Abstract
Microcirculatory perfusion is disturbed in sepsis. Recent research has shown that maintaining systemic blood pressure is associated with inadequate perfusion of the microcirculation in sepsis. Microcirculatory perfusion is regulated by an intricate interplay of many neuroendocrine and paracrine pathways, which makes blood flow though this microvascular network a heterogeneous process. Owing to an increased microcirculatory resistance, a maldistribution of blood flow occurs with a decreased systemic vascular resistance due to shunting phenomena. Therapy in shock is aimed at the optimization of cardiac function, arterial hemoglobin saturation and tissue perfusion. This will mean the correction of hypovolemia and the restoration of an evenly distributed microcirculatory flow and adequate oxygen transport. A practical clinical score for the definition of shock is proposed and a novel technique for bedside visualization of the capillary network is discussed, including its possible implications for the treatment of septic shock patients with vasodilators to open the microcirculation.
Similar content being viewed by others
Introduction
The initial treatment of trauma and critically ill patients is aimed at securing the airway and establishing adequate breathing, followed by the correction of circulatory abnormalities ('ABC') [1]. These basic principles underline the fact that optimization of oxygen delivery to the tissues is one of the cornerstones of critical care medicine, thus preventing cellular dysfunction and cellular death, and subsequent organ dysfunction. Disturbance of the delicate balance between oxygen delivery (DO2) and oxygen consumption (VO2) to the tissues can be defined as a state of shock. Impairment of DO2 can be caused by severe anemia, hypoxia, or a low cardiac output. To preserve tissue DO2 in several states of shock, especially to the heart and brain, many compensating physiological reserve mechanisms come into play. This leads to microvascular derecruitment in compliant vascular beds such as the skin and the splanchnic area, redirecting blood flow to more crucial body areas. During this process, systemic hemodynamics can be maintained at the expense of impaired microcirculatory perfusion. Nevertheless, if this microcirculatory state of hypoperfusion is not reversed in a timely manner, multiple organ failure can develop, with a high probability of death. This line of thought can be found in a recent general guideline for the treatment of patients with septic shock, in which infusion of volume is judged to be critical to basic care in these patients [2].
Systemic inflammatory response syndrome (SIRS) is seen after trauma, major surgery or hemorrhage. A similar phenomenon is seen in sepsis as a response to infection, and is still an important cause of death in critically ill patients. Both can progress to severe shock and multiple organ dysfunction syndrome (MODS) [3]. This progression is currently thought to be due to an increased VO2, a decreased peripheral vascular resistence and a maldistribution of tissue blood flow to preserve central blood volume. As a result, microcirculatory perfusion is shut down and is the final common pathway in shock. Especially in septic shock, alterations in metabolic pathways called 'cytopathic hypoxia' can lead to additional tissue damage [4]. This review discusses briefly the importance of microcirculatory flow in the pathogenesis of sepsis and the progression to MODS.
Heterogeneous microcirculatory perfusion
The measurement of global hemodynamics reflects only a tiny part of whole-body circulatory blood flow. The microcirculation, with its huge endothelial surface, is in fact the largest 'organ' in the human body. We have come a long way since the disclosure of human bodily circulation by Harvey [5] and Malpighi [6]. The number of publications concerning the microcirculation in humans is steadily increasing (Fig. 1). However, the microcirculation remains difficult to investigate. In clinical practice, microcirculatory perfusion is judged on aspects such as the color, capillary refill and temperature of the distal parts of the body (i.e. fingers, toes, earlobes and nose).
