ill. The underlying reasons for these inequalities remain unclear, but unconscious or implicit bias may influence the treating clinicians, leading to delays in diagnosis and care with harmful consequences. The latter is supported by the fact that ICU mortality of women is higher in diagnoses with a lower percentage of female patients. It underscores the importance of workforce diversity, educational measures and most importantly, male and female physicians’ awareness that unconscious biases exist. Besides biology, gender factors influence critical illness at various stages. Socio-economic status, access and use of healthcare resources, social roles and personality traits may impact illness severity at presentation, ICU admission, treatment decisions (treatment limitation), and thus outcomes. The implementation of validated gender scores and the SAGER guidelines in critical care research may help to detangle the impact of biological and gender factors on critical illness.
Conclusion
Taken together, sex and gender differences in critical illness are complex and further challenged by unmeasurable existing biases. Current evidence is still scarce, and data remain conflicting. Healthcare providers and researchers need to be aware of these sex/gender differences and unconscious biases and work toward addressing them in their practice and clinical studies to improve ICU management for both, men and women, in a personalized, patient-centered manner. To further improve understanding, we encourage to use the terms sex and gender carefully to avoid confusion, to include information about hormonal status and to consider sociocultural factors as modifiers of diseases and outcomes.
References
Modra LJ, Higgins AM, Abeygunawardana VS, Vithanage RN, Bailey MJ, Bellomo R (2022) Sex differences in treatment of adult intensive care patients: a systematic review and meta-analysis. Crit Care Med 50:913–923
Arslani K, Tontsch J, Todorov A, Gysi B, Kaufmann M, Kaufmann F, Hollinger A, Wildi K, Merdji H, Helms J, Siegemund M, Gebhard C, Gebhard CE, Swiss Society of Intensive Care M (2023) Temporal trends in mortality and provision of intensive care in younger women and men with acute myocardial infarction or stroke. Crit Care 27:14
Healy B (1991) The Yentl syndrome. N Engl J Med 325:274–276
Goldstein RH, Walensky RP (2019) Where were the women? Gender parity in clinical trials. N Engl J Med 381:2491–2493
Modra L, Higgins A, Vithanage R, Abeygunawardana V, Bailey M, Bellomo R (2021) Sex differences in illness severity and mortality among adult intensive care patients: a systematic review and meta-analysis. J Crit Care 65:116–123
Mauvais-Jarvis F, Bairey Merz N, Barnes PJ, Brinton RD, Carrero JJ, DeMeo DL, De Vries GJ, Epperson CN, Govindan R, Klein SL, Lonardo A, Maki PM, McCullough LD, Regitz-Zagrosek V, Regensteiner JG, Rubin JB, Sandberg K, Suzuki A (2020) Sex and gender: modifiers of health, disease, and medicine. Lancet 396:565–582
Regitz-Zagrosek V, Gebhard C (2023) Gender medicine: effects of sex and gender on cardiovascular disease manifestation and outcomes. Nat Rev Cardiol 20:236–247
Heidari S, Babor TF, De Castro P, Tort S, Curno M (2016) Sex and gender equity in research: rationale for the SAGER guidelines and recommended use. Res Integr Peer Rev 1:2
