Abstract
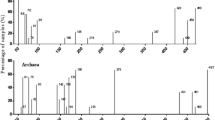
Plant-microorganism interaction in the rhizosphere is important for nutrient cycling, carbon sequestration in natural ecosystems, contaminant elimination and ecosystem functioning. Abundance of microbial communities and variation in species composition can be an imperative determinant of phytoremediation capability. In the present study we have assessed the bacterial community structure in the rhizoplane of wetland plants, Acorus calamus, Typha latifolia, and Phragmites karka using Terminal restriction fragment length polymorphism technique. The most dominant phylum, in the plants under study, was phylum Firmicutes, followed by Proteobacteria and Actinobacteria. Bacterial groups belonging to phylum Chloroflexi, Acidobacteria, Deferribacteres and Thermotogae also showed their presence in P. karka and T. latifolia but were absent in A. calamus. Diversity indices of bacterial community were assessed. The results of this study show the presence of bacterial phyla which play an important role in bioremediation of contaminants. Thus these plants can be used as potential candidates of phytoremediation.



Similar content being viewed by others
References
Abed RMM, Al-Kharusi S, Gkorezis P, Prigent P, Headley T (2018) Bacterial communities in the rhizosphere of Phragmites australis from an oil-polluted wetland. Arch Agron Soil Sci 64(3):360–370
Alvarez A, Saez JM, Costa JSD, Colin VL, Fuentes MS, Cuozzo SA, Benimeli CS, Poltri MA, Amoroso MJ (2017) Actinobacteria: current research and perspectives for bioremediation of pesticides and heavy metals. Chemosphere 166:41–62
Arroyo P, Ansola G, Blanco I, Molleda P, de Luis Calabuig E, de Miera LES (2010) Comparative analysis of the composition of bacterial communities from two constructed wetlands for municipal and swine wastewater treatment. J Water Health 8(1):147–157
Barns SM, Cain EC, Sommerville L, Kuske CR (2007) Acidobacteria phylum sequences in uranium-contaminated subsurface sediments greatly expand the known diversity within the phylum. Appl Environ Microbiol 73(9):3113–3116
Baz SE (2017) Bioremediation of heavy metals by actinobacteria: review. Am J Innov Res Appl Sci 5(5):359–369
Behera P, Mohapatra M, Adhya TK, Suar M, Pattnaik AK, Rastogi G (2018) Structural and metabolic diversity of rhizosphere microbial communities of Phragmites karka in a tropical coastal lagoon. Appl Soil Ecol 125:202–212
Borsodi AK, Vladár P, Cech G, Gedeon G, Beszteri B, Micsinai A, Reskone N, Márialigeti K (2003) Bacterial activities in the sediment of Lake Velencei, Hungary. Hydrobiologia 506(1):721–728
Borsodi AK, Rusznyák A, Molnár P, Vladár P, Reskóné MN, Tóth EM, Sipos R, Gedeon G, Márialigeti K (2007) Metabolic activity and phylogenetic diversity of reed (Phragmites australis) periphyton bacterial communities in a Hungarian shallow soda lake. Microb Ecol 53(4):612–620
Calheiros CS, Duque AF, Moura A, Henriques IS, Correia A, Rangel AO, Castro PM (2009) Changes in the bacterial community structure in two-stage constructed wetlands with different plants for industrial wastewater treatment. Bioresour Technol 100(13):3228–3235
Chaparro JM, Badri DV, Vivanco JM (2014) Rhizosphere microbiome assemblage is affected by plant development. ISME J 8(4):790–803
Chen S, Luo J, Hu M, Lai K, Geng P, Huang H (2012b) Enhancement of cypermethrin degradation by a coculture of Bacillus cereus ZH-3 and Streptomyces aureus HP-S-01. Bioresour Technol 110:97–104
Daubin V, Moran NA, Ochman H (2003) Phylogenetics and the cohesion of bacterial genomes. Science 301(5634):829–832
Doolotkeldieva T, Konurbaeva M, Bobusheva S (2017) Microbial communities in pesticide-contaminated soils in Kyrgyzstan and bioremediation possibilities. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-017-0048-5
Evans TN, Watson G, Rees GN, Seviour RJ (2014) Comparing activated sludge fungal community population diversity using denaturing gradient gel electrophoresis and terminal restriction fragment length polymorphism. Antonie Van Leeuwenhoek 105(3):559–569
Fauziah SH, Jayanthi B, Emenike CU, Agamuthu (2017) Remediation of heavy metal contaminated soil using potential microbes isolated from a closed disposal site. Int J Biosci Biochem Bioinform 7(4):230–237
Fawzy MA, Mohamed AKSH (2017) Bioremediation of heavy metals from municipal sewage by cyanobacteria and its effects on growth and some metabolites of Beta vulgaris. J Plant Nutr 40(18):2550–2561
Fiedler HP, Bruntner C, Bull AT, Ward AC, Goodfellow M, Potterat O, Puder C, Mihm G (2005) Marine actinomycetes as a source of novel secondary metabolites. Antonie Van Leeuwenhoek 87(1):37–42
Gupta RK, Choudhary KK, Kumar M, Negi A, Rai H (2012) Bioremediation and cyanobacteria an overview. Bionano Front 9:190–196
Hugenholtz P, Goebel BM, Pace NR (1998) Impact of culture-independent studies on the emerging phylogenetic view of bacterial diversity. J Bacteriol 180(18):4765–4774