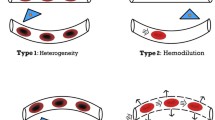
Perfusion of the microcirculation is regulated by an intricate interplay of many neuroendocrine, paracrine, and mechanosensory pathways [7]. These mechanisms adapt to the balance between locoregional tissue oxygen transport and metabolic needs to ensure that supply matches demand. In sepsis, this process is severely compromised because of decreased deformability of red blood cells with inherent increased viscosity [8], an increased percentage of activated neutrophils with decreased deformability and increased aggregability due to the upregulation of adhesion molecules [9], activation of the clotting cascade with fibrin deposition and the formation of microthrombi [10], dysfunction of vascular autoregulatory mechanisms [11], and finally, the secondary enhanced perfusion of large arteriovenous shunts [12] (Fig. 2). These processes result in tissue dysoxia, either from impaired microcirculatory oxygen delivery and/or from mitochondrial dysfunction [4, 13]. Clinically this process is perceived as an oxygen extraction defect, a prominent feature of sepsis. A possible mechanism accounting for this phenomenon could be the shut-down of vulnerable microcirculatory units in the organ beds, promoting the shunting of oxygen transport from the arterial to the venous compartment leaving the microcirculation hypoxic [14]. This might be an explanation for the different findings regarding locoregional tissue perfusion in shock (Fig. 3). In this so-called shunting theory of sepsis, correction of this condition should occur by recruitment of the shunted microcirculatory units. Applying strategies to 'open the microcirculation' by vasodilation would be expected to promote microcirculatory flow by increasing the driving pressure at the entrance of the microcirculation and/or decreasing the capillary afterload [15].
The shunting theory of sepsis accounts for the condition in which apparently adequate oxygen delivery is not successful in delivering oxygen to microcirculatory weak units that are shunted. This leads to an oxygen extraction deficit of these shunted units with raised levels of venous partial pressure of CO2, lactate and gastric CO2, whereas input oxygen delivery seems adequate. Vasodilation would be expected to recruit these shunted units by increasing the driving pressure to the microcirculation and possibly to these shunted units.
Indeed, in animal studies, these effects occur during hemorrhage and sepsis caused by microcirculatory shunting with associated tissue dysoxia [16–18]. Such microcirculatory shunting was reversed by vasodilation [14] and by improvement in regional flow in an animal sepsis model [19]. In addition, oxygen extraction was improved [20] and microcirculatory shunting was reversed [21] by the use of nitric donors. To redirect microvascular flow, matters become more complicated if one realizes that sepsis causes heterogeneous effects in constriction and dilation in different organs and at different levels of the microcirculation [22]. Although cardiac output is frequently increased in sepsis, high lactate levels and increased tonometric partial pressure of CO2 (pCO2) in tissues indicate at least regional tissue dysoxia. This has been termed oxygen extraction deficit in sepsis and has been well documented in different animal models of shock [23–25]. It is still a matter of debate whether it can be explained by pathologic flow heterogeneity due to dysfunctional autoregulatory mechanisms and microcirculatory dysfunction causing hypoxic pockets, or by mitochondrial dysfunction with associated impaired oxidative phosphorylation [4], or by a combination of both.
How is critical microcirculatory dysfunction assessed?
Especially in critical illness, function and dysfunction of the microcirculatory network are of utmost importance in the cause of disease and the development of organ failure. In sepsis, all three elements of the microvascular network are compromised, namely arteriolar hyporesponsiveness to vasocontrictors and vasodilators, a reduced number of perfused capillaries, and venular obstruction by the sequestration of activated neutrophils [22]. However, an objective and reliable method of monitoring microcirculatory organ perfusion is still not available. 'Downstream' global derivatives of microcirculatory dysfunction such as lactate, tonometry, and mixed venous oxygen saturation (SvO2), in addition to measurements of DO2 and oxygen uptake VO2, are used in daily intensive care clinical practice. But which parameters should be used to prevent further deterioration of organ function in a critically ill patient with septic shock? In this section we discuss the reasons for, and limitations of, several parameters that have been used to assess microcirculatory perfusion.
Lactate levels are thought to reflect anaerobic metabolism associated with tissue dysoxia and might predict a response to therapy and prognosis [26]. The balance between lactate production due to global (shock, hypoxia), local (tissue ischemia), and cellular (mitochondrial dysfunction) factors on the one hand, and lactate clearance depending on metabolic liver function on the other hand, make the interpretation of lactate levels uncertain and difficult [27]. SvO2 can be measured with a pulmonary artery catheter and is thought to reflect the average oxygen saturation of all perfused microvascular beds. In sepsis, microcirculatory shunting can cause normal SvO2 while severe local tissue dysoxia is present [14]. Delayed therapy aimed at the normalization of SvO2 failed to demonstrate a survival benefit [28, 29]. Optimization of oxygen delivery might have been instituted too late in these studies, when irreversible cellular damage was already present. In addition, the frequent use of dobutamine to obtain preset goals of oxygen delivery might have affected the outcome, because dobutamine has been implicated in the impairment of hepatosplanchnic perfusion in sepsis [30]. Nevertheless, besides ongoing discussions about the use of a pulmonary artery catheter in sepsis, the sole use of SvO2 seems an inadequate parameter as a guideline for therapy in the restoration of local tissue oxygenation in septic shock patients. However, if an integrative approach is used in the early stage of treating critically ill patients, states of hypoperfusion are recognized earlier [31] and, if early treatment is started, can even improve survival [32]. It is likely that the results of the Rivers study [32] are due largely to the prevention of irreversible cellular damage, in contrast to the earlier findings by Hayes and Gattinoni, who targeted high oxygen delivery levels during later phases of sepsis [28, 29].
