Abstract
Diabetes in pregnancy affects 20 million women per year and is associated with increased risk of obesity in offspring, leading to insulin resistance and cardiometabolic disease. Despite the substantial public health ramifications, relatively little is known about the pathophysiological mechanisms underlying obesity in these high-risk children, which creates a barrier to successful intervention. While maternal glucose itself is undeniably a major stimulus upon intrauterine growth, the degree of offspring hyperinsulinism and disturbed lipid metabolism in mothers and offspring are also likely to be implicated in the disease process. The aim of this review is to summarise current understanding of the pathophysiology of childhood obesity after intrauterine exposure to maternal hyperglycaemia and to highlight possible opportunities for intervention. I present here a new unified hypothesis for the pathophysiology of childhood obesity in infants born to mothers with diabetes, which involves self-perpetuating twin cycles of pancreatic beta cell hyperfunction and altered lipid metabolism, both acutely and chronically upregulated by intrauterine exposure to maternal hyperglycaemia.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.

Introduction
Diabetes in pregnancy affects one in six pregnancies internationally and is associated with short-term and long-term health sequelae for both mother and child [1, 2]. Infants born to mothers with diabetes are commonly large-for-gestational-age at birth and have a higher risk of obesity in childhood [2, 3]. Obesity in childhood has many deleterious consequences upon future cardiometabolic health, including early-onset insulin resistance [4] and type 2 diabetes [5]. Rapid childhood growth is also associated with increased risk of type 1 diabetes [6]. The early development of obesity in children with existing environmental and genetic susceptibilities to diabetes should be a major public health concern [7].
Unfortunately, very few interventions with proven effectiveness are available to reduce the risk of obesity in high-risk children [5]. Barriers to successful intervention include knowledge gaps about the mechanisms of disease, the optimal timing for effective action and the most suitable short-term or long-term measures to modify the disease process. The aim of this review is to summarise current understanding of the pathophysiology of childhood obesity after intrauterine exposure to maternal hyperglycaemia and to highlight possible opportunities for intervention.
Childhood obesity after diabetes in pregnancy: the scale of the problem
There is a growing consensus that the risk of obesity (BMI ≥95th centile for age [5]) is elevated in offspring exposed to gestational diabetes, type 1 diabetes or type 2 diabetes in utero [8, 9], although the data remain controversial [10, 11]. Many studies are limited by incomplete adjustment for important confounding factors such as maternal obesity or socioeconomic status, insufficient sample size or inadequate duration of follow-up. Maternal obesity is a particularly important confounding factor and is likely to exert effects both genetically and environmentally upon intrauterine development and postnatal lifestyle and behaviour. A recent metanalysis identified that maternal obesity was associated with offspring overweight or obesity in early-, mid- and late childhood with odds ratios (OR) of 2.43, 3.12 and 4.47, respectively [12].
Despite these limitations, there is consistent evidence of an association between maternal diabetes and child obesity, described in diverse populations including from China [13], Denmark [14], Sweden [15], Turkey [16], South Korea [17] and in the multi-ethnic Hyperglycemia and Adverse Pregnancy Outcomes (HAPO) cohort [10]. There is also emerging evidence of a dose-dependent effect of maternal hyperglycaemia upon offspring obesity, with risk increasing proportionately with maternal glucose concentrations in pre-gestational and gestational diabetes [11, 14]. This dose-dependent effect is also evident in pregnancies with lower levels of hyperglycaemia in pregnancy, below the diagnostic thresholds for gestational diabetes [18, 19], with elevated obesity rates in affected offspring, even after adjustment for maternal BMI. A sibling study suggests that the effect of maternal hyperglycaemia upon offspring obesity risk is likely to be primarily a developmental effect [15].
Childhood obesity after diabetes in pregnancy: body composition
The importance of developmental effects upon obesity risk suggests that large-for-gestational-age and childhood obesity may be two manifestations of the same mechanistic process. Size at birth is an important determinant of adiposity in adults [20, 21], and large-for-gestational-age is associated with a twofold increase in the rate of obesity in childhood [9, 22]. Excessive growth postnatally (not unique to diabetes in pregnancy) also contributes to child obesity [23].
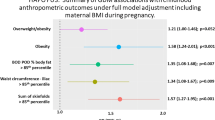
Although increases in child BMI are evident, it is unclear how this relates to body composition. BMI has limitations as a marker of adiposity in children, especially during active growth. The risk of childhood adiposity after gestational diabetes was assessed in over 4000 children aged 10 to 14 years old from the HAPO cohort [2, 24, 25]. Associations were identified between gestational diabetes and child obesity, percentage body fat and sum of skinfold thickness, independent of maternal BMI [2]. These data suggest that the increase in child BMI represents a true change in body composition, characterised by increased fat mass.
Questions still remain about fat distribution and type (white, brown and beige adipose cells) after exposure to intrauterine hyperglycaemia. Data from the multi-ethnic Growing Up in Singapore Towards healthy Outcomes (GUSTO) cohort suggest that the increased abdominal circumference seen in hyperglycaemia-exposed neonates is due to increased deep and subcutaneous adipose tissue, with increases in liver fat [26]. Santos and colleagues found no association between maternal diabetes and infant fat distribution using anthropometry [27] (Generation XXI, Portugal; n=4747). However, rapid fetal growth, a key characteristic of intrauterine exposure to maternal diabetes [28], has been associated with specific increases in central fat accretion [29] and linked to future risk of liver steatosis [30].
Childhood obesity after diabetes in pregnancy: does the type of diabetes matter?
Although type 1, type 2 and gestational diabetes have distinct mechanistic causes, they all demonstrate similar associations with childhood obesity, broadly proportionate to the severity of hyperglycaemia in pregnancy [11, 14, 18, 19]. This suggests that there is a common pathway to childhood obesity, regardless of diabetes aetiology. Offspring of mothers with type 2 diabetes in pregnancy or gestational diabetes may be exposed to less severe hyperglycaemia but may have additional genetic and socioeconomic factors which predispose to obesity.
