Abstract
Purpose
Shock is a life-threatening condition characterized by substantial alterations in the microcirculation. This study tests the hypothesis that considering sublingual microcirculatory perfusion variables in the therapeutic management reduces 30-day mortality in patients admitted to the intensive care unit (ICU) with shock.
Methods
This randomized, prospective clinical multicenter trial-recruited patients with an arterial lactate value above two mmol/L, requiring vasopressors despite adequate fluid resuscitation, regardless of the cause of shock. All patients received sequential sublingual measurements using a sidestream-dark field (SDF) video microscope at admission to the intensive care unit (± 4 h) and 24 (± 4) hours later that was performed blindly to the treatment team. Patients were randomized to usual routine or to integrating sublingual microcirculatory perfusion variables in the therapy plan. The primary endpoint was 30-day mortality, secondary endpoints were length of stay on the ICU and the hospital, and 6-months mortality.
Results
Overall, we included 141 patients with cardiogenic (n = 77), post cardiac surgery (n = 27), or septic shock (n = 22). 69 patients were randomized to the intervention and 72 to routine care. No serious adverse events (SAEs) occurred. In the interventional group, significantly more patients received an adjustment (increase or decrease) in vasoactive drugs or fluids (66.7% vs. 41.8%, p = 0.009) within the next hour. Microcirculatory values 24 h after admission and 30-day mortality did not differ [crude: 32 (47.1%) patients versus 25 (34.7%), relative risk (RR) 1.39 (0.91–1.97); Cox-regression: hazard ratio (HR) 1.54 (95% confidence interval (CI) 0.90–2.66, p = 0.118)].
Conclusion
Integrating sublingual microcirculatory perfusion variables in the therapy plan resulted in treatment changes that do not improve survival at all.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
In different types of cardiocirculatory shock, considering sublingual microcirculatory perfusion variables in the therapeutic management has been proposed to improve outcome. In this prospective randomized-controlled multicenter study, integrating sublingual microcirculatory perfusion variables in the therapy plan resulted in significantly more treatment changes that did not improve 30-day survival. |
Introduction
Shock is a life-threatening condition defined as a mismatch between oxygen supply and demand in tissue. [1]. The network of microvessels with a diameter < 100 μm is defined as microcirculation and is responsible for tissue supply [2] and homeostasis [3]. Thus, microcirculation is of great importance for clinical routine [4]. Intensive care medicine often tried to optimize systemic blood pressure to improve microcirculation, but several recent studies remained neutral [2, 5]. Indirect methods to assess the microcirculation have been proposed, i.e. serum lactate [6], the capillary refill time [7] or the mottling score [8], that might be suitable resuscitation targets in shock [9]. In parallel, image-based direct visualization with intravital microscopy such as sidestream darkfield-cameras (SDF) have been developed [10]. Several studies showed a correlation between the severity of microcirculatory disturbances and the outcome of critically ill patients [11,12,13,14]. These hand-held devices offer a standardized fast, and non-invasive assessment of the microcirculation at the sublingual mucosa. The obtained results may correlate with the microcirculation of relevant territories such as the gut mucosa and has therefore been suggested as suitable surrogate parameter of whole-body microcirculation [2, 15, 16]. The automated analysis of the video sequences by the AVA 4.3C software should allow a quick objectification of the microcirculatory variables without being dependent on a subjective visual interpretation [17]. However, data on the potential impact of microcirculatory assessment on the decision-making process during shock resuscitation is missing.
The objective of this prospective randomized-controlled trial was to test the hypothesis that considering sublingual microcirculatory perfusion variables in the therapy plan reduces 30-day mortality in patients admitted to the intensive care unit (ICU) with circulatory shock.
Methods
Study design and oversight
The trial was registered on clinicaltrials.gov (NCT04173221) on November 21, 2019. This randomized, prospective clinical multicenter DAMIS (Direct Assessment of Microcirculation in Shock) trial was conducted at different hospitals in Germany (see supplemental Table 1). The primary competent ethics committee was the Ethics Committee of the University of Duesseldorf, Germany. Institutional research ethic board approval was acquired from each study site. Written informed consent was obtained from all patients or surrogates. The detailed study protocol is attached as electronic supplement. An overview on the study plan is depicted in supplemental Fig. 1. The trial was logistically supported by MicroVision Medical (Amsterdam, Netherlands); however, protocol design and data interpretation were independent of the company. The members of the steering committee designed the trial and analyzed the data.
Patient selection and randomization
To be included, patients had to be adults (18 years old or older) suffering from circulatory shock on intensive care unit (ICU) admission. Shock was operationally defined as requirement of vasopressors despite adequate fluid resuscitation plus an elevated lactate value (> 2 mmol/L) as markers for hypotension and hypoperfusion [6]. Adequate fluid substitution was defined at the discretion of the attending intensivist. To be included, a signed informed consent was mandatory. In non-consenting patients, a medical witness or legal guardian signed a provisional informed consent form. Exclusion criteria included inaccessibility of the sublingual mucosa, lack of informed consent, impracticality of measurement during the inclusion period due to examinations/treatments/patient already dying, uncooperative/weakly sedated patients, or infectious reasons such as coronavirus disease 2019 (COVID-19). Upon meeting the criteria for inclusion in the study, the patient was randomized to the intervention or control group. Patients were randomized to routine care or to integrating sublingual microcirculatory perfusion variables in the therapy plan. Randomization was performed by blind drawing of sealed envelopes by a neutral person in blocks of 50 patients (25 per group).