An appealing alternative to the evaluation of tissue dysoxia might be regional intestinal capnography as introduced by Fiddian-Green and Baker [33]. This method relies on the principle of CO2 diffusion from the local anaerobic production site across tissue and cell membranes. Measurement of the difference between intestinal pCO2 and arterial pCO2 has been found to be better than that of pHi alone, because arterial pCO2 fluctuates in ventilated patients [34]. In sepsis, the interpretation of tonometric results is affected by microcirculatory shunting. This complicates the clear establishment of impaired perfusion, because areas with reduced perfusion and CO2 offloading are next to hypoxic regions [35]. Recently, gastric intramucosal pCO2 values were found to be well correlated with sublingual pCO2 values [36]. The baseline difference between sublingual pCO2 and arterial pCO2 values was a better predictor of survival than the change in lactate or SvO2 [37]. Further studies should demonstrate whether this parameter can be used in clinical management of patients with septic shock.
All parameters discussed are indirect and downstream from the pathological process in the microcirculatory network. Direct assessment of microcirculatory perfusion seems a superior and more direct approach and has been extensively studied in vivo by intravital microscopy (IVM) in animals. In humans, IVM studies are restricted to the eye, the skin and the nail fold owing to the size of the IVM equipment and the use of fluorescent dyes for contrast enhancement. IVM depends on trans- or epi-illumination and thus observations are limited to superficial layers of thin tissues only. By using fluorescent dyes a higher contrast is possible as well as labelling specific cells for visualization and quantification. Because of the potentially toxic effects of these dyes in humans, studies are mostly limited to animals [38, 39]. We recently introduced [40, 41], validated [42], and clinically applied [43] a new method for observing the microcirculation in patients, called orthogonal polarization spectral (OPS) imaging (CYTOSCAN™; Cytometrics Inc., Philadelphia, PA), which creates high-contrast images without the use of fluorescent dyes. This technique is based on the reflection of light from the tissues. Contrast is obtained from the absorption of linearly polarized light by the haemoglobin in the blood. As a consequence, red blood cells in the microcirculation appear black on the white background of the surrounding tissue. For OPS imaging a 5 × objective (on-screen magnification of × 326) is used during measurements. Data are recorded on a digital video recorder for later analysis and displayed on a black and white monitor. Because the OPS machine is a small hand-held device (Fig. 4), it can be used at the bedside for humans in the visualization of unique in vivo images of the microcirculation [44]. Although nailfold microcirculatory blood flow as established by OPS imaging correlates very well with IVM microvascular flow when analysed by specific video-analysis-software [42], this quantitative approach proved not to be usable with sublingual images owing to movement artefacts induced by tongue movements or respiration. A semi-quantitative approach was therefore used successfully to analyse changes in microcirculatory flow [45, 46].
Despite these shortcomings in the assessment of local tissue oxygenation, several studies have been performed aiming at recruitment of the tissue microcirculatory flow.