Bartz D, Chitnis T, Kaiser UB, Rich-Edwards JW, Rexrode KM, Pennell PB, Goldstein JM, O’Neal MA, LeBoff M, Behn M, Seely EW, Joffe H, Manson JE (2020) Clinical advances in sex- and gender-informed medicine to improve the health of all: a review. JAMA Intern Med 180:574–583
Pelletier R, Khan NA, Cox J, Daskalopoulou SS, Eisenberg MJ, Bacon SL, Lavoie KL, Daskupta K, Rabi D, Humphries KH, Norris CM, Thanassoulis G, Behlouli H, Pilote L (2016) Sex versus gender-related characteristics: which predicts outcome after acute coronary syndrome in the young? J Am Coll Cardiol 67:127–135
Gebhard CE, Hamouda N, Gebert P, Regitz-Zagrosek V, Gebhard C, Investigators C (2022) Sex versus gender-related characteristics: which predicts clinical outcomes of acute COVID-19? Intensive Care Med 48:1652–1655
Lat TI, McGraw MK, White HD (2021) Gender differences in critical illness and critical care research. Clin Chest Med 42:543–555
Thornton J (2019) WHO report shows that women outlive men worldwide. BMJ 365:l1631
Modra L, Pilcher D, Bailey M, Bellomo R (2021) Sex differences in intensive care unit admissions in Australia and New Zealand. Crit Care Resusc 23:86–93
Gacouin A, Maamar A, Fillatre P, Sylvestre E, Dolan M, Le Tulzo Y, Tadie JM (2017) Patients with preexisting psychiatric disorders admitted to ICU: a descriptive and retrospective cohort study. Ann Intensive Care 7:1
Hollinger A, Gayat E, Feliot E, Paugam-Burtz C, Fournier MC, Duranteau J, Lefrant JY, Leone M, Jaber S, Mebazaa A, Arrigo M, Investigators FIS (2019) Gender and survival of critically ill patients: results from the FROG-ICU study. Ann Intensive Care 9:43
Dias SP, Brouwer MC, van de Beek D (2022) Sex and gender differences in bacterial infections. Infect Immun 90:e0028322
Lomauro A, Aliverti A (2021) Sex and gender in respiratory physiology. Eur Respir Rev 30(162):210038. https://doi.org/10.1183/16000617.0038-2021
Gaudino M, Di Franco A, Cao D et al (2022) Sex-related outcomes of medical, percutaneous, and surgical interventions for coronary artery disease: JACC focus seminar 3/7. J Am Coll Cardiol 79(14):1407–1425. https://doi.org/10.1016/j.jacc.2021.07.066
Ansdell P, Thomas K, Hicks KM, Hunter SK, Howatson G, Goodale S (2020) Physiological sex differences affect the integrative response to exercise: acute and chronic complications. Exp Physiol 105(12):2007–2025. https://doi.org/10.1113/EP088548
Sperrin M, Marshall AD, Higgins V, Renehan AG, Buchan IE (2016) Body mass index relates weight to height differently in women and older adults: serial cross-sectional surveys in England (1992-2011). J Public Health (Oxf) 38(3):607–613. https://doi.org/10.1093/pubmed/fdv067
Fowler RA, Sabur N, Li P, Juurlink DN, Pinto R, Hladunewich MA, Adhikari NK, Sibbald WJ, Martin CM (2007) Sex-and age-based differences in the delivery and outcomes of critical care. CMAJ 177:1513–1519
Todorov A, Kaufmann F, Arslani K, Haider A, Bengs S, Goliasch G, Zellweger N, Tontsch J, Sutter R, Buddeberg B, Hollinger A, Zemp E, Kaufmann M, Siegemund M, Gebhard C, Gebhard CE, Swiss Society of Intensive Care M (2021) Gender differences in the provision of intensive care: a Bayesian approach. Intensive Care Med 47:577–587
Samuelsson C, Sjoberg F, Karlstrom G, Nolin T, Walther SM (2015) Gender differences in outcome and use of resources do exist in Swedish intensive care, but to no advantage for women of premenopausal age. Crit Care 19:129
Modra LJ, Higgins AM, Pilcher DV, Bailey MJ, Bellomo R (2022) Sex differences in mortality of ICU patients according to diagnosis-related sex balance. Am J Respir Crit Care Med 206:1353–1360