Study interventions
The sublingual measurement of the microcirculation was performed using the MicroScan USB3 (MS-U) SDF video microscope at ICU admission (time frame: maximal 4 h) and 24 h (± 4 h) after ICU admission. After the measurement, the four highest-quality video sequences recorded at different locations of the sublingual mucosa were evaluated using the AVA 4.3C algorithm. The average of these variables was then calculated [17]. No variables were manually calculated by investigators. For this type of microcirculation measurement, a proof-of-concept study could confirm the feasibility [18]. The quality of the videos was later assessed by blinded trained personnel using the microcirculation-imaging-quality-score (MIQS) [19]. In addition, further information about the patient was collected in both cohorts, including macrocirculatory variables such as blood pressure, additional microcirculatory variables such as mottling score or capillary refill time, blood gas analysis values, and medications The microcirculatory assessment was performed blindly to the treatment team. The same investigator performed both consecutive measurements (with the exemption of one single measurement). In the intervention group, the percentage of perfused small vessels (sPPV) as key value and other microcirculatory measurement results (De Backer Density, Number of Crossings, Perfused De Backer Density, Perfused Number of Crossings for all and for small vessels) were reported immediately to the treating physicians, whereas in the control group, they were withheld from the medical team. The De Backer Density and the Number of Crossings describe the number of vessels that can be detected. The perfused De Backer Density and the Perfused Number of Crossings are all vessels with visible microcirculatory flow. The Percentage of Perfused Vessels is calculated as the percentage of perfused in relation to the total number of all vessels. Small vessels (capillaries) are defined as smaller than 20 µm [20]. Physicians could adjust the therapy to the current microcirculation in the intervention group. The study protocol divided all patients into three risk categories according to their sPPV on admission (≤ 80%: red; 81–94%: yellow and ≥ 95%: green). The cut-offs were chosen a priori according to the manufacturer’s recommendation, which considered an sPPV greater than 95% as “healthy,” from 81–94% as “unhealthy,” and values less than 80% as “dangerous.” This classification corresponds to the data known from the literature and our own clinical experience for critically ill patients [21], patients suffering from shock [22], and healthy volunteers [23,24,25], although in other studies a PPV < 90% were considered as abnormal [10, 26]. Recommendations on fluid and vasopressor dosing according to the microcirculatory status are shown in supplemental Fig. 2. The study protocol did not restrict the use of inotropes or vasodilators.
Data collection regarding therapy adjustments after microcirculation-oriented consultation
One hour after the first microcirculation-oriented consultation, dosing data on fluids and vasoactive drugs were extracted from medical records and compared with those registered at the time of the microcirculatory assessment, and determining if these were increased, reduced, started or stopped. Reduced administration rates of 0.1 ml/h or of 0 ml/h were defined as “stop”, and newly started rates of greater than 0.1 ml/h were defined as “start”. Changes of less than 0.5 ml per hour for the vasoactive perfusion pump, and less than 10 ml per hour for the volumetric infusion pump were ignored. Patients who were assigned to the control (routine care) group were treated according to usual practice at their study sites including decisions about hemodynamic and perfusion monitoring, and all treatments.
Definition of matching between announced and performed treatment changes after microcirculatory consultations
If the change in therapy announced after the SDF measurement and microcirculatory consultations corresponded to the actual change documented later, this was noted as a “match”; otherwise, it was noted as a “mismatch.” Therapy changes one hour after the SDF measurements were also documented in the control group according to the procedure mentioned above.
Definition of limitation of life-sustaining therapy
If the patient had a medical indication to undergo certain intensive care measures (usually: intubation and ventilation, cardiopulmonary resuscitation, catecholamine therapy, hemodialysis), but these were not administered to the patient due to the patient's will or for ethical reasons or in the case of a palliative situation, this was defined as a “withheld therapy”. If one of these measures is already performed, but during intensive care therapy the patient's will to terminate this therapy was expressed, then in this case a “withdrawn therapy” was defined [27].
Cause of shock and comorbidities
The cause of shock was defined primarily from the etiology and not from the underlying physiology: Patients with a primary infectious etiology were defined as “septic shock” according to the Sepsis-3 criteria [6] (for example, pneumonia or urinary tract infection), and those with a primary cardiac etiology (e.g., acute myocardial infarction or acute heart failure) as “cardiogenic shock”. Patients after cardiac surgery as “post cardiac surgery”, which is widely described in the literature [28] and can be caused by vasoplegia [29, 30], hypovolemia or reduced cardiac pump function [31]. Comorbidities were extracted from health records. If the disease was present, regardless of severity or etiology, it was recorded as “present” in a binary way, otherwise as “not present”.