Hussain F, Mustufa G, Zia R, Faiq A, Matloob M, Shah H, Ali WR, Irfan JA (2018) Constructed wetlands and their role in remediation of industrial effluents via plant-microbe interaction—a mini review. J Bioremediat Biodegrad 9(4):1–8
James A, Singh DK, Khankhane PJ (2018) Enhanced atrazine removal by hydrophyte—bacterium associations and in vitro screening of the isolates for their plant growth-promoting potential. Int J Phytorem 20(2):89–97
Knuteson SL, Wilson PC, Whitwell T, Klaine SJ (2000) Constructed wetlands using ornamental plants to remediate golf course pesticides. SNA Res Conf 45:375
Kumar A, Bhoot N, Soni I, John PJ (2014) Isolation and characterization of a Bacillus subtilis strain that degrades endosulfan and endosulfan sulfate. 3 Biotech 4(5):467–475
Li YH, Zhu JN, Liu QF, Liu Y, Liu M, Liu L, Zhang Q (2013) Comparison of the diversity of root-associated bacteria in Phragmites australis and Typha angustifolia L. in artificial wetlands. W J Microbiol Biotechnol 29(8):1499–1508
Liu WT, Marsh TL, Cheng H, Forney LJ (1997) Characterization of microbial diversity by determining terminal restriction fragment length polymorphisms of genes encoding 16S rRNA. Appl Environ Microbiol 63(11):4516–4522
Margalef R (1958) Information theory in ecology. Gen Syst 3:36–71
Nithya C, Pandian SK (2012) Evaluation of bacterial diversity in Palk Bay sediments using terminal-restriction fragment length polymorphisms (T-RFLP). Appl Biochem Biotechnol 167(6):1763–1777
Nongkhlaw FMW, Joshi SR (2014) Distribution pattern analysis of epiphytic bacteria on ethnomedicinal plant surfaces: a micrographical and molecular approach. J Microsc Ultrastruct 2(1):34–40
Pandey VC, Prakash P, Bajpai O, Kumar A, Singh N (2015) Phytodiversity on fly ash deposits: evaluation of naturally colonized species for sustainable phytorestoration. Environ Sci Pollut Res 22(4):2776–2787
Pielou EC (1966) The measurement of diversity in different types of biological collections. J Theor Biol 13:131–144
Richter-Heitmann T, Eickhorst T, Knauth S, Friedrich MW, Schmidt H (2016) Evaluation of strategies to separate root-associated microbial communities: a crucial choice in rhizobiome research. Front Microbiol 7:773
Sarkar J, Kazy SK, Gupta A, Dutta A, Mohapatra B, Roy A, Bera P, Mitra A, Sar P (2016) Biostimulation of indigenous microbial community for bioremediation of petroleum refinery sludge. Front Microbiol 7:1407
Shannon CE, Weaver W (1963) The measurement theory of communication. University of Illinois Press, Urbana
Simpson EH (1949) Measurement of diversity. Nature 163:688
Tanaka Y, Tamaki H, Matsuzawa H, Nigaya M, Mori K, Kamagata Y (2012) Microbial community analysis in the roots of aquatic plants and isolation of novel microbes including an organism of the candidate phylum OP10. Microbes Environ 27(2):149–157
Ter Braak CJ (1986) Canonical correspondence analysis: a new eigenvector technique for multivariate direct gradient analysis. Ecology 67(5):1167–1179
Vik U, Logares R, Blaalid R, Halvorsen R, Carlsen T, Bakke I, Kolsto A-B, Okstad OA, Kauserud H (2013) Different bacterial communities in ectomycorrhizae and surrounding soil. Sci Rep 3:3471
Vladár P, Rusznyák A, Márialigeti K, Borsodi AK (2008) Diversity of sulfate-reducing bacteria inhabiting the rhizosphere of Phragmites australis in Lake Velencei (Hungary) revealed by a combined cultivation-based and molecular approach. Microb Ecol 56(1):64–75
Wilson PC, Whitwell T, Klaine SJ (2000) Phytotoxicity, uptake, and distribution of 14C-simazine in Acorus gramenius and Pontederia cordata. Weed Sci 48(6):701–709
Yan Z, Jiang H, Cai H, Zhou Y, Krumholz LR (2015) Complex interactions between the macrophyte Acorus calamus and microbial fuel cells during pyrene and benzo [a] pyrene degradation in sediments. Sci Rep 5:10709
Yang A, Liu N, Tian Q, Bai W, Williams M, Wang Q, Li LH, Zhang WH (2015) Rhizosphere bacterial communities of dominant steppe plants shift in response to a gradient of simulated nitrogen deposition. Front Microbiol 6:789
Zehra A, Khan MA (2007) Comparative effect of NaCl and sea salt on germination of halophytic grass Phragmites karka at different temperature regimes. Pak J Bot 39(5):1681–1694
Zhang B, Zhang P, Chen X (2000) Factors affecting colonization of introduced microorganisms on plant roots. Ying yong sheng tai xue bao = J Appl Ecol/Zhongguo sheng tai xue xue hui, Zhongguo ke xue yuan Shenyang ying yong sheng tai yan jiu suo zhu ban 11(6):951–953
Acknowledgements
We gratefully acknowledge financial support by Indian Council of Agricultural Research, New Delhi, India, as research project entitled “Bioremediation of contaminants in polluted sites: Use of weedy plants” NFBSFARA/ WQ-3032/2013-14.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Singh, T., Singh, D.K. Assessing the Bacterial Community Structure in the Rhizoplane of Wetland Plants. Bull Environ Contam Toxicol 101, 521–526 (2018). https://doi.org/10.1007/s00128-018-2426-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-018-2426-1