Microcirculatory perfusion as an endpoint
Data from several studies support the idea that the impairment of microcirculatory perfusion results in organ failure and increases the risk of death [17, 18, 22, 45, 47–50]. In this line of thought, restoring perfusion in disturbed microcirculatory networks might improve outcome. Indeed, survival was related to microcirculatory shutdown in rats that were bled and in which the blood volume was subsequently resuscitated, although whole-body hemodynamic parameters were comparable in survivors and non-survivors [51]. Comparable findings have been reported in humans with septic shock. Bihari found that vasodilation might unmask a preexisting tissue oxygen debt. After increasing DO2 with the vasodilator prostacyclin, all patients survived when the increase in DO2 did not coincide with an increase in VO2, whereas all patients died who showed increasing VO2 [52]. By recruitment of the microcirculation, oxygen might have become available to previously hypoxic tissues that had shut down. De Backer and colleagues [45] reported that sublingual microcirculatory perfusion was compromised to a greater extent in non-surviving than in surviving septic shock patients. We observed normal sublingual microcirculatory perfusion in a septic patient with hepatic failure who received high doses of norepinephrine (P Spronk, unpublished observation). Dubois recently reported a comparable observation in a septic patient treated with vasopressin [53], whereas others observed sublingual microcirculatory shutdown with the use of vasopressin (C Boerma, personal communication). Larger studies should demonstrate why these patients behave differently from those in previous reports. Nevertheless, De Backer and colleagues showed that microcirculatory perfusion improved over time in survivors, whereas the disturbance of perfusion in the microvessels of the non-survivors remained. In addition, they showed that sublingual microcirculatory perfusion abnormalities could be corrected by the topical application of acetylcholine, showing that the local endothelium was still responsive to nitric oxide (NO), whereas vasoplegia due to ongoing sepsis might be expected.
NO has been implicated as the major cause for hypotension, generated from endothelial cells through the expression of inducible NO synthase (NOS) [54], thus contributing to many of the manifestations of septic shock such as vasoplegia, diminished myocardial contractility, hepatic damage, and vascular and intestinal hyperpermeability. Others, however, found decreased NO production during sepsis [55], and, more recently, that NOS activity is diminished in mononuclear cells from sepsis patients [56]. On the basis of the hypothesis that NO production is increased in sepsis, experiments in septic animal models were performed and indicated that hypotension could be prevented by inhibiting NOS. This led to clinical studies with several compounds capable of inhibiting NO synthesis. Early promising data showed increasing blood pressures and decreasing doses of vasopressors in septic shock patients treated with NOS inhibitors [57]. However, a subsequent randomized controlled multicenter phase III trial was stopped when interim analysis showed increased mortality in the NG-monomethyl-L-arginine group compared with placebo [58]. Inhibition of NOS activity seems to result in an improvement in the general hemodynamic situation, but at the cost of increased mortality [59]. Apparently, completely inhibiting vasodilation is not the proper answer to sepsis. A more specific approach by inhibiting only the inducible form of NOS might be an attractive alternative. Indeed, after the application of 1400 W (a synthetic blocker of inducible NOS) in a pig endotoxemia model, microvascular perfusion was restored by a redistribution within the gut wall and/or an amelioration of the cellular respiration [60].
NO is an important vasodilator in the microcirculation during sepsis [61]. Indeed, Ince and colleagues showed recently that NO donors were highly effective in correcting microcirculatory oxygenation after endotoxemia in a pig model of sepsis, with both mucosal and serosal microvascular PO2 as well as intraluminal gastric pCO2 being restored to baseline values [21]. In addition, the glucose oxidation rate improves in septic patients after treatment with prostacyclin [62]. Apparently, the microcirculation in sepsis fails to support adequate tissue oxygenation. Optimizing DO2 can result in lower mortality rates, especially when therapy is started without delay [63, 64]. Others, however, showed comparable mortality rates [29] or even a higher hospital mortality [65] in septic shock patients whose treatment sought to increase DO2. In these studies, oxygen supply to the tissues was increased by manipulating macrohemodynamic endpoints such as cardiac output, hemoglobin, and central venous pressure and/or pulmonary artery wedge pressure. Radermacher and colleagues [66] treated septic shock patients with prostacyclin when no further increase in DO2 could be obtained by volume resuscitation and dobutamine infusion. Gastric pHi improved after starting prostacyclin, suggesting an increase in splanchnic blood flow.
These findings led us to propose that the addition of systemic NO to adequately volume resuscitated patients with septic shock results in an improvement of microcirculatory perfusion. In a small observational study in septic shock patients, we were indeed able to show an improvement in sublingual microcirculatory perfusion after the injection of 0.5 mg of nitroglycerin [46]. The observation of capillary shutdown next to sustained flow in the larger vessels corroborates the shunting theory of sepsis. Upon the administration of nitroglycerin, microcirculatory flow increased not only in large microvessels but also in small microvessels. The latter finding argues against NO donation's inducing even more shunting flow. All patients except one, owing to late cerebral hemorrhage, were discharged from the hospital alive. This suggests that one can actively open up the microcirculatory network and keep it open by volume and vasodilator therapy. One might argue that oxygen consumption increases with a concurrent increase in DO2 under nitrate administration [67]. However, concentrations of nitrate/nitrite seem to be increased in septic shock patients anyway [68]. We administered 1 mg/kg dexamethasone intravenously to all our patients at admission, which might well have attenuated the production of NO by inhibiting excessive activation of inducible NOS. With this background, a controlled opening strategy using NO donors might be a rational approach. Further studies should demonstrate whether this line of thought regarding therapy in sepsis can be guided by microcirculatory flow patterns and might result in a better outcome.