Vincent JL, Moreno R, Takala J, Willatts S, De Mendonca A, Bruining H, Reinhart CK, Suter PM, Thijs LG (1996) The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 22:707–710
Le Gall JR, Lemeshow S, Saulnier F (1993) A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA 270:2957–2963
Waikar SS, Bonventre JV (2009) Creatinine kinetics and the definition of acute kidney injury. J Am Soc Nephrol 20:672–679
Jacobson S, Liedgren E, Johansson G, Ferm M, Winso O (2012) Sequential organ failure assessment (SOFA) scores differ between genders in a sepsis cohort: cause or effect? Upsala J Med Sci 117:415–425
Wenzl FA, Kraler S, Ambler G, Weston C, Herzog SA, Raber L, Muller O, Camici GG, Roffi M, Rickli H, Fox KAA, de Belder M, Radovanovic D, Deanfield J, Luscher TF (2022) Sex-specific evaluation and redevelopment of the GRACE score in non-ST-segment elevation acute coronary syndromes in populations from the UK and Switzerland: a multinational analysis with external cohort validation. Lancet 400:744–756
Sarma AA, Braunwald E, Cannon CP, Guo J, Im K, Antman EM, Gibson CM, Newby LK, Giugliano RP, Morrow DA, Wiviott SD, Sabatine MS, O’Donoghue ML (2019) Outcomes of women compared with men after non-ST-segment elevation acute coronary syndromes. J Am Coll Cardiol 74:3013–3022
McPherson K, Carlos WG 3rd, Emmett TW, Slaven JE, Torke AM (2019) Limitation of life-sustaining care in the critically ill: a systematic review of the literature. J Hosp Med 14:303–310
Tamargo J, Caballero R and Delpón E (2022) Sex-related differences in the pharmacological treatment of heart failure. Pharmacol Ther 229:107891
Filipescu D, Stefan M (2021) Sex and gender differences in anesthesia: relevant also for perioperative safety? Best Pract Res Clin Anesthesiol 35(1):141–153. https://doi.org/10.1016/j.bpa.2020.12.006
Whutley H, Lindsey W (2009) Sex-based differences in drug activity. Am Fam Physician 80(11):1254–1258
FitzGerald C, Hurst S (2017) Implicit bias in healthcare professionals: a systematic review. BMC Med Ethics 18:19
Martin GS, Mannino DM, Eaton S, Moss M (2003) The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med 348:1546–1554
Thompson KJ, Finfer SR, Woodward M, Leong RNF, Liu B (2022) Sex differences in sepsis hospitalisations and outcomes in older women and men: a prospective cohort study. J Infect 84:770–776
Campanelli F, Landoni G, Cabrini L, Zangrillo A (2018) Gender differences in septic intensive care unit patients. Minerva Anestesiol 84:504–508
Sunden-Cullberg J, Nilsson A, Inghammar M (2020) Sex-based differences in ED management of critically ill patients with sepsis: a nationwide cohort study. Intensive Care Med 46:727–736
Grimaldi CM, Hill L, Xu X, Peeva E, Diamond B (2005) Hormonal modulation of B cell development and repertoire selection. Mol Immunol 42:811–820
Wagenvoort GH, Sanders EA, Vlaminckx BJ, de Melker HE, van der Ende A, Knol MJ (2017) Sex differences in invasive pneumococcal disease and the impact of pneumococcal conjugate vaccination in the Netherlands, 2004–2015. Euro Surveill 22:30481
Laupland KB, Gregson DB, Zygun DA, Doig CJ, Mortis G, Church DL (2004) Severe bloodstream infections: a population-based assessment. Crit Care Med 32:992–997
Fukushima S, Hagiya H, Otsuka Y, Koyama T, Otsuka F (2021) Trends in the incidence and mortality of legionellosis in Japan: a nationwide observational study, 1999–2017. Sci Rep 11:7246