Sample size calculation
The study aimed to detect a difference of 19% in 30-day survival rates, with an expected rate of 43% in the control group. The sample size was calculated using a beta of 80% and an alpha of 5%, with a total of 186 patients divided equally between the two groups through 1:1 randomization. We planned to randomize 200 patients allowing for dropouts. After 70% (140) of the planned number of patients were included, a pre-planned interim analysis was conducted using a group sequential design and an O’Brien-Fleming boundary [32]. The trial was stopped since futility stopping boundaries were met [33].
Statistical analysis
The primary outcome was 30-day mortality. Secondary endpoints were length of stay on the ICU and the hospital, and 6-months mortality. Primary analyses were performed on the intention-to-treat population. Continuous data points were expressed as median ± interquartile range. Statistical significance in differences between independent groups were calculated using the Mann–Whitney U-test. Categorical data are expressed as numbers (percentages). The Chi2 test was applied to test differences between groups for their statistical significance. Proportional hazard ratios with 95% confidence interval (CI) were calculated using univariate Cox-regression analysis, and Kaplan–Meier curves were depicted to compare the cumulative incidence of mortality 30 days after randomization. Missing values were defined as “missing values”, and a complete-cases analyses was conducted. Unless otherwise stated, there were no missing values for the variables examined in the statistical analysis. Baseline variables and other variables that are not outcome had not been tested for statistically significant differences. All tests were two-sided, and a p-value of < 0.05 was considered statistically significant. Analyses were conducted using IBM SPSS Statistics (Version 28) and GraphPad Prism 9.4.1.681 (GraphPad Software, Boston, USA).
Post hoc analyses
Additional proportional hazard ratios with 95% CI were calculated after exclusion of patients from the interventional group with mismatches using univariate Cox-regression analysis, and Kaplan–Meier curves were depicted to compare the cumulative incidence of mortality 30 days after randomization. Post hoc sensitivity analyses were performed for different subgroups of patients (with and without any treatment limitation, gender, age and Sequential Organ Failure Assessment (SOFA) score according to their median). Mixed-effects repeated measures analyses of variance were used to compare within-group, and between-group (sPPV-category on admission or randomized group) mean changes in SOFA, lactate, and capillary refill time over time to assess the effect of time on the organ function and microcirculation. The sphericity was tested for using Mauchly’s test and a Huyn–Feldt correction was applied to the one-factorial repeated measures ANOVA results in case of not fulfilling the spherical assumption. Greenhouse–Geisser correction was used to correct for the risk for type II error [34, 35].
Results
Patients’ baseline characteristics
From 21st February 2020 to 27th August 2022, 987 patients were screened, with 176 patients meeting inclusion criteria for shock. 35 patients could not be included because of various reasons (28 patients: no investigator ready for measurement within the time limits; 2 patients with a baseline lactate ≤ 2 mmol/L at ICU admission; 3 patients with no sufficient mouth opening; 2 patients with infectious diseases (COVID-19). Thus, 141 patients (69 patients in the interventional and 72 in the control group) from four tertiary referral hospitals were included and randomized (Fig. 1). Most patients were admitted to medical ICUs (111 patients, 79%). There were no significant differences regarding age, pre-existing comorbidities, SOFA score on admission, and the main cause of ICU admission between groups. The most frequent reasons for admission were cardiogenic and septic shock (Table 1). An overview of the missing values for additional analyses is given in supplemental Table 3.
Macrocirculation and microcirculation on admission
The mean heart rate was similar in both groups (83 ± 20beats per minute). Regarding microcirculation, there was no difference in capillary refill time (3 ± 2 s in the control group compared to 3 ± 2 s in the interventional group). Serum lactate did not differ after 6, 24 or 48 h, and lactate kinetics did not show significant differences after six hours (supplemental Tables 4 and 5). The baseline SDF measurement showed impaired perfusion and capillary density without significant differences between both groups. After 24 h, there were no statistically significant differences in the microcirculatory values from the SDF measurement between both groups. In the complete cohort, there was no association between sPPV on ICU admission and 30-day mortality (odds ratio (OR) 1.0, 95% CI 0.97–1.03, p = 0.975; area under curve (AUC) 0.498). There was only weak to no correlation between sPPV on the one hand, and serum lactate on admission, after 6 h, mottling score or capillary refill time (see supplemental Fig. 3), and there was no correlation between the mean arterial pressure (MAP) and the sPPV (see supplemental Fig. 4).
Intensive care treatment
During ICU stay, most patients received mechanical ventilation. Only a minority underwent renal replacement therapy or extracorporeal life support. Life-sustaining therapy was limited in almost half of the patients on day two. In the interventional group, therapy was withheld or withdrawn in more patients, but there was no statistically significant difference compared to the control group. Furthermore, there was no difference regarding the length of ICU stay or length of hospital stay (Table 2). All patients received norepinephrine as vasopressor without differences in the administration rate on admission (0.38 µg/kg/min (± 0.5) versus 0.33 µg/kg/min (± 0.3), p = 0.502). Additional inotropic drugs (dobutamine, epinephrine, milrinone, levosimendan) were used in both groups without statistical differences (supplemental Table 6). In sum, 8 patients underwent placement of mechanical support devices (3 in the interventional, 5 in the control group, see supplemental Table 7). The median arterial pressure after 24 h under vasopressor support did not differ between the groups (see supplemental Table 8).