Future aspects
Therapy in shock should be aimed at the optimization of cardiac function, arterial hemoglobin saturation, and tissue perfusion. This will mean the correction of hypovolemia and the restoration of an evenly distributed microcirculatory flow and inadequate oxygen transport. How can the latter goals in particular be accomplished? Discussions about the role of vasodilators, particularly NO, in sepsis with microcirculatory disturbance will continue. Will the optimization of sublingual microcirculation become a novel resuscitation endpoint? Do we need to take mitochondrial function and tissue respiration into account [69]? Or should we use an integrative approach incorporating both macrocirculatory and microcirculatory hemodynamic data, as proposed in Table 1? Several tools will become available for improving the assessment of regional oxygen demands in critical illness. This will create new challenges for the clinician to improve bedside critical care and optimization of microcirculatory perfusion, thus preventing the further deterioration of organ function and keeping the old principle of primum non nocere alive.
Abbreviations
- DO:
-
oxygen delivery
- IVM:
-
intravital microscopy
- MODS:
-
multiple organ dysfunction syndrome
- NO:
-
nitric oxide
- NOS:
-
NO synthase
- OPS:
-
orthogonal polarization spectral
- pCO:
-
partial pressure of CO2
- SIRS:
-
systemic inflammatory response syndrome
- SvO:
-
mixed venous oxygen saturation
- VO:
-
oxygen consumption.
References
Carley S, Driscoll P: Trauma education. Resuscitation 2001, 48: 47-56. 10.1016/S0300-9572(00)00317-8
Vincent JL: Hemodynamic support in septic shock. Intensive Care Med 2001,27(Suppl 1):S80-S92.
Bone RC: The pathogenesis of sepsis. Ann Intern Med 1991, 115: 457-469.
Fink M: Cytopathic hypoxia in sepsis. Acta Anaesthesiol Scand Suppl 1997, 110: 87-95.
Harvey W: Exercitatio Anatomica de Motu Cordis et Sanguinis in Animalibus 1628.
Malpighi M: Opera Omnia 1687.
Lehr HA, Bittinger F, Kirkpatrick CJ: Microcirculatory dysfunction in sepsis: a pathogenetic basis for therapy? J Pathol 2000, 190: 373-386. 10.1002/(SICI)1096-9896(200002)190:3<373::AID-PATH593>3.3.CO;2-V
Astiz ME, DeGent GE, Lin RY, Rackow EC: Microvascular function and rheologic changes in hyperdynamic sepsis. Crit Care Med 1995, 23: 265-271. 10.1097/00003246-199502000-00011
Linderkamp O, Ruef P, Brenner B, Gulbins E, Lang F: Passive deformability of mature, immature, and active neutrophils in healthy and septicemic neonates. Pediatr Res 1998, 44: 946-950.
Diaz NL, Finol HJ, Torres SH, Zambrano CI, Adjounian H: Histochemical and ultrastructural study of skeletal muscle in patients with sepsis and multiple organ failure syndrome (MOFS). Histol Histopathol 1998, 13: 121-128.
Avontuur JA, Bruining HA, Ince C: Nitric oxide causes dysfunction of coronary autoregulation in endotoxemic rats. Cardiovasc Res 1997, 35: 368-376. 10.1016/S0008-6363(97)00132-6
Cronenwett JL, Lindenauer SM: Direct measurement of arteriovenous anastomotic blood flow in the septic canine hindlimb. Surgery 1979, 85: 275-282.
Ince C: Microcirculatory weak units – an alternative explanation. Crit Care Med 2000, 28: 3127-3129.