Part 1: potential mechanisms
Maternal glucose homeostasis and offspring body size and composition
Maternal glucose is the main fuel substrate for both fetus and placenta during a healthy pregnancy [31], with a direct effect upon offspring intrauterine growth in gestational diabetes, type 1 diabetes and type 2 diabetes [32]. We previously demonstrated strong associations between birthweight and maternal hyperglycaemia, assessed using continuous glucose monitoring metrics or biochemical measures [33].
The Pedersen hypothesis from 1952 states that a fetus will develop hyperinsulinism in response to maternal hyperglycaemia, as maternal glucose can travel freely across the placenta, while maternal insulin cannot [34]. Abundant glucose from the maternal circulation and abundant insulin from the fetal pancreas produce an environment with enhanced glycolysis and plentiful cellular energy for fetal growth.
Maternal lipid homeostasis and offspring body size and composition
While established data highlight the importance of maternal glucose, not maternal lipids, in the development of offspring adipose tissue [35], there is emerging evidence that maternal hyperglycaemia may also directly or indirectly increase offspring adiposity through altered lipid metabolism. Using the UK Pregnancies Better Eating and Activity Trial (UPBEAT) cohort, we recently identified that lipid species associated with de novo lipogenesis (species containing fatty acids 16:0, 16:1, 18:0 and 18:1) were elevated in mothers with gestational diabetes, and directly associated with offspring adiposity (abdominal circumference) independently of maternal hyperglycaemia [36]. These findings were further supported by evidence in women with type 1 diabetes, which demonstrated strong associations between offspring skinfold sum and lipid species in maternal serum, independently of maternal glucose [37]. The strongest independent associations were identified with species including fatty acids 16:0 or 18:1, consistent with increased or upregulated de novo lipogenesis. A mediation analysis suggested that maternal lipids are important but do not solely mediate the relationship between maternal hyperglycaemia and offspring adiposity [37].
Recent work has identified a reduction in brown adipose tissue (BAT) in mothers with gestational diabetes, with reduction in concentrations of BAT-derived adipokines, neuregulin-4 [38, 39] and angiopoietin-like protein 8 (ANGPTL8) [40]. Although these data merit further investigation, the significance of maternal or offspring BAT upon offspring obesity risk in childhood remains unclear.
The fetal response to maternal hyperglycaemia: offspring insulin secretion
Hyperinsulinism, or augmented fetal beta cell function, is undoubtedly a key pathophysiological mechanism in diabetes in pregnancy and directly associated with many clinical sequelae, including neonatal hypoglycaemia and large-for-gestational age [34, 41]. However, it is difficult to study potential genetic and environmental variations in the fetal response to maternal hyperglycaemia because a meaningful assessment of fetal metabolism in humans is so challenging.
Although it is logical to assume that neonatal hyperinsulinism, neonatal hypoglycaemia and large-for-gestational age are closely related sequelae of late pregnancy hyperglycaemia, our recent metabolomics analysis of the Continuous Glucose Monitoring in Women with Type 1 Diabetes in Pregnancy Trial (CONCEPTT) cohort suggests there are important differences in these conditions and in the timing of onset [37]. For example, neonatal hypoglycaemia was associated with marked increases in lipid abundance in maternal blood in the first trimester, suggesting maternal lipolysis (e.g. due to insufficient insulin dosing or energy restriction due to nausea and vomiting in pregnancy). Neonatal hyperinsulinism was also associated with first-trimester changes in maternal metabolites, showing positive associations with phenolic compounds (saccharin, metabolites from phenolic compounds in tea, coffee, chocolate and olives). Taken together, these findings suggest that maternal metabolism and nutrition at the time of fetal pancreatic development may have an important effect upon offspring pancreatic function, but these data require corroboration with other cohorts.
New translational opportunities may arise from a better understanding of the determinants of fetal hyperinsulinism longitudinally during pregnancy, especially if fetal metabolism can be measured and monitored. Recent work in the CONCEPTT cohort identified that pregnancies with the most hyperinsulinaemic offspring had a third-trimester increase in C-peptide in maternal blood, unexpected in women with no evidence of beta cell function at baseline, providing an opportunity to assess C-peptide as a potential biomarker for fetal hyperinsulinism [42], or fragments of C-peptide, insulin or proinsulin [43].
The longer-term significance of neonatal hyperinsulinism upon children’s metabolic health is also unclear, as current neonatal glucose testing stops 24 h after birth. As clinically relevant episodes of hypoglycaemia seem to happen rarely after the first week of life, the increased insulin production either normalises or becomes less clinically evident, through insulin resistance. Counter-regulatory hormones such as cortisol and glucagon are important in the acute response to neonatal hypoglycaemia and may have a role in determining longer-term insulin sensitivity in childhood.
The fetal response to maternal hyperglycaemia: offspring insulin resistance
Several studies have identified associations between maternal diabetes and offspring insulin resistance in childhood and adolescence. Boney and colleagues studied children aged 11 years old and identified that exposure to maternal gestational diabetes was associated with insulin resistance (OR 10.4; 95% CI 1.5, 74.4) [44]. The presence of large-for-gestational-age at birth appeared to have an additive effect upon risk [44]. Similar findings were obtained by Sauder et al, who identified increased insulin resistance (18% increase in HOMA-IR) and increased beta cell function (9% increase in HOMA-B) in 10–16 year old children after exposure to intrauterine hyperglycaemia in the Exploring Perinatal Outcomes among Children (EPOCH) study [4]. This study showed that the relationship between maternal diabetes and offspring insulin resistance was not mediated by offspring BMI. The HAPO follow-up study has corroborated these findings [25, 45]. Maternal glucose concentrations in pregnancy showed inverse linear associations between child insulin sensitivity and maternal pregnancy glucose concentrations in the fasting state and 1 h or 2 h after a glucose load. Importantly, these associations were independent of maternal and child BMI [25, 45].
However, since most studies have included older children and adolescents, it remains unclear if insulin resistance is an early or late feature in the development of obesity in children exposed to intrauterine hyperglycaemia. The optimal method for assessing insulin sensitivity is the hyperinsulinaemic–euglycaemic clamp (reference standard) [46]. Surrogate measures such as the hyperglycaemic clamp, the minimal model of the frequently sampled intravenous glucose tolerance test, oral glucose tolerance test or biomarker combinations are more convenient but still unachievable at scale in infants or very young children [46, 47]. Biomarkers associated with insulin resistance have been identified at birth after exposure to gestational diabetes [48] or after antenatal steroids in individuals with type 1 diabetes in pregnancy [49].