Microcirculatory adjustments following SDF measurement
The proportion of patients without any treatment change after receiving microcirculatory consultation was significantly lower in the interventional arm (16.7% versus 40%, p = 0.007). Significantly more patients received either an adjustment in vasoactive drugs or fluid resuscitation (66.7% versus 41.8%, p = 0.009), although the proportion of patients with a treatment change in both vasopressors and fluids was similar (16.7% versus 18.2, p = 0.083, Fig. 2). There was no difference in the treatment changes after the re-assessment (no change: 53.1% in the interventional versus 43.5% in the control group; one change in 50% versus 46.9%; and two changes in 6.5% versus 0%, respectively, p = 0.158). There were no significant differences in the distribution of the microcirculatory risk classes as defined by their sPPV on admission (≤ 80%: red; 81–94%: yellow and ≥ 95%: green) between the interventional and the control group [interventional group: red 20 (29%), yellow 38 (55%), green 11 (16%); control group: red 25 (35%), yellow 37 (52%), green 9 (13%), p = 0.691]. The change of the individual patients from one risk class to another after 24 hours occurred in a similar pattern in both groups (see supplemental Fig. 5).
Primary outcome
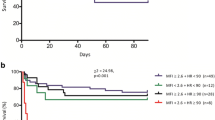
Crude 30-day mortality was without statistically significant difference between both groups [34.7% in the control groups versus 47.1% in the interventional group, relative risk (RR) 1.39 (0.91–1.97)]. Likewise, in the Cox-regression analysis, 30-day mortality did not differ between both groups (hazard ratio (HR) 1.54, 95% CI 0.90–2.66, p = 0.118, Fig. 3).
Six months follow-up
After 6 months, all-cause mortality did not differ between both groups (crude: 44.4% in the control groups versus 55.1% in the interventional group, RR 1.23 (95% CI 0.89–1.71), Cox-regression analysis, (HR 1.38, 95% CI 0.86–2.11, p = 0.189).
Post hoc analyses
The supplemental File 1 and supplemental Figs. 6 to 8 provide additional post-hoc analyses including additional sensitivity analyses, an outcome analysis excluding patients with mismatches in the interventional group, and treatment changes according to the risk category.
Video quality
865 videos underwent retrospective quality control by blinded investigators. 72% of the videos were good or acceptable. The most frequent error points from all MIQS error points were assigned for the criteria “Stability” (30%) and “Pressure” (23%) (see supplemental Fig. 9). The main reasons for being judged as “not acceptable” were stability (12.8%), pressure (8.1%), and content (7.2%). There were no significant differences regarding the video quality (quantified as MIQS) between the investigators (illumination p = 0.436; focus 0.436; content p = 0.453; stability p = 0.483; pressure 0.453).
Discussion
The present study represents the largest prospective randomized-controlled trial that investigated the therapeutic impact of real-time knowledge of the status of sublingual microcirculation in patients suffering from different types of shock. The sequential microcirculatory assessment resulted in the decision of treatment changes for fluids or vasopressors in the interventional group, without evidencing any effect on microcirculatory parameters at 24 hours, macrocirculatory and perfusion variables, or 30-day mortality.
In 2002, De Backer et al. described key alterations of the microcirculation in septic [10] and – two years afterwards – in cardiogenic shock [36]. More than 20 years later, DAMIS is the first study that integrated sublingual microcirculatory assessment into the clinical decision-making process in critically ill patients. Our trial demonstrated that real-time knowledge of the microcirculatory status significantly influenced therapeutic decisions of clinicians at the bedside, not only in cardiogenic and septic shock, but in a wide variety of shock patients. The strategy proposed in DAMIS used a multimodal approach to improve tissue perfusion, and provides the clinicians with a microcirculatory consultation that is integrated with standard practice monitoring signals and resuscitation targets, potentially avoiding a reductionist view of the resuscitation process. Apart from this, our study results offer valuable technical data on the use and applicability of real-time microcirculatory assessment in a randomized-controlled clinical trial, in a wide set of patients, such as sepsis, cardiogenic shock, or dehydration [5, 12, 37,38,39].
In daily practice, shock resuscitation mainly focuses on normalizing MAP and serum lactate [40]. Nonetheless, achieving a normal MAP does not warrant improvement of microcirculatory flow and tissue perfusion, particularly in patients in which there is a loss of coherence between macro- and microcirculation [5]. Sakr et al. demonstrated in 49 patients suffering from shock that patients with an impaired microcirculation had a significantly worse outcome despite normalizing macrohemodynamic values [37]. On the other hand, persistent hyperlactatemia is not always directly related with tissue hypoperfusion and should be interpreted with caution [41]. Although a high blood-lactate level is associated with an increased odds of death [42, 43], the use of blood-lactate as a single resuscitation target could increase the risk of “over-resuscitation” and induce harm [44, 45]. Therefore, real-time microcirculatory assessment might fill this gap providing valuable information on the status of tissue perfusion, aiding clinicians in the contextual interpretation of standard monitoring signals and in therapeutic decision-making process, namely adjusting fluids and vasopressors. However, in the present study treatment changes after microcirculatory assessment did not improve outcome but showed a trend towards higher mortality compared to the usual care control group.