Lei H, Hu J, Liu L, Xu D (2020) Sex differences in survival after out-of-hospital cardiac arrest: a meta-analysis. Crit Care 24:613
Jerkeman M, Sultanian P, Lundgren P, Nielsen N, Helleryd E, Dworeck C, Omerovic E, Nordberg P, Rosengren A, Hollenberg J, Claesson A, Aune S, Stromsoe A, Ravn-Fischer A, Friberg H, Herlitz J, Rawshani A (2022) Trends in survival after cardiac arrest: a Swedish nationwide study over 30 years. Eur Heart J 43:4817–4829
Lakbar I, Ippolito M, Nassiri A, Delamarre L, Tadger P, Leone M, Einav S (2022) Sex and out-of-hospital cardiac arrest survival: a systematic review. Ann Intensive Care 12:114
Ko DT, Qiu F, Koh M, Dorian P, Cheskes S, Austin PC, Scales DC, Wijeysundera HC, Verbeek PR, Drennan I, Ng T, Tu JV, Morrison LJ (2016) Factors associated with out-of-hospital cardiac arrest with pulseless electric activity: a population-based study. Am Heart J 177:129–137
Liu N, Ning Y, Ong MEH, Saffari SE, Ryu HH, Kajino K, Lin CH, Karim SA, Rao GVR, Ho AFW, Lim SL, Siddiqui FJ, Investigators PCRN (2022) Gender disparities among adult recipients of layperson bystander cardiopulmonary resuscitation by location of cardiac arrest in Pan-Asian communities: a registry-based study. EClinicalMedicine 44:101293
Perman SM, Shelton SK, Knoepke C, Rappaport K, Matlock DD, Adelgais K, Havranek EP, Daugherty SL (2019) Public perceptions on why women receive less bystander cardiopulmonary resuscitation than men in out-of-hospital cardiac arrest. Circulation 139:1060–1068
Vallabhajosyula S, Dunlay SM, Barsness GW, Miller PE, Cheungpasitporn W, Stulak JM, Rihal CS, Holmes DR Jr, Bell MR, Miller VM (2020) Sex disparities in the use and outcomes of temporary mechanical circulatory support for acute myocardial infarction-cardiogenic shock. CJC Open 2:462–472
Safdar B, Stolz U, Stiell IG, Cone DC, Bobrow BJ, deBoehr M, Dreyer J, Maloney J, Spaite DW (2014) Differential survival for men and women from out-of-hospital cardiac arrest varies by age: results from the OPALS study. Acad Emerg Med 21:1503–1511
Kuhar P, Lunder M, Drevensek G (2007) The role of gender and sex hormones in ischemic-reperfusion injury in isolated rat hearts. Eur J Pharmacol 561:151–159
Simpkins JW, Wang J, Wang X, Perez E, Prokai L, Dykens JA (2005) Mitochondria play a central role in estrogen-induced neuroprotection. Curr Drug Targets CNS Neurol Disord 4:69–83
Gorman EA, O’Kane CM, McAuley DF (2022) Acute respiratory distress syndrome in adults: diagnosis, outcomes, long-term sequelae, and management. Lancet 400:1157–1170
Heffernan DS, Dossett LA, Lightfoot MA, Fremont RD, Ware LB, Sawyer RG, May AK (2011) Gender and acute respiratory distress syndrome in critically injured adults: a prospective study. J Trauma 71:878–883
Bindl L, Buderus S, Dahlem P, Demirakca S, Goldner M, Huth R, Kohl M, Krause M, Kuhl P, Lasch P, Lewandowski K, Merz U, Moeller J, Mohamad Y, Peters M, Porz W, Vierzig A, Ruchard J, Scharf J, Varnholt V, Group EAD (2003) Gender-based differences in children with sepsis and ARDS: the ESPNIC ARDS Database Group. Intensive Care Med 29:1770–1773
Villar J, Martinez D, Mosteiro F, Ambros A, Anon JM, Ferrando C, Soler JA, Montiel R, Vidal A, Conesa-Cayuela LA, Blanco J, Arrojo R, Solano R, Capilla L, Del Campo R, Civantos B, Fernandez MM, Aldecoa C, Parra L, Gutierrez A, Martinez-Jimenez C, Gonzalez-Martin JM, Fernandez RL, Kacmarek RM, Stratification, Outcome of Acute Respiratory Distress Syndrome N (2018) Is overall mortality the right composite endpoint in clinical trials of acute respiratory distress syndrome? Crit Care Med 46:892–899