The fetal response to maternal hyperglycaemia: epigenetic influences
Epigenetic changes such as methylation of cytosines in CG dinucleotides (CpG methylation), histone modifications and non-coding RNA may contribute to the effect of the intrauterine environment upon offspring obesity. The Environmental Versus Genetic and Epigenetic Influences on Growth, Metabolism and Cognitive Function in Offspring of Mothers With Type 1 Diabetes (EPICOM) study identified methylation patterns in adolescents exposed to maternal diabetes [50]. Kelstrup and colleagues identified that offspring exposed to gestational diabetes had lower expression of the peroxisome proliferator-activated receptor-γ coactivator-1α (PPARGC1A) in skeletal muscle [51]. Non-coding RNAs such as miRNA and long non-coding RNA (lncRNA) may mediate the effect of maternal diabetes on offspring obesity and pancreatic beta cell dysfunction (reviewed in Saeedi Borujeni et al and Fernandez-Twinn et al [52, 53]). Further progress is this field is limited by technical issues restricting validation across cohorts and the need to adjust for confounders such as maternal obesity [54, 55] and offspring age [56].
The fetal response to paternal hyperglycaemia: social, genetic and epigenetic influences
Fathers with diabetes pass on genetic and epigenetic traits which influence the metabolic health of their offspring, but they also contribute to social cues for diet and health. Sperm quality, sperm motility, DNA integrity, semen composition and the efficacy of the acrosome reaction are all affected by diabetes [57], but many of the exact mechanisms are unclear in humans. Our own previous work identified that the lipid composition of sperm, required for energy and cell membranes, is associated with sperm motility, suggesting that men’s metabolic health is intrinsically important to fertility [58]. Data from animal models suggest that paternal high-fat diet is associated with changes in DNA methylation and miRNA activity in sperm [59], while exercise may have a beneficial effect [60].
A unified hypothesis on the effect of intrauterine exposure to hyperglycaemia upon offspring body composition and metabolism
Although we know that maternal and fetal glucose and lipid homeostasis are mechanistically involved in the regulation of body composition after diabetes in pregnancy, many knowledge gaps have yet to be addressed. For example, how is it possible that exposure to maternal hyperglycaemia for a brief period in utero (e.g. 3 months in gestational diabetes, after the period of organogenesis) can have adverse consequences on health across the life course? In addition, why does neonatal metabolism not return to normal once the stimulus of exposure to maternal hyperglycaemia is removed at birth?
While the first trimester is considered the key window for organogenesis in general, the formation and distribution of fetal adipose tissue, a metabolically active endocrine organ, is not confined to the first trimester (reviewed in Desoye and Herrera [35]). Early fat lobules are identifiable in the human fetus from 14 weeks’ gestation, which gradually increase in size due to increasing triglyceride storage throughout the remainder of gestation [35]. Lipid metabolism undoubtedly occurs throughout pregnancy and is likely to be highly regulated, but there is little information on the exact timing and regulation of key processes such as lipid mobilisation or lipid accretion. The third trimester is a key time for lipid deposition in fetal adipose tissue, as demonstrated by the marked differences in body composition at birth and postnatally in preterm infants [61]. With rising maternal insulin resistance through the second trimester, exposure to maternal hyperglycaemia and fetal hyperinsulinaemia in late pregnancy may contribute to increased de novo lipogenesis (making fatty acids) and adipogenesis (making adipose tissue) in offspring. Increased lipoprotein lipase activity in fetal adipose tissue is a potential mechanism behind the enhanced lipid storage [35].
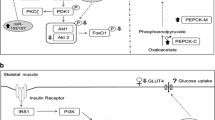
One possible explanation for the persisting effect of pregnancy exposure to maternal diabetes is that, in addition to these short-term acute effects, it initiates subtle, chronic changes in body composition predisposing to obesity in childhood. Mechanistically, this could involve two self-perpetuating cycles affecting lipid metabolism and pancreatic function (Fig. 1). Intrauterine exposure to maternal diabetes may result in acute changes in body composition but may also cause subtle upregulation of biological pathways in childhood which support continued lipogenesis and adipogenesis, fed by ongoing excess insulin secretion and resistance, placing children on a trajectory towards later-life cardiometabolic disease.
A unifying hypothesis for the development of large-for-gestational-age and childhood obesity after exposure to intrauterine hyperglycaemia. Hypothesis: the developmental effects of maternal hyperglycaemia upon childhood obesity are mediated directly or indirectly through altered offspring lipid metabolism/distribution and increased offspring pancreatic function from birth. These factors exert short-term effects upon body composition but also chronically upregulate key pathways in postnatal life (the lipid cycle and pancreatic cycle), resulting in obesity and cardiometabolic disease. This figure is available as a downloadable slide
Part 2: Potential interventions
Babies born to mothers with diabetes often have multiple risk factors for childhood obesity, which appear to have an additive effect upon risk. Effective interventions targeting maternal hyperglycaemia, maternal obesity and offspring health in the preconception, pregnancy and postnatal periods are needed to prevent childhood cardiometabolic disease. Interventions in the preconception, pregnancy and postnatal periods will be considered in turn.
Preconception interventions
Pre-pregnancy BMI is a strong modifiable predictor of both birthweight and future childhood obesity [12]. Since many pregnancies are unplanned, and most women do not have access to individualised health promotion advice, preconception interventions are challenging. Population wide strategies [62] and individual diet and lifestyle interventions are reviewed in detail elsewhere [63, 64].
However, preconception advice is more accessible to women with type 1 or type 2 diabetes in pregnancy, but this focuses on improving blood glucose levels and reducing risks of congenital anomalies, rather than reducing rates of childhood obesity [32, 65]. A UK-wide national audit recently found that only 22% of women with type 2 diabetes and 44% of women with type 1 diabetes were taking folic acid preconception (marker of optimal pre-pregnancy care), suggesting that access to preconception advice is still limited [32].