Early fluid administration in hypovolemic patients may recruit microcirculatory vascular beds and increase overall perfusion parameters [31]. Ospina-Tascon et al. evaluated 60 patients with severe sepsis using SDF microscopy comparing the impact of an early (within 24 h after diagnosing a severe sepsis) to a delayed (more than 48 h) on the sublingual microcirculation [46]. They found that fluid administration enhanced microvascular perfusion in the early, but not late phase of sepsis independently of global hemodynamic effects and of the type of solution. Moreover, in later stages of the disease, fluids could even deteriorate microcirculatory flow by inducing interstitial edema and venous congestion, thus decreasing convective oxygen flow to cells [47]. Vasopressors are often used to increase mean arterial pressure in shock but should be used with caution and considering the microcirculation [13]. Potter et al. collected in their systematic review 6 randomized-controlled trials, 12 interventional, 3 observational, and 1 pilot study with 572 patients [48]. They conclude that there “is no robust evidence to date that any one agent can reproducibly lead to improved microvascular flow” when using mean arterial pressure or cardiac index as target goals. Indeed, as shown by Dubin et al., in patients with abnormal baseline microcirculatory flow, a norepinephrine-induced increase in MAP could recruit perfused vessels [49], while in other patients it could have a detrimental effect, eventually due to excessive precapillary vasoconstriction [50]. Due to these reasons, real-time knowledge of microcirculation emerges as a relevant clinical tool and could further aid physicians to titrate fluids and vasopressors more accurately [51, 52].
In the present study, microcirculatory values were not significantly associated with the outcome. These results are in line with the microSOAP trial (n = 501 ICU patients) that found in a mixed ICU population, lactate levels and several macrohaemodynamic variables, but not microcirculatory variables to be independently associated with hospital mortality [21]. On the contrary, the MicroDAIMON study (n = 97 ICU patients) demonstrated an independent association between baseline MFI < 2.6 and outcome (OR 4.59 95% CI 1.34–15.75, p = 0.015) [53]). In another study with 252 patients suffering from severe sepsis, PPV was normal (≥ 90%) in only 9 (4%) patients and moderately altered (between 80 and 90%) in 35 (15%) patients [54]. In DAMIS, all patients received immediate hemodynamic stabilization before the first SDF measurement. This might explain the relatively high sPPV values, and the lack of association between sPPV and the main outcome.
Microvascular abnormalities differ between different types of shock. Especially septic shock is characterized by typical microvascular inhomogeneous flow [55], while patients with cardiogenic shock suffer – for example—from decreasing vascular density, although heterogeneity also occurs in cardiogenic shock [56, 57]. However, DAMIS considered shock as a clinical syndrome defined by hypoperfusion and consecutive microcirculatory impairment. Capillary refill time and serum lactate are established parameters in septic and cardiogenic shock [7, 58]. Usually, variables of flow rather than variables that represent capillary density are recommended to be used for risk stratification and managing adjustments in – for example – fluid therapy [59]. The most used flow variable is the microvascular flow index (MFI). Especially on the first day of ICU admission, an abnormal and low MFI (defined as < 2.6) is associated with adverse outcomes [53]. MFI needs the manual interpretation of the investigator, but AVA 4.3C does not provide any information about the microcirculatory flow pattern. Other approaches, such as the Point of Care Microcirculation (POEM) score, consider the flow pattern with major attention [60]. The DAMIS study protocol had intentionally omitted a subjective assessment of the flow pattern by the investigator since the software cannot calculate this parameter automatically. Using this automatic algorithm surpasses this individual (subjective) evaluation providing (independent) information about microvascular perfusion (yes/no), density, and the percentage of perfused vessels. Previous studies found a sufficient correspondence between a low PPV and low MFI with a PPV of 88% in the low MFI group and a PPV 94% in the high MFI group [59]. Furthermore, PPV has a low observer variability [61].
The post-hoc sensitivity analyses revealed no significant effects in the different subgroups except for the subgroup with low SOFA score, but the clinical relevance seems negligible. As evolution of microcirculation was similar in all subgroups, the results are probably more a sign for the failure of the selected interventions than the failure of microcirculatory monitoring. The suggested treatment adjustments after microcirculatory counseling did not routinely include inotropic drugs to improve the microcirculation, because the study protocol basically decided to prevent potential heart injury in a mixed population. In the present study, we found a trend towards higher mortality in the interventional group. However, the post-hoc analysis excluding patients with a mismatch between announced and performed treatment changes showed no differences between both groups. It is also possible that treatment consequences of microcirculatory improvement attempts led to a clinical under- or overtreatment resulting in patient harm.
This study opens the road to further analyze the impact of real-time microcirculatory assessment in shock resuscitation. Future studies should explore the best therapeutic interventions to address each pattern of microcirculatory derangement, and its’ relationship with patient-centered outcomes.