Lott N, Gebhard CE, Bengs S, Haider A, Kuster GM, Regitz-Zagrosek V, Gebhard C (2023) Sex hormones in SARS-CoV-2 susceptibility: key players or confounders? Nat Rev Endocrinol 19:217–231
Han S, Martin GS, Maloney JP, Shanholtz C, Barnes KC, Murray S, Sevransky JE (2011) Short women with severe sepsis-related acute lung injury receive lung protective ventilation less frequently: an observational cohort study. Crit Care 15:R262
McNicholas BA, Madotto F, Pham T, Rezoagli E, Masterson CH, Horie S, Bellani G, Brochard L, Laffey JG, Investigators LS, the ETG (2019) Demographics, management and outcome of females and males with acute respiratory distress syndrome in the LUNG SAFE prospective cohort study. Eur Respir J. https://doi.org/10.1183/13993003.00609-2019
Walkey AJ, Wiener RS (2012) Risk factors for underuse of lung-protective ventilation in acute lung injury. J Crit Care 27(323):e321-329
Sasko B, Thiem U, Christ M, Trappe HJ, Ritter O, Pagonas N (2018) Size matters: an observational study investigating estimated height as a reference size for calculating tidal volumes if low tidal volume ventilation is required. PLoS ONE 13:e0199917
Berg DD, Bohula EA, van Diepen S, Katz JN, Alviar CL, Baird-Zars VM, Barnett CF, Barsness GW, Burke JA, Cremer PC, Cruz J, Daniels LB, DeFilippis AP, Haleem A, Hollenberg SM, Horowitz JM, Keller N, Kontos MC, Lawler PR, Menon V, Metkus TS, Ng J, Orgel R, Overgaard CB, Park JG, Phreaner N, Roswell RO, Schulman SP, Jeffrey Snell R, Solomon MA, Ternus B, Tymchak W, Vikram F, Morrow DA (2019) Epidemiology of shock in contemporary cardiac intensive care units. Circ Cardiovasc Qual Outcomes 12:e005618
Delco A, Portmann A, Mikail N, Rossi A, Haider A, Bengs S, Gebhard C (2023) Impact of sex and gender on heart failure. Cardiovasc Med 26:88–94
Berg DD, Bohula EA, Morrow DA (2021) Epidemiology and causes of cardiogenic shock. Curr Opin Crit Care 27:401–408
Goldberg RJ, Spencer FA, Gore JM, Lessard D, Yarzebski J (2009) Thirty-year trends (1975–2005) in the magnitude of, management of, and hospital death rates associated with cardiogenic shock in patients with acute myocardial infarction: a population-based perspective. Circulation 119:1211–1219
Murphy E (2011) Estrogen signaling and cardiovascular disease. Circ Res 109:687–696
Vallabhajosyula S, Verghese D, Desai VK, Sundaragiri PR, Miller VM (2022) Sex differences in acute cardiovascular care: a review and needs assessment. Cardiovasc Res 118:667–685
Vallabhajosyula S, Patlolla SH, Dunlay SM, Prasad A, Bell MR, Jaffe AS, Gersh BJ, Rihal CS, Holmes DR Jr, Barsness GW (2020) Regional variation in the management and outcomes of acute myocardial infarction with cardiogenic shock in the United States. Circ Heart Fail 13:e006661
Vallabhajosyula S, Ya’Qoub L, Singh M, Bell MR, Gulati R, Cheungpasitporn W, Sundaragiri PR, Miller VM, Jaffe AS, Gersh BJ, Holmes DR Jr, Barsness GW (2020) Sex disparities in the management and outcomes of cardiogenic shock complicating acute myocardial infarction in the young. Circ Heart Fail 13:e007154