Ince C, Sinaasappel M: Microcirculatory oxygenation and shunting in sepsis and shock. Crit Care Med 1999, 27: 1369-1377. 10.1097/00003246-199907000-00031
Buwalda M, Ince C: Opening the microcirculation: can vasodilators be useful in sepsis? Intensive Care Med 2002, 28: 1208-1217. 10.1007/s00134-002-1407-2
Lam C, Tyml K, Martin C, Sibbald W: Microvascular perfusion is impaired in a rat model of normotensive sepsis. J Clin Invest 1994, 94: 2077-2083.
Sinaasappel M, van Iterson M, Ince C: Microvascular oxygen pressure in the pig intestine during haemorrhagic shock and resuscitation. J Physiol 1999, 514: 245-253. 10.1111/j.1469-7793.1999.245af.x
van Iterson M, Sinaasappel M, Burhop K, Trouwborst A, Ince C: Low-volume resuscitation with a hemoglobin-based oxygen carrier after hemorrhage improves gut microvascular oxygenation in swine. J Lab Clin Med 1998, 132: 421-431. 10.1016/S0022-2143(98)90113-5
Erdmann E: The effect of positive inotropes on the failing human myocardium. Cardiology 1997,88(Suppl 2):7-11.
Zhang H, Rogiers P, Smail N, Cabral A, Preiser JC, Peny MO, Vincent JL: Effects of nitric oxide on blood flow distribution and O2 extraction capabilities during endotoxic shock. J Appl Physiol 1997, 83: 1164-1173.
Siegemund M, van Bommel J, Ince C: Influence of NO donor SIN-1 on the gut oxygenation in a normodynamic, porcine model of low-dose endotoxaemia. Intensive Care Med 2000, 26: S362.
Lush CW, Kvietys PR: Microvascular dysfunction in sepsis. Microcirculation 2000, 7: 83-101. 10.1038/sj.mn.7300096
Cain SM, Curtis SE: Experimental models of pathologic oxygen supply dependency. Crit Care Med 1991, 19: 603-612.
Nelson DP, Samsel RW, Wood LDH, Schumacker PT: Experimental models of pathologic oxygen supply dependency. J Appl Physiol 1988, 64: 2410-2419.
Vallet B, Lund N, Curtis SE, Kelly D, Cain SM: Gut and muscle tissue pO 2 in endotoxemic dogs during shock and resuscitation. J Appl Physiol 1994, 76: 793-800.
Bakker J, Coffernils M, Leon M, Gris P, Vincent JL: Blood lactate levels are superior to oxygen-derived variables in predicting outcome in human septic shock. Chest 1991, 99: 956-962.
De Backer D: Lactic acidosis. Intensive Care Med 2003, 29: 699-702.
Hayes MA, Timmins AC, Yau EH, Palazzo M, Hinds CJ, Watson D: Elevation of systemic oxygen delivery in the treatment of critically ill patients. N Engl J Med 1994, 330: 1717-1722. 10.1056/NEJM199406163302404
Gattinoni L, Brazzi L, Pelosi P, Latini R, Tognoni G, Pesenti A, Fumagalli R: A trial of goal-oriented hemodynamic therapy in critically ill patients. SvO2 Collaborative Group. N Engl J Med 1995, 333: 1025-1032. 10.1056/NEJM199510193331601
Creteur J, De Backer D, Vincent JL: A dobutamine test can disclose hepatosplanchnic hypoperfusion in septic patients. Am J Respir Crit Care Med 1999, 160: 839-845.
Kaplan LJ, McPartland K, Santora TA, Trooskin SZ: Start with a subjective assessment of skin temperature to identify hypoperfusion in intensive care unit patients. J Trauma 2001, 50: 620-627.
Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, Peterson E, Tomlanovich M: Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med 2001, 345: 1368-1377. 10.1056/NEJMoa010307
Fiddian-Green RG, Baker S: Predictive value of the stomach wall pH for complications after cardiac operations: comparison with other monitoring. Crit Care Med 1987, 15: 153-156.
Lowes BD, Tsvetkova T, Eichhorn EJ, Gilbert EM, Bristow MR: Milrinone versus dobutamine in heart failure subjects treated chronically with carvedilol. Int J Cardiol 2001, 81: 141-149. 10.1016/S0167-5273(01)00520-4
Vallet B, Ince C: Noninvasive assessment of tissue oxygenation. Semin Respir Crit Care Med 1999, 20: 3-10.