Limitations
Some limitations of this study should be acknowledged. First, there was no additional re-evaluation of the impact of the interventions after 24 hours, which might imply that an ineffective or partially effective treatment was sometimes applied, and no alternative treatment was used in case of failure. Sublingual microcirculation was measured only on two time points. However, previous studies showed that on the first day of ICU admission, an impaired sublingual microcirculation is associated with adverse outcome, while later measurements provided no prognostic information [53]. Measuring on admission might give the opportunity to understand the microcirculation directly after the immediate resuscitation to unmask hemodynamic incoherence. The measurement after 24 hours was meant as control if the treatment changes after the first measurement had been able to improve the microcirculatory values. All patients were hemodynamically stabilized immediately at ICU admission. Consequently, the mean arterial pressure was controlled with vasoactive drugs, which was necessary for inclusion (see supplemental Table 9). A minority of videos evidenced lower technical quality, which might have an impact on the generalizability of the microcirculatory data, although our quality scores obtained are in line with other studies [62], and it must be underlined that videos were performed by experienced trained investigators who were not part of the ICU team. The study protocol was not binding. The counseling in the intervention group was deliberately not binding, as this was neither possible nor reasonable from a scientific point of view with weak evidence, nor for medico-legal reasons. If a mandatory algorithm had been required, this would have decisively changed the character of the study. However, the results clearly show that the consultation had an influence on the therapy adjustments for volume and catecholamine therapy, although there was a not insignificant proportion of patients in whom the announced therapy adjustments were not implemented at all, but in some cases contrary measures were taken. This discrepancy had been identified only a posteriori. The reasons for this are unclear, but the decision about therapy rested exclusively with the intensive care treatment team. We hypothesize that the treating team apparently considered many factors other than microcirculation data, such as serum lactate, organ function, hemodynamics, and other factors, to adjust therapy. We can only speculate that other aspects outweighed microcirculatory advice in some patients during the assessment. Furthermore, the SDF-assessment focused on the sPPV as most promising and reproducible value reflecting the patient’s microcirculatory tissue perfusion. Density values might be important as well, but there exist even less defined cut-off values in the literature, while density parameters are mostly used intra-individually, but not inter-individually. It must be mentioned that, currently, there is no established standard approach for optimizing the microcirculation. Furthermore, there was a considerable percentage of patients with various degrees of limitations for life-sustaining therapy, which are known to be a significant confounder for short-term mortality in critically ill patients [63]. The study provided data about the type and day of the limitation, but not about the cause and motivation for life-sustaining therapy. Of course, the study protocol allowed including different types of shock leading to a certain etiological heterogeneity, but they all lead to an impaired microcirculation. All these factors represent major confounders that could not be adequately adjusted due to the low sample size. Furthermore, the sensitivity analysis did not reveal any difference between the different etiologies. Another limitation is that recruitment occurred competitively resulting in an imbalance of inclusion rates, mostly because the University Hospital of Duesseldorf was the coordinating center with a superior number of trained teams. This distribution did not allow to adjust for any center effects. Furthermore, the randomization process that used blocks of 50 seals. Thus, the other study sites did not used a full block, which might contribute to an imbalanced allocation.
The study protocol anticipated that an early microcirculatory-driven adjustment of catecholamine- and fluid therapy provides a significant benefit for 30-day survival of 19% compared to usual care. However, the result was unable to reject the null hypothesis. Thus even with a less pronounced anticipated effect and – consequently – larger sample size, any positive effect in this study setting cannot be expected. Even after excluding all patients from the interventional group with mismatches regarding the announced and performed adjustments, there was no benefit for the (remaining) interventional compared to the control group. Last, as outlined by Ospina-Tascón et al., choosing mortality as primary endpoint in an intensive care setting might not be the optimal strategy evaluating the effectiveness of interventions [64].
Conclusion
Knowledge of sublingual microcirculatory status resulted in significantly more treatment adjustments during shock resuscitation. However, these adjustments did not impact any microcirculatory variable after 24 hours or patient-centered outcomes although we could not exclude a small reduction in mortality or a large increase in mortality with the integration of microcirculatory assessments in the therapy plan of critically ill patients.
Data availability
The anonymized data can be requested from the authors if required.