Schrage B, Sundermeyer J, Beer BN, Bertoldi L, Bernhardt A, Blankenberg S, Dauw J, Dindane Z, Eckner D, Eitel I, Graf T, Horn P, Kirchhof P, Kluge S, Linke A, Landmesser U, Luedike P, Lusebrink E, Mangner N, Maniuc O, Winkler SM, Nordbeck P, Orban M, Pappalardo F, Pauschinger M, Pazdernik M, Proudfoot A, Kelham M, Rassaf T, Reichenspurner H, Scherer C, Schulze PC, Schwinger RHG, Skurk C, Sramko M, Tavazzi G, Thiele H, Villanova L, Morici N, Wechsler A, Westenfeld R, Winzer E, Westermann D (2023) Use of mechanical circulatory support in patients with non-ischaemic cardiogenic shock. Eur J Heart Fail 25:562–572
Jneid H, Fonarow GC, Cannon CP, Hernandez AF, Palacios IF, Maree AO, Wells Q, Bozkurt B, Labresh KA, Liang L, Hong Y, Newby LK, Fletcher G, Peterson E, Wexler L, Get With the Guidelines Steering C, Investigators (2008) Sex differences in medical care and early death after acute myocardial infarction. Circulation 118:2803–2810
Hutchens MP, Dunlap J, Hurn PD, Jarnberg PO (2008) Renal ischemia: does sex matter? Anesth Analg 107:239–249
Medina D, Mehay D, Arnold AC (2020) Sex differences in cardiovascular actions of the renin-angiotensin system. Clin Auton Res 30:393–408
Neugarten J, Golestaneh L (2018) Female sex reduces the risk of hospital-associated acute kidney injury: a meta-analysis. BMC Nephrol 19:314
Ely EW, Shintani A, Truman B, Speroff T, Gordon SM, Harrell FE Jr, Inouye SK, Bernard GR, Dittus RS (2004) Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA 291:1753–1762
Mehta S, Cook D, Devlin JW, Skrobik Y, Meade M, Fergusson D, Herridge M, Steinberg M, Granton J, Ferguson N, Tanios M, Dodek P, Fowler R, Burns K, Jacka M, Olafson K, Mallick R, Reynolds S, Keenan S, Burry L, Investigators S, Canadian Critical Care Trials G (2015) Prevalence, risk factors, and outcomes of delirium in mechanically ventilated adults. Crit Care Med 43:557–566
Li Y, Yuan D, Li X, Wang S (2020) Risk factors for delirium in intensive care unit and its duration. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 32:62–66
Trzepacz PT, Franco JG, Meagher DJ, Lee Y, Kim JL, Kishi Y, Furlanetto LM, Negreiros D, Huang MC, Chen CH, Kean J, Leonard M (2018) Delirium phenotype by age and sex in a pooled data set of adult patients. J Neuropsychiatry Clin Neurosci 30:294–301
Krewulak KD, Stelfox HT, Ely EW, Fiest KM (2020) Risk factors and outcomes among delirium subtypes in adult ICUs: a systematic review. J Crit Care 56:257–264
Marshall J, Herzig SJ, Howell MD, Le SH, Mathew C, Kats JS, Stevens JP (2016) Antipsychotic utilization in the intensive care unit and in transitions of care. J Crit Care 33:119–124
Melk A, Schmidt BMW, Geyer S, Epping J (2020) Sex disparities in dialysis initiation, access to waitlist, transplantation and transplant outcome in German patients with renal disease—a population based analysis. PLoS ONE 15:e0241556
Allen AM, Heimbach JK, Larson JJ, Mara KC, Kim WR, Kamath PS, Therneau TM (2018) Reduced access to liver transplantation in women: role of height, MELD exception scores, and renal function underestimation. Transplantation 102:1710–1716
Tejada S, Martinez-Reviejo R, Nogueira TA, Gomez A, Pont T, Liao X, Zhang Z, Manuel O, Rello J (2023) The effect of sex inequality on solid organ transplantation: a systematic review and meta-analysis. Eur J Intern Med 109:58–67
Abi-Gerges N, Philp K, Pollard C, Wakefield I, Hammond TG, Valentin JP (2004) Sex differences in ventricular repolarization: from cardiac electrophysiology to Torsades de Pointes. Fundam Clin Pharmacol 18:139–151