Pregnancy interventions: addressing hyperglycaemia
Addressing maternal hyperglycaemia through diabetes treatment or prevention may be key to reducing the burden of childhood obesity. Preventing maternal diabetes would be the optimal approach, but there is currently no clear way to prevent gestational diabetes, as trials of lifestyle interventions in pregnancy have had variable results [66, 67]. Effective treatment of gestational diabetes is a helpful contribution to reducing child obesity [19]. The Programming of Enhanced Adiposity Risk in Childhood–Early Screening (PEACHES) study identified that offspring obesity risk was lower in women treated for gestational diabetes, compared with untreated women with hyperglycaemia [19].
Dietary management of gestational diabetes reduces hyperglycaemia in pregnancy and has been associated with lower birthweight, suggesting benefits upon future offspring body composition [68]. A metanalysis demonstrated that women who adhered to one of several dietary approaches to gestational diabetes management had babies 170 g lighter than those who did not follow a specific diet [68]. However, the optimal pregnancy diet to promote good pregnancy outcomes and favourable cardiometabolic health in mothers and offspring is unclear. It is also unknown if dietary strategies for gestational diabetes, type 1 diabetes and type 2 diabetes should be the same. Our RCT, the dietary intervention in gestational diabetes (DiGest) should soon provide some new evidence about the role of maternal energy restriction in short-term and longer-term maternal and offspring outcomes [69].
The role of medication in pregnancy as a means of addressing future child obesity is controversial. Metformin is the most commonly used medication for diabetes in pregnancy, and is economical, convenient and safe. However, a previous meta-analysis identified associations between metformin and accelerated growth postnatally, leading to an increased risk of obesity in childhood, compared with children of women treated with insulin during pregnancy [70]. However, the latest findings on the role of metformin in childhood obesity, from the Metformin in Women with Type 2 diabetes in Pregnancy trial (MITy) follow-up study, have demonstrated no difference in BMI at 2 years of age in children of women who were randomly assigned to receive metformin or placebo during pregnancies affected by type 2 diabetes [71]. While these findings are reassuring, it also suggests that despite treating hyperglycaemia, metformin treatment does not confer any specific benefit upon childhood obesity rates after diabetes in pregnancy. Further follow-up is required to assess the effects of metformin exposure upon BMI in older children.
Novel technologies such as continuous glucose monitoring or closed loop systems have reduced maternal glucose or reduced hypoglycaemic episodes in type 1 diabetes [72, 73] (reviewed in detail [74]). However, technological options are underexplored in type 2 diabetes or gestational diabetes but may improve glycaemic control in the future.
Pregnancy interventions: addressing maternal gestational weight gain
Some weight gain in pregnancy is expected, but excessive gestational weight gain is very common and has repercussions for women’s BMI for 15 years or more after the pregnancy [75]. Landon and colleagues found that gestational weight gain was strongly related to obesity in children aged 5–10 years old [76], with results confirmed by a meta-analysis [77]. Relatively few studies have attempted weight loss in pregnancy, although several observational studies have identified benefits from restricted gestational weight gain [78]. The DiGest study will provide new data in this field soon [69].
Postpartum interventions: addressing maternal obesity
Women with excessive gestational weight gain are more likely to retain weight postpartum, which has a long-term effect on maternal BMI [75]. The importance of postpartum weight loss to improve BMI and reduce risk of type 2 diabetes is an area needing much greater attention. Relatively few interventions have shown efficacy in the postpartum period, but lactation remains an important opportunity to improve glucose tolerance, and possibly BMI, in women postnatally. Gunderson and colleagues identified that breastfeeding intensity and duration in women after gestational diabetes were associated with a lower weight gain trajectory in infants [79] and reduced de novo lipogenesis activity in mothers and offspring, downregulating a crucial step in the disease process (Fig. 1). Recent work by Ma and colleagues, in 12 countries internationally, identified that longer breastfeeding duration was associated with reduced risk of obesity in children aged 9–11 years [80].
Internationally, high parity and short inter-pregnancy intervals (<12 months) are substantial contributors to obesity in women. One study in the USA demonstrated an increased risk of obesity in 3422 multiparous women with short inter-pregnancy intervals [81]. Another study has identified a significant effect of parity upon obesity, due in part to cumulative effects of excessive gestational weight gain [82]. Although there is limited evidence on effective interventions, education of women and their partners about reproductive health with improved contraceptive availability may be useful [83].
Postpartum interventions: addressing child obesity
Guidelines from the Endocrine Society, endorsed by the European Society of Endocrinology and the Paediatric Endocrine Society recognise the lack of good long-term evidence for prevention and amelioration of child obesity [5]. Promoting a healthy diet [84], regular physical activity [85] and a built environment to support a healthy lifestyle [86] are all important, but large, well-controlled studies of specific interventions with prolonged follow-up are lacking [86, 87]. New pharmacological treatments such as semaglutide [88] and an increasing acceptance of bariatric surgery [89, 90] may help adolescents with obesity. The psychological effects of child obesity are significant and also need to be consistently assessed and addressed [5].
Conclusions
The early development of obesity in children with existing environmental and genetic susceptibilities to type 2 diabetes needs to be addressed to prevent multimorbidity in future generations. Exposure to maternal diabetes and/or obesity in utero is likely to influence offspring body composition, insulin sensitivity and beta cell function. While the mechanisms behind this are underexplored, the complex interplay between maternal and offspring insulin and lipid metabolism are likely to be involved. Effective intervention will require a new focus on maternal health before, during and after pregnancy to halt the intergenerational cycle of obesity.