Change history
28 July 2023
A Correction to this paper has been published: https://doi.org/10.1007/s00134-023-07162-0
References
Standl T et al (2018) The nomenclature, definition and distinction of types of shock. Dtsch Arztebl Int 115(45):757–768
Dilken O, Ergin B, Ince C (2020) Assessment of sublingual microcirculation in critically ill patients: consensus and debate. Ann Transl Med 8(12):793
Guven G, Hilty MP, Ince C (2020) Microcirculation: physiology, pathophysiology, and clinical application. Blood Purif 49(1–2):143–150
Jung C, Kelm M (2015) Evaluation of the microcirculation in critically ill patients. Clin Hemorheol Microcirc 61(2):213–224
Arnold RC et al (2012) Discordance between microcirculatory alterations and arterial pressure in patients with hemodynamic instability. J Crit Care 27(5):531 e1-537
Singer M et al (2016) The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 315(8):801–810
Hernandez G et al (2019) Effect of a resuscitation strategy targeting peripheral perfusion status vs serum lactate levels on 28-day mortality among patients with septic shock: the andromeda-shock randomized clinical trial. JAMA 321(7):654–664
Ait-Oufella H et al (2011) Mottling score predicts survival in septic shock. Intensive Care Med 37(5):801–807
Bakker J et al (2022) Current practice and evolving concepts in septic shock resuscitation. Intensive Care Med 48(2):148–163
De Backer D et al (2002) Microvascular blood flow is altered in patients with sepsis. Am J Respir Crit Care Med 166(1):98–104
Spronk PE, Zandstra DF, Ince C (2004) Bench-to-bedside review: sepsis is a disease of the microcirculation. Crit Care 8(6):462–468
Massey MJ et al (2018) Microcirculatory perfusion disturbances in septic shock: results from the ProCESS trial. Crit Care 22(1):308
Ince C (2005) The microcirculation is the motor of sepsis. Crit Care 9(4):S13–S19
Jung C et al (2015) Intraaortic balloon counterpulsation and microcirculation in cardiogenic shock complicating myocardial infarction: an IABP-SHOCK II substudy. Clin Res Cardiol 104(8):679–687
Verdant CL et al (2009) Evaluation of sublingual and gut mucosal microcirculation in sepsis: a quantitative analysis. Crit Care Med 37(11):2875–2881
Qian J et al (2014) Post-resuscitation intestinal microcirculation: its relationship with sublingual microcirculation and the severity of post-resuscitation syndrome. Resuscitation 85(6):833–839
Bruno RR et al (2020) Evaluation of a shorter algorithm in an automated analysis of sublingual microcirculation. Clin Hemorheol Microcirc 76(2):287–297
Bruno RR et al (2020) Sublingual microcirculation in prehospital critical care medicine: a proof-of-concept study. Microcirculation 27(5):e12614
Massey MJ et al (2013) The microcirculation image quality score: development and preliminary evaluation of a proposed approach to grading quality of image acquisition for bedside videomicroscopy. J Crit Care 28(6):913–917
De Backer D et al (2007) How to evaluate the microcirculation: report of a round table conference. Crit Care 11(5):R101
Vellinga NA et al (2015) International study on microcirculatory shock occurrence in acutely ill patients. Crit Care Med 43(1):48–56
Spanos A et al (2010) Early microvascular changes in sepsis and severe sepsis. Shock 33(4):387–391
Kanoore Edul VS et al (2015) The effects of arterial hypertension and age on the sublingual microcirculation of healthy volunteers and outpatients with cardiovascular risk factors. Microcirculation 22(6):485–492
Donadello K et al (2011) Sublingual and muscular microcirculatory alterations after cardiac arrest: a pilot study. Resuscitation 82(6):690–695
Pranskunas A et al (2015) Effects of whole body heat stress on sublingual microcirculation in healthy humans. Eur J Appl Physiol 115(1):157–165
Favory R et al (2010) Can normal be more normal than normal? Crit Care Med 38(2):737–738
Guidet B et al (2018) Withholding or withdrawing of life-sustaining therapy in older adults (≥ 80 years) admitted to the intensive care unit. Intensive Care Med 44(7):1027–1038
Jochberger S et al (2009) The vasopressin and copeptin response in patients with vasodilatory shock after cardiac surgery: a prospective, controlled study. Intensive Care Med 35(3):489–497
Egi M et al (2007) Selecting a vasopressor drug for vasoplegic shock after adult cardiac surgery: a systematic literature review. Ann Thorac Surg 83(2):715–723
Hajjar LA et al (2017) Vasopressin versus norepinephrine in patients with vasoplegic shock after cardiac surgery: the VANCS randomized controlled trial. Anesthesiology 126(1):85–93
Vallabhajosyula S et al (2019) Trends, predictors, and outcomes of temporary mechanical circulatory support for postcardiac surgery cardiogenic shock. Am J Cardiol 123(3):489–497
O’Brien PC, Fleming TR (1979) A multiple testing procedure for clinical trials. Biometrics 35(3):549–556
Schuler S, Kieser M, Rauch G (2017) Choice of futility boundaries for group sequential designs with two endpoints. BMC Med Res Methodol 17(1):119
Vasey MW, Thayer JF (1987) The continuing problem of false positives in repeated measures ANOVA in psychophysiology: a multivariate solution. Psychophysiology 24(4):479–486
Lakens D (2013) Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs. Front Psychol 4:863
De Backer D et al (2004) Microvascular alterations in patients with acute severe heart failure and cardiogenic shock. Am Heart J 147(1):91–99
Sakr Y et al (2004) Persistent microcirculatory alterations are associated with organ failure and death in patients with septic shock. Crit Care Med 32(9):1825–1831
Bruno RR et al (2020) Sublingual microcirculation detects impaired perfusion in dehydrated older patients. Clin Hemorheol Microcirc 75(4):475–487