Roosendaal LC, Wiersema AM, Smit JW, Doganer O, Blankensteijn JD, Jongkind V (2022) Editor’s choice—sex differences in response to administration of heparin during non-cardiac arterial procedures. Eur J Vasc Endovasc Surg 64:557–565
Myatra SN, Tripathy S, Einav S (2021) Global health inequality and women—beyond maternal health. Anaesthesia 76(Suppl 4):6–9
Bernard AM, Hayward RA, Rosevear JS, McMahon LJ (1993) Gender and hospital resource use. Unexpected differences. Eval Health Prof 16:177–189
Block L, Petzold M, Syrous AN, Lindqvist B, Odenstedt Herges H, Naredi S (2019) Age, SAPS 3 and female sex are associated with decisions to withdraw or withhold intensive care. Acta Anaesthesiol Scand 63:1210–1215
Herridge MS, Azoulay E (2023) Outcomes after critical illness. N Engl J Med 388:913–924
De Jonghe B, Sharshar T, Lefaucheur JP, Authier FJ, Durand-Zaleski I, Boussarsar M, Cerf C, Renaud E, Mesrati F, Carlet J, Raphael JC, Outin H, Bastuji-Garin S, Groupe de Reflexion et d’Etude des Neuromyopathies en R (2002) Paresis acquired in the intensive care unit: a prospective multicenter study. JAMA 288:2859–2867
Lee M, Kang J, Jeong YJ (2020) Risk factors for post-intensive care syndrome: a systematic review and meta-analysis. Aust Crit Care 33:287–294
Pandharipande PP, Girard TD, Jackson JC, Morandi A, Thompson JL, Pun BT, Brummel NE, Hughes CG, Vasilevskis EE, Shintani AK, Moons KG, Geevarghese SK, Canonico A, Hopkins RO, Bernard GR, Dittus RS, Ely EW, Investigators B-IS (2013) Long-term cognitive impairment after critical illness. N Engl J Med 369:1306–1316
Girard TD, Thompson JL, Pandharipande PP, Brummel NE, Jackson JC, Patel MB, Hughes CG, Chandrasekhar R, Pun BT, Boehm LM, Elstad MR, Goodman RB, Bernard GR, Dittus RS, Ely EW (2018) Clinical phenotypes of delirium during critical illness and severity of subsequent long-term cognitive impairment: a prospective cohort study. Lancet Respir Med 6:213–222
Parker AM, Sricharoenchai T, Raparla S, Schneck KW, Bienvenu OJ, Needham DM (2015) Posttraumatic stress disorder in critical illness survivors: a metaanalysis. Crit Care Med 43:1121–1129
Hauer D, Kaufmann I, Strewe C, Briegel I, Campolongo P, Schelling G (2014) The role of glucocorticoids, catecholamines and endocannabinoids in the development of traumatic memories and posttraumatic stress symptoms in survivors of critical illness. Neurobiol Learn Mem 112:68–74
Funding
Open access funding provided by University of Basel.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
HM: no relevant disclosures. MH: no relevant disclosures. MO: no relevant disclosures. MTL: no relevant disclosures. SNM: no relevant disclosures. SDR: received honorary for lectures from Estor, Fresenius Medical, and Toray. VM: no relevant conflicts of interest. KK: no relevant disclosures. CR: received honorary for lectures from Masimo and Edwards. ADJ: received honoraria for lectures from Medtronic, Viatris, Drager and Fisher & Paykel. JH: received honoraria for lectures from Diagnostica Stago, Pfizer PFE France, Sanofi Aventis France, Inotrem, MSD and Shionogi. CEG: no relevant disclosures.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Merdji, H., Long, M.T., Ostermann, M. et al. Sex and gender differences in intensive care medicine. Intensive Care Med 49, 1155–1167 (2023). https://doi.org/10.1007/s00134-023-07194-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-023-07194-6