Abbreviations
- BAT:
-
Brown adipose tissue
- CONCEPTT:
-
Continuous Glucose Monitoring in Women with Type 1 Diabetes in Pregnancy Trial
- HAPO:
-
Hyperglycemia and Adverse Pregnancy Outcomes
References
Metzger BE, Coustan DR (1998) Summary and recommendations of the fourth international workshop-conference on gestational diabetes mellitus. The organizing committee. Diabetes Care 21(Suppl 2):B161-167
Lowe WL Jr, Lowe LP, Kuang A et al (2019) Maternal glucose levels during pregnancy and childhood adiposity in the hyperglycemia and adverse pregnancy outcome follow-up study. Diabetologia 62(4):598–610. https://doi.org/10.1007/s00125-018-4809-6
Metzger BE, Lowe LP, Dyer AR et al (2008) Hyperglycemia and adverse pregnancy outcomes. N Engl J Med 358(19):1991–2002. https://doi.org/10.1056/NEJMoa0707943
Sauder KA, Hockett CW, Ringham BM, Glueck DH, Dabelea D (2017) Fetal overnutrition and offspring insulin resistance and β-cell function: the Exploring Perinatal Outcomes among Children (EPOCH) study. Diabet Med 34(10):1392–1399. https://doi.org/10.1111/dme.13417
Styne DM, Arslanian SA, Connor EL et al (2017) Pediatric obesity-assessment, treatment, and prevention: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 102(3):709–757. https://doi.org/10.1210/jc.2016-2573
Nucci AM, Virtanen SM, Cuthbertson D et al (2021) Growth and development of islet autoimmunity and type 1 diabetes in children genetically at risk. Diabetologia 64(4):826–835. https://doi.org/10.1007/s00125-020-05358-3
Blake-Lamb TL, Locks LM, Perkins ME, Woo Baidal JA, Cheng ER, Taveras EM (2016) Interventions for childhood obesity in the first 1,000 days a systematic review. Am J Prev Med 50(6):780–789. https://doi.org/10.1016/j.amepre.2015.11.010
Pitchika A, Jolink M, Winkler C et al (2018) Associations of maternal type 1 diabetes with childhood adiposity and metabolic health in the offspring: a prospective cohort study. Diabetologia 61(11):2319–2332. https://doi.org/10.1007/s00125-018-4688-x
Kaul P, Bowker SL, Savu A, Yeung RO, Donovan LE, Ryan EA (2019) Association between maternal diabetes, being large for gestational age and breast-feeding on being overweight or obese in childhood. Diabetologia 62(2):249–258. https://doi.org/10.1007/s00125-018-4758-0
Lowe WL Jr, Scholtens DM, Lowe LP et al (2018) Association of gestational diabetes with maternal disorders of glucose metabolism and childhood adiposity. JAMA 320(10):1005–1016. https://doi.org/10.1001/jama.2018.11628
Wang X, Martinez MP, Chow T, Xiang AH (2020) BMI growth trajectory from ages 2 to 6 years and its association with maternal obesity, diabetes during pregnancy, gestational weight gain, and breastfeeding. Pediatr Obes 15(2):e12579. https://doi.org/10.1111/ijpo.12579
Voerman E, Santos S, PatroGolab B et al (2019) Maternal body mass index, gestational weight gain, and the risk of overweight and obesity across childhood: an individual participant data meta-analysis. PLoS Med 16(2):e1002744. https://doi.org/10.1371/journal.pmed.1002744
Zhang Y, Chen Z, Cao Z et al (2020) Associations of maternal glycemia and prepregnancy BMI with early childhood growth: a prospective cohort study. Ann N Y Acad Sci 1465(1):89–98. https://doi.org/10.1111/nyas.14258
Zhu Y, Olsen SF, Mendola P et al (2016) Growth and obesity through the first 7 y of life in association with levels of maternal glycemia during pregnancy: a prospective cohort study. Am J Clin Nutr 103(3):794–800. https://doi.org/10.3945/ajcn.115.121780
Lawlor DA, Lichtenstein P, Långström N (2011) Association of maternal diabetes mellitus in pregnancy with offspring adiposity into early adulthood: sibling study in a prospective cohort of 280,866 men from 248,293 families. Circulation 123(3):258–265. https://doi.org/10.1161/circulationaha.110.980169
Ardıç C, Çolak S, Uzun K, Salı G, Aydemir T, Telatar G (2020) Maternal gestational diabetes and early childhood obesity: a retrospective cohort study. Child Obes 16(8):579–585. https://doi.org/10.1089/chi.2020.0183
Choi MJ, Yu J, Choi J (2022) Maternal pre-pregnancy obesity and gestational diabetes mellitus increase the risk of childhood obesity. Children (Basel) 9(7):928. https://doi.org/10.3390/children9070928
Deierlein AL, Siega-Riz AM, Chantala K, Herring AH (2011) The association between maternal glucose concentration and child BMI at age 3 years. Diabetes Care 34(2):480–484. https://doi.org/10.2337/dc10-1766
Gomes D, von Kries R, Delius M et al (2018) Late-pregnancy dysglycemia in obese pregnancies after negative testing for gestational diabetes and risk of future childhood overweight: an interim analysis from a longitudinal mother-child cohort study. PLoS Med 15(10):e1002681. https://doi.org/10.1371/journal.pmed.1002681
Curhan GC, Chertow GM, Willett WC et al (1996) Birth weight and adult hypertension and obesity in women. Circulation 94(6):1310–1315. https://doi.org/10.1161/01.cir.94.6.1310
Curhan GC, Willett WC, Rimm EB, Spiegelman D, Ascherio AL, Stampfer MJ (1996) Birth weight and adult hypertension, diabetes mellitus, and obesity in US men. Circulation 94(12):3246–3250. https://doi.org/10.1161/01.cir.94.12.3246
Asher P (1966) Fat babies and fat children. The prognosis of obesity in the very young. Arch Dis Child 41(220):672–673
Charney E, Goodman HC, McBride M, Lyon B, Pratt R (1976) Childhood antecedents of adult obesity. Do chubby infants become obese adults? N Engl J Med 295(1):6–9. https://doi.org/10.1056/nejm197607012950102
Metzger BE, Coustan DR, Trimble ER (2019) Hyperglycemia and adverse pregnancy outcomes. Clin Chem 65(7):937–938. https://doi.org/10.1373/clinchem.2019.303990