Chommeloux J et al (2020) Microcirculation evolution in patients on venoarterial extracorporeal membrane oxygenation for refractory cardiogenic shock. Crit Care Med 48(1):e9–e17
Evans L et al (2021) Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med 47(11):1181–1247
Gutierrez G, Williams JD (2009) The riddle of hyperlactatemia. Crit Care 13(4):176
Casserly B et al (2015) Lactate measurements in sepsis-induced tissue hypoperfusion: results from the surviving sepsis campaign database. Crit Care Med 43(3):567–573
Nichol A et al (2011) Dynamic lactate indices as predictors of outcome in critically ill patients. Crit Care 15(5):R242
Vincent JL et al (2016) The value of blood lactate kinetics in critically ill patients: a systematic review. Crit Care 20(1):257
Levy B (2006) Lactate and shock state: the metabolic view. Curr Opin Crit Care 12(4):315–321
Ospina-Tascon G et al (2010) Effects of fluids on microvascular perfusion in patients with severe sepsis. Intensive Care Med 36(6):949–955
Hernandez G et al (2014) When to stop septic shock resuscitation: clues from a dynamic perfusion monitoring. Ann Intensive Care 4:30
Potter EK et al (2019) Manipulating the microcirculation in sepsis - the impact of vasoactive medications on microcirculatory blood flow: a systematic review. Shock 52(1):5–12
Dubin A et al (2009) Increasing arterial blood pressure with norepinephrine does not improve microcirculatory blood flow: a prospective study. Crit Care 13(3):R92
Segal SS (2005) Regulation of blood flow in the microcirculation. Microcirculation 12(1):33–45
Thooft A et al (2011) Effects of changes in arterial pressure on organ perfusion during septic shock. Crit Care 15(5):R222
Marik PE, Weinmann M (2019) Optimizing fluid therapy in shock. Curr Opin Crit Care 25(3):246–251
Scorcella C et al (2018) MicroDAIMON study: microcirculatory DAIly MONitoring in critically ill patients: a prospective observational study. Ann Intensive Care 8(1):64
De Backer D et al (2013) Microcirculatory alterations in patients with severe sepsis: impact of time of assessment and relationship with outcome. Crit Care Med 41(3):791–799
Boerma EC et al (2005) Quantifying bedside-derived imaging of microcirculatory abnormalities in septic patients: a prospective validation study. Crit Care 9(6):R601–R606
Akin S et al (2017) Functional evaluation of sublingual microcirculation indicates successful weaning from VA-ECMO in cardiogenic shock. Crit Care 21(1):265
Wijntjens GW et al (2020) Prognostic implications of microcirculatory perfusion versus macrocirculatory perfusion in cardiogenic shock: a CULPRIT-SHOCK substudy. Eur Heart J Acute Cardiovasc Care 9(2):108–119
Merdji H et al (2022) Performance of early capillary refill time measurement on outcomes in cardiogenic shock: an observational, prospective multicentric study. Am J Respir Crit Care Med 206(10):1230–1238
Pranskunas A et al (2013) Microcirculatory blood flow as a tool to select ICU patients eligible for fluid therapy. Intensive Care Med 39(4):612–619
Naumann DN et al (2016) Real-time point of care microcirculatory assessment of shock: design, rationale and application of the point of care microcirculation (POEM) tool. Crit Care 20(1):310
Hubble SM et al (2009) Variability in sublingual microvessel density and flow measurements in healthy volunteers. Microcirculation 16(2):183–191
Damiani E et al (2017) Impact of microcirculatory video quality on the evaluation of sublingual microcirculation in critically ill patients. J Clin Monit Comput 31(5):981–988
Flaatten H et al (2022) The importance of revealing data on limitation of life sustaining therapy in critical ill elderly Covid-19 patients. J Crit Care 67:147–148
Ospina-Tascon GA, Buchele GL, Vincent JL (2008) Multicenter, randomized, controlled trials evaluating mortality in intensive care: doomed to fail? Crit Care Med 36(4):1311–1322
Funding
Open Access funding enabled and organized by Projekt DEAL. This work was supported by the Forschungskommission of the Medical Faculty of the Heinrich-Heine-University Düsseldorf No. 2020–21 to RRB for a Clinician Scientist Track. Furthermore, institutional support has been received by the German Research Council (SFB 1116, B06) as well as the State of North Rhine Westphalia (Giga for Health: 5GMedizincampus. NRW, Project number 005–2008-0055 and PROFILNRW-2020–107-A, TP4). No (industry) sponsorship has been received for this investigator-initiated study. MicroVision Medical (Amsterdam, Netherlands) provided SDF-measurement equipment, but was not involved in the study plan or analysis.
Author information
Authors and Affiliations
Contributions
RRB and CJ analyzed the data and wrote the first draft of the manuscript. CW and JW and KF and MF and BS and SA and HT and MS and JH and HM and FD and LP contributed data and contributed to statistical analysis and improved the paper. DB and JB and GH and EK and MK and MF and JB contributed to statistical analysis, improved the paper, gave guidance and improved the paper. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no competing interests.
Ethical approval
The primary competent ethics committee was the Ethics Committee of the University of Duesseldorf, Germany. Institutional research ethic board approval was obtained from each study site.
Consent for publication
The manuscript does not contain any individual person’s data in any form.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: In this article the author Marcus Franz has been affiliated wrongly. This has been corrected.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Bruno, R.R., Wollborn, J., Fengler, K. et al. Direct assessment of microcirculation in shock: a randomized-controlled multicenter study. Intensive Care Med 49, 645–655 (2023). https://doi.org/10.1007/s00134-023-07098-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-023-07098-5