Marik PE: Sublingual capnography: a clinical validation study. Chest 2001, 120: 923-927. 10.1378/chest.120.3.923
Marik PE, Bankov A: Sublingual capnometry versus traditional markers of tissue oxygenation in critically ill patients. Crit Care Med 2003, 31: 818-822. 10.1097/01.CCM.0000054862.74829.EA
Saetzler RK, Jallo J, Lehr HA, Philips CM, Vasthare U, Arfors KE, Tuma RF: Intravital fluorescence microscopy: impact of light-induced phototoxicity on adhesion of fluorescently labeled leukocytes. J Histochem Cytochem 1997, 45: 505-513.
Steinbauer M, Harris AG, Abels C, Messmer K: Characterization and prevention of phototoxic effects in intravital fluorescence microscopy in the hamster dorsal skinfold model. Langenbecks Arch Surg 2000, 385: 290-298. 10.1007/s004239900108
Groner W, Winkelman JW, Harris AG, Ince C, Bouma GJ, Messmer K, Nadeau RG: Orthogonal polarization spectral imaging: a new method for study of the microcirculation. Nat Med 1999, 5: 1209-1212. 10.1038/13529
Mathura KR, Alic L, Ince C: Initial clinical experience with OPS imaging for observation of the human microcirculation. In Yearbook of Intensive Care and Emergency Medicine (Edited by: Vincent JL). Berlin: Springer Verlag 2001, 233-245.
Mathura KR, Vollebregt KC, Boer K, De Graaff JC, Ubbink DT, Ince C: Comparison of OPS imaging and conventional capillary microscopy to study the human microcirculation. J Appl Physiol 2001, 91: 74-78.
Liu L, Zhao SP: The changes of circulating tumor necrosis factor levels in patients with congestive heart failure influenced by therapy. Int J Cardiol 1999, 69: 77-82. 10.1016/S0167-5273(99)00008-X
Robin ED: Of men and mitochondria: coping with hypoxic dysoxia. The 1980 J Burns Amberson Lecture. Am Rev Respir Dis 1980, 122: 517-531.
De Backer D, Creteur J, Preiser JC, Dubois MJ, Vincent JL: Microvascular blood flow is altered in patients with sepsis. Am J Respir Crit Care Med 2002, 166: 98-104. 10.1164/rccm.200109-016OC
Spronk PE, Ince C, Gardien MJ, Mathura KR, Oudemans-van Straaten HM, Zandstra DF: Nitroglycerin in septic shock after intravascular volume resuscitation. Lancet 2002, 360: 1395-1396. 10.1016/S0140-6736(02)11393-6
Baskurt OK, Temiz A, Meiselman HJ: Red blood cell aggregation in experimental sepsis. J Lab Clin Med 1997, 130: 183-190. 10.1016/S0022-2143(97)90094-9
Siegemund M, Racovitza I, Ince C: The rationale for vasodilator therapy in sepsis. In Yearbook of Intensive Care and Emergency Medicine (Edited by: Vincent JL). Berlin: Springer Verlag 2002, 221-231.
Piagnerelli M, Boudjeltia KZ, Vanhaeverbeek M, Vincent JL: Red blood cell rheology in sepsis. Intensive Care Med 2003, 29: 1052-1061. 10.1007/s00134-003-1783-2
Bateman RM, Sharpe MD, Ellis CG: Bench-to-bedside review: microvascular dysfunction in sepsis – hemodynamics, oxygen transport, and nitric oxide. Crit Care 2003, 7: 359-373. 10.1186/cc2353
Zhao KS, Junker D, Delano FA, Zweifach BW: Microvascular adjustments during irreversible hemorrhagic shock in rat skeletal muscle. Microvasc Res 1985, 30: 143-153. 10.1016/0026-2862(85)90046-9
Bihari D, Smithies M, Gimson A, Tinker J: The effects of vasodilation with prostacyclin on oxygen delivery and uptake in critically ill patients. N Engl J Med 1987, 317: 397-403.
Dubois MJ, De Backer D, Creteur J, Anane S, Vincent JL: Effect of vasopressin on sublingual microcirculation in a patient with distributive shock. Intensive Care Med 2003, 29: 1020-1023.
Vallance P: Exploring vascular nitric oxide in health and disease. The Goulstonian Lecture 1996. J R Coll Physicians Lond 1997, 31: 321-327.
