Abstract
Key message
Rfo is located on a radish chromosome fragment (~ 108 Kb), which is seated in the middle of a pretty large C genome translocation at the distal region of chromosome A09 of B. juncea.
Abstract
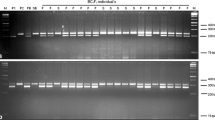
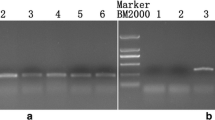
Ogura cytoplasmic male sterility (CMS) is used to produce hybrids in Indian mustard (Brassica juncea L.). Fertility restorers for this CMS were developed by cross-hybridizing B. juncea (AABB; 2n = 36) with B. napus (AACC; 2n = 38) carrying radish Rfo gene. This hybrid production system is normally stable, but many commercial mustard hybrids show male sterile contaminants. We aimed to identify linkage drag associated with Rfo by comparing hybridity levels of 295 handmade CMS x Rfo crosses. Although Rfo was stably inherited