Scholtens DM, Kuang A, Lowe LP et al (2019) Hyperglycemia and Adverse Pregnancy Outcome Follow-up Study (HAPO FUS): maternal glycemia and childhood glucose metabolism. Diabetes Care 42(3):381–392. https://doi.org/10.2337/dc18-2021
Tint MT, Sadananthan SA, Soh SE et al (2020) Maternal glycemia during pregnancy and offspring abdominal adiposity measured by MRI in the neonatal period and preschool years: the Growing Up in Singapore Towards healthy Outcomes (GUSTO) prospective mother-offspring birth cohort study. Am J Clin Nutr 112(1):39–47. https://doi.org/10.1093/ajcn/nqaa055
Santos S, Severo M, Gaillard R, Santos AC, Barros H, Oliveira A (2016) The role of prenatal exposures on body fat patterns at 7 years: intrauterine programming or birthweight effects? Nutr Metab Cardiovasc Dis 26(11):1004–1010. https://doi.org/10.1016/j.numecd.2016.06.010
Sovio U, Murphy HR, Smith GC (2016) Accelerated fetal growth prior to diagnosis of gestational diabetes mellitus: a prospective cohort study of nulliparous women. Diabetes Care 39(6):982–987. https://doi.org/10.2337/dc16-0160
Durmuş B, Mook-Kanamori DO, Holzhauer S et al (2010) Growth in foetal life and infancy is associated with abdominal adiposity at the age of 2 years: the generation R study. Clin Endocrinol (Oxf) 72(5):633–640. https://doi.org/10.1111/j.1365-2265.2009.03708.x
Parente DB, Oliveira Neto JA, Brasil P et al (2018) Preperitoneal fat as a non-invasive marker of increased risk of severe non-alcoholic fatty liver disease in patients with type 2 diabetes. J Gastroenterol Hepatol 33(2):511–517. https://doi.org/10.1111/jgh.13903
Hay WW Jr (2006) Placental-fetal glucose exchange and fetal glucose metabolism. Trans Am Clin Climatol Assoc 117:321–339 (discussion 339-340)
Murphy HR, Howgate C, O’Keefe J et al (2021) Characteristics and outcomes of pregnant women with type 1 or type 2 diabetes: a 5-year national population-based cohort study. Lancet Diabetes Endocrinol 9(3):153–164. https://doi.org/10.1016/S2213-8587(20)30406-X
Meek CL, Tundidor D, Feig DS et al (2021) Novel biochemical markers of glycemia to predict pregnancy outcomes in women with type 1 diabetes. Diabetes Care 44(3):681–689. https://doi.org/10.2337/dc20-2360
Pedersen J (1952) Course of diabetes during pregnancy. Acta Endocrinol (Copenh) 9(4):342–364. https://doi.org/10.1530/acta.0.0090342
Desoye G, Herrera E (2021) Adipose tissue development and lipid metabolism in the human fetus: the 2020 perspective focusing on maternal diabetes and obesity. Prog Lipid Res 81:101082. https://doi.org/10.1016/j.plipres.2020.101082
Furse S, Koulman A, Ozanne SE, Poston L, White SL, Meek CL (2022) Altered lipid metabolism in obese women with gestational diabetes and associations with offspring adiposity. J Clin Endocrinol Metab 107(7):e2825–e2832. https://doi.org/10.1210/clinem/dgac206
Meek CL, Stewart ZA, Feig DS et al (2023) Metabolomic insights into maternal and neonatal complications in type 1 diabetes pregnancies. Diabetologia (in press)
Attique H, Baig S, Ishtiaque S, Rehman R, Ahmed ST, Ali Shahid M (2022) Neuregulin 4 (NRG4) - the hormone with clinical significance in gestational diabetes mellitus. J Obstet Gynaecol 42(6):1931–1936. https://doi.org/10.1080/01443615.2022.2054683
Kralisch S, Hoffmann A, Kratzsch J et al (2018) The brown-fat-secreted adipokine neuregulin 4 is decreased in gestational diabetes mellitus. Diabetes Metab 44(2):150–154. https://doi.org/10.1016/j.diabet.2017.06.001
Martinez-Perez B, Ejarque M, Gutierrez C et al (2016) Angiopoietin-like protein 8 (ANGPTL8) in pregnancy: a brown adipose tissue-derived endocrine factor with a potential role in fetal growth. Transl Res 178:1–12. https://doi.org/10.1016/j.trsl.2016.06.012
Yamamoto JM, Corcoy R, Donovan LE et al (2019) Maternal glycaemic control and risk of neonatal hypoglycaemia in Type 1 diabetes pregnancy: a secondary analysis of the CONCEPTT trial. Diabet Med 36(8):1046–1053. https://doi.org/10.1111/dme.13988
Meek CL, Oram RA, McDonald TJ et al (2021) Reappearance of C-peptide during the third trimester of pregnancy in type 1 diabetes: pancreatic regeneration or fetal hyperinsulinism? Diabetes Care 44(8):1826–1834. https://doi.org/10.2337/dc21-0028
Kay RG, Challis BG, Casey RT et al (2018) Peptidomic analysis of endogenous plasma peptides from patients with pancreatic neuroendocrine tumours. Rapid Commun Mass Spectrom 32(16):1414–1424. https://doi.org/10.1002/rcm.8183
Boney CM, Verma A, Tucker R, Vohr BR (2005) Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics 115(3):e290-296. https://doi.org/10.1542/peds.2004-1808
Lowe WL Jr, Scholtens DM, Kuang A et al (2019) Hyperglycemia and Adverse Pregnancy Outcome Follow-up Study (HAPO FUS): maternal gestational diabetes mellitus and childhood glucose metabolism. Diabetes Care 42(3):372–380. https://doi.org/10.2337/dc18-1646
Brown RJ, Yanovski JA (2014) Estimation of insulin sensitivity in children: methods, measures and controversies. Pediatr Diabetes 15(3):151–161. https://doi.org/10.1111/pedi.12146
Dabelea D, D’Agostino RB Jr, Mason CC et al (2011) Development, validation and use of an insulin sensitivity score in youths with diabetes: the SEARCH for Diabetes in Youth study. Diabetologia 54(1):78–86. https://doi.org/10.1007/s00125-010-1911-9
Chen Q, Francis E, Hu G, Chen L (2018) Metabolomic profiling of women with gestational diabetes mellitus and their offspring: review of metabolomics studies. J Diabetes Complications 32(5):512–523. https://doi.org/10.1016/j.jdiacomp.2018.01.007