Wang P, Ba ZF, Chaudry IH: Endothelium-dependent relaxation is depressed at the macro- and microcirculatory levels during sepsis. Am J Physiol 1995, 269: R988-R994.
Reade MC, Young D, Boyd CAR: Nitric oxide synthases are decreased in mononuclear cells from sepsis patients. Crit Care Med 2003, 31: A52.
Lopez A, Lorente JA, Steingrub J, Bakker J, McLuckie A, Willatts S, Brockway M, Anzueto A, Holzapfel L, Breen D, Silverman MS, Takala J, Donaldson J, Arneson C, Grove G, Grossman S, Grover R: Multiple-center, randomized, placebo-controlled, double-blind study of the nitric oxide synthase inhibitor 546C88: effect on survival in patients with septic shock. Crit Care Med 2004, 32: 21-30. 10.1097/01.CCM.0000105581.01815.C6
Grover R, Lopez A, Lorente JA, Grossman S: Multicenter, randomized, placebo controlled, double-blind study of the nitric oxide synthase inhibitor 546C88: effect on survival in patients with septic shock. Crit Care Med 1999,27(Suppl):A33. 10.1097/00003246-199901001-00021
Vincent JL, Zhang H, Szabo C, Preiser JC: Effects of nitric oxide in septic shock. Am J Respir Crit Care Med 2000, 161: 1781-1785.
Pittner A, Nalos M, Asfar P, Yang Y, Ince C, Georgieff M, Bruckner UB, Radermacher P, Froba G: Mechanisms of inducible nitric oxide synthase (iNOS) inhibition-related improvement of gut mucosal acidosis during hyperdynamic porcine endo-toxemia. Intensive Care Med 2003, 29: 312-316.
Li H, Förstermann U: Nitric oxide in the pathogenesis of vascular disease. J Pathol 2000, 190: 244-254. 10.1002/(SICI)1096-9896(200002)190:3<244::AID-PATH575>3.0.CO;2-8
Siostrzonek P, Koreny M, Delle-Karth G, Haumer M, Koller-Strametz J, Heinz G: Milrinone therapy in catecholamine-dependent critically ill patients with heart failure. Acta Anaesthesiol Scand 2000, 44: 403-409. 10.1034/j.1399-6576.2000.440408.x
Tuchschmidt J, Fried J, Astiz M, Rackow E: Elevation of cardiac output and oxygen delivery improves outcome in septic shock. Chest 1992, 102: 216-220.
Milani RV, Mehra MR, Endres S, Eigler A, Cooper ES, Lavie CJ Jr, Ventura HO: The clinical relevance of circulating tumor necrosis factor-alpha in acute decompensated chronic heart failure without cachexia. Chest 1996, 110: 992-995.
Hayes MA, Yau EH, Timmins AC, Hinds CJ, Watson D: Response of critically ill patients to treatment aimed at achieving supra-normal oxygen delivery and consumption. Relationship to outcome. Chest 1993, 103: 886-895.
Radermacher P, Buhl R, Santak B, Klein M, Kniemeyer HW, Becker H, Tarnow J: The effects of prostacyclin on gastric intramucosal pH in patients with septic shock. Intensive Care Med 1995, 21: 414-421.
Cerra FB, Hassett J, Siegel JH: Vasodilator therapy in clinical sepsis with low output syndrome. J Surg Res 1978, 25: 180-183. 10.1016/0022-4804(78)90074-4
Groeneveld AB, Hartemink KJ, de Groot MC, Visser J, Thijs LG: Circulating endothelin and nitrate-nitrite relate to hemodynamic and metabolic variables in human septic shock. Shock 1999, 11: 160-166.
Brealey D, Brand M, Hargreaves I, Heales S, Land J, Smolenski R, Davies NA, Cooper CE, Singer M: Association between mitochondrial dysfunction and severity and outcome of septic shock. Lancet 2002, 360: 219-223. 10.1016/S0140-6736(02)09459-X
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
None declared.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
About this article
Cite this article
Spronk, P.E., Zandstra, D.F. & Ince, C. Bench-to-bedside review: Sepsis is a disease of the microcirculation. Crit Care 8, 462 (2004). https://doi.org/10.1186/cc2894
Published:
DOI: https://doi.org/10.1186/cc2894