Meek CL, Stewart Z, Furse S et al (2022) Antenatal glucocorticoids and neonatal outcomes in type 1 diabetes pregnancy. Int J Obs Gyn 10(3):1–4
Knorr S, Skakkebæk A, Just J et al (2022) Epigenetic and transcriptomic alterations in offspring born to women with type 1 diabetes (the EPICOM study). BMC Med 20(1):338. https://doi.org/10.1186/s12916-022-02514-x
Kelstrup L, Hjort L, Houshmand-Oeregaard A et al (2016) Gene expression and DNA methylation of PPARGC1A in muscle and adipose tissue from adult offspring of women with diabetes in pregnancy. Diabetes 65(10):2900–2910. https://doi.org/10.2337/db16-0227
Saeedi Borujeni MJ, Esfandiary E, Baradaran A et al (2019) Molecular aspects of pancreatic β-cell dysfunction: oxidative stress, microRNA, and long noncoding RNA. J Cell Physiol 234(6):8411–8425. https://doi.org/10.1002/jcp.27755
Fernandez-Twinn DS, Hjort L, Novakovic B, Ozanne SE, Saffery R (2019) Intrauterine programming of obesity and type 2 diabetes. Diabetologia 62(10):1789–1801. https://doi.org/10.1007/s00125-019-4951-9
Jönsson J, Renault KM, García-Calzón S et al (2021) Lifestyle intervention in pregnant women with obesity impacts cord blood DNA methylation, which associates with body composition in the offspring. Diabetes 70(4):854–866. https://doi.org/10.2337/db20-0487
Opsahl JO, Moen GH, Qvigstad E, Böttcher Y, Birkeland KI, Sommer C (2021) Epigenetic signatures associated with maternal body mass index or gestational weight gain: a systematic review. J Dev Orig Health Dis 12(3):373–383. https://doi.org/10.1017/s2040174420000811
Manitta E, Fontes Marques IC, StokholmBredgaard S et al (2022) DNA methylation and gene expression in blood and adipose tissue of adult offspring of women with diabetes in pregnancy-a validation study of DNA methylation changes identified in adolescent offspring. Biomedicines 10(6):1244. https://doi.org/10.3390/biomedicines10061244
Ding GL, Liu Y, Liu ME et al (2015) The effects of diabetes on male fertility and epigenetic regulation during spermatogenesis. Asian J Androl 17(6):948–953. https://doi.org/10.4103/1008-682x.150844
Furse S, Kusinski LC, Ray A et al (2022) Relative abundance of lipid metabolites in spermatozoa across three compartments. Int J Mol Sci 23(19):11655. https://doi.org/10.3390/ijms231911655
Slyvka Y, Zhang Y, Nowak FV (2015) Epigenetic effects of paternal diet on offspring: emphasis on obesity. Endocrine 48(1):36–46. https://doi.org/10.1007/s12020-014-0328-5
Kusuyama J, Alves-Wagner AB, Makarewicz NS, Goodyear LJ (2020) Effects of maternal and paternal exercise on offspring metabolism. Nat Metab 2(9):858–872. https://doi.org/10.1038/s42255-020-00274-7
Perrin T, Pradat P, Larcade J, Masclef-Imbert M, Pastor-Diez B, Picaud JC (2023) Postnatal growth and body composition in extremely low birth weight infants fed with individually adjusted fortified human milk: a cohort study. Eur J Pediatr. https://doi.org/10.1007/s00431-022-04775-3
Stephenson J, Heslehurst N, Hall J et al (2018) Before the beginning: nutrition and lifestyle in the preconception period and its importance for future health. Lancet 391(10132):1830–1841. https://doi.org/10.1016/s0140-6736(18)30311-8
Lyons-Reid J, Derraik JGB, Kenealy T et al (2023) The effect of a preconception and antenatal nutritional supplement on children’s BMI and weight gain over the first 2 years of life: findings from the NiPPeR randomised controlled trial. Lancet Glob Health 11(Suppl 1):S11-s12. https://doi.org/10.1016/s2214-109x(23)00095-5
Moholdt T, Hawley JA (2020) Maternal lifestyle interventions: targeting preconception health. Trends Endocrinol Metab 31(8):561–569. https://doi.org/10.1016/j.tem.2020.03.002
Yamamoto JM, Hughes DJF, Evans ML et al (2018) Community-based pre-pregnancy care programme improves pregnancy preparation in women with pregestational diabetes. Diabetologia 61(7):1528–1537. https://doi.org/10.1007/s00125-018-4613-3
Koivusalo SB, Rönö K, Klemetti MM et al (2016) Gestational diabetes mellitus can be prevented by lifestyle intervention: the finnish gestational diabetes prevention study (RADIEL): a randomized controlled trial. Diabetes Care 39(1):24–30. https://doi.org/10.2337/dc15-0511
Poston L, Bell R, Croker H et al (2015) Effect of a behavioural intervention in obese pregnant women (the UPBEAT study): a multicentre, randomised controlled trial. Lancet Diabetes Endocrinol 3(10):767–777. https://doi.org/10.1016/S2213-8587(15)00227-2
Yamamoto JM, Kellett JE, Balsells M et al (2018) Gestational diabetes mellitus and diet: a systematic review and meta-analysis of randomized controlled trials examining the impact of modified dietary interventions on maternal glucose control and neonatal birth weight. Diabetes Care 41(7):1346–1361. https://doi.org/10.2337/dc18-0102
Kusinski LC, Murphy HR, De Lucia Rolfe E et al (2020) Dietary intervention in pregnant women with gestational diabetes; protocol for the DiGest randomised controlled trial. Nutrients 12(4):1165. https://doi.org/10.3390/nu12041165
Tarry-Adkins JL, Aiken CE, Ozanne SE (2019) Neonatal, infant, and childhood growth following metformin versus insulin treatment for gestational diabetes: a systematic review and meta-analysis. PLoS Med 16(8):e1002848. https://doi.org/10.1371/journal.pmed.1002848
Feig DS, Sanchez JJ, Murphy KE et al (2023) Outcomes in children of women with type 2 diabetes exposed to metformin versus placebo during pregnancy (MiTy Kids): a 24-month follow-up of the MiTy randomised controlled trial. Lancet Diabetes Endocrinol 11(3):191–202. https://doi.org/10.1016/s2213-8587(23)00004-9
Feig DS, Donovan LE, Corcoy R et al (2017) Continuous glucose monitoring in pregnant women with type 1 diabetes (CONCEPTT): a multicentre international randomised controlled trial. Lancet 390(10110):2347–2359. https://doi.org/10.1016/S0140-6736(17)32400-5
Stewart ZA, Wilinska ME, Hartnell S et al (2018) Day-and-night closed-loop insulin delivery in a broad population of pregnant women with type 1 diabetes: a randomized controlled crossover trial. Diabetes Care 41(7):1391–1399. https://doi.org/10.2337/dc17-2534
Yamamoto JM, Murphy HR (2023) Technology and pregnancy. Diabetes Technol Ther 25(S1):S109-s117. https://doi.org/10.1089/dia.2023.2507
Nehring I, Schmoll S, Beyerlein A, Hauner H, von Kries R (2011) Gestational weight gain and long-term postpartum weight retention: a meta-analysis. Am J Clin Nutr 94(5):1225–1231. https://doi.org/10.3945/ajcn.111.015289
Landon MB, Mele L, Varner MW et al (2018) The relationship of maternal glycemia to childhood obesity and metabolic dysfunction(double dagger). J Matern Fetal Neonatal Med 1–9. https://doi.org/10.1080/14767058.2018.1484094
Woo Baidal JA, Locks LM, Cheng ER, Blake-Lamb TL, Perkins ME, Taveras EM (2016) Risk factors for childhood obesity in the first 1,000 days: a systematic review. Am J Prev Med 50(6):761–779. https://doi.org/10.1016/j.amepre.2015.11.012
Feng Y, Zhao Z, Fu D, Gao W, Zhang F (2021) Maternal and neonatal outcomes after energy-restricted diet for women with gestational diabetes mellitus: a systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore) 100(14):e25279. https://doi.org/10.1097/md.0000000000025279
Gunderson EP, Greenspan LC, Faith MS, Hurston SR, Quesenberry CP Jr (2018) Breastfeeding and growth during infancy among offspring of mothers with gestational diabetes mellitus: a prospective cohort study. Pediatr Obes 13(8):492–504. https://doi.org/10.1111/ijpo.12277
Ma J, Qiao Y, Zhao P et al (2020) Breastfeeding and childhood obesity: a 12-country study. Matern Child Nutr 16(3):e12984. https://doi.org/10.1111/mcn.12984
Davis EM, Babineau DC, Wang X et al (2014) Short inter-pregnancy intervals, parity, excessive pregnancy weight gain and risk of maternal obesity. Matern Child Health J 18(3):554–562. https://doi.org/10.1007/s10995-013-1272-3
Harris HE, Ellison GT, Holliday M (1997) Is there an independent association between parity and maternal weight gain? Ann Hum Biol 24(6):507–519. https://doi.org/10.1080/03014469700005272
Lassi ZS, Kedzior SGE, Tariq W, Jadoon Y, Das JK, Bhutta ZA (2021) Effects of preconception care and periconception interventions on maternal nutritional status and birth outcomes in low- and middle-income countries: a systematic review. Campbell Syst Rev 17(2):e1156. https://doi.org/10.1002/cl2.1156
Council on School Health, Committee on Nutrition (2015) Snacks, sweetened beverages, added sugars, and schools. Pediatrics 135(3):575–583. https://doi.org/10.1542/peds.2014-3902
Davis CL, Tomporowski PD, McDowell JE et al (2011) Exercise improves executive function and achievement and alters brain activation in overweight children: a randomized, controlled trial. Health Psychol 30(1):91–98. https://doi.org/10.1037/a0021766
Daniels KM, Schinasi LH, Auchincloss AH, Forrest CB, Diez Roux AV (2021) The built and social neighborhood environment and child obesity: a systematic review of longitudinal studies. Prev Med 153:106790. https://doi.org/10.1016/j.ypmed.2021.106790
Pereira AR, Oliveira A (2021) Dietary interventions to prevent childhood obesity: a literature review. Nutrients 13(10):3447. https://doi.org/10.3390/nu13103447
Weghuber D, Barrett T, Barrientos-Pérez M et al (2022) Once-weekly semaglutide in adolescents with obesity. N Engl J Med 387(24):2245–2257. https://doi.org/10.1056/NEJMoa2208601
Inge TH, Courcoulas AP, Jenkins TM et al (2016) Weight loss and health status 3 years after bariatric surgery in adolescents. N Engl J Med 374(2):113–123. https://doi.org/10.1056/NEJMoa1506699
Olbers T, Gronowitz E, Werling M et al (2012) Two-year outcome of laparoscopic Roux-en-Y gastric bypass in adolescents with severe obesity: results from a Swedish Nationwide Study (AMOS). Int J Obes (Lond) 36(11):1388–1395. https://doi.org/10.1038/ijo.2012.160
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Acknowledgements
The author wishes to thank Dr Laura Kusinski and Dr Clive Petry (University of Cambridge) for proof-reading and constructive feedback on the final manuscript. This was an invited review after a presentation of the same name delivered at the EASD conference in 2022.
Funding
CLM receives funding from Diabetes UK (17/0005712), the BMA Foundation (Helen H Lawson Award), the European Foundation for the Study of Diabetes–Novo Nordisk Foundation (NNF19SA058974) and the National Institute of Health Research (NIHR) Cambridge Biomedical Research Centre (BRC). The views expressed are those of the author and not necessarily those of the NIHR or the Department of Health and Social Care.
Authors’ relationships and activities
The author declares that there are no relationships or activities that might bias, or be perceived to bias, their work.
Contribution statement
The author was the sole contributor to this manuscript.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Meek, C.L. An unwelcome inheritance: childhood obesity after diabetes in pregnancy. Diabetologia 66, 1961–1970 (2023). https://doi.org/10.1007/s00125-023-05965-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-023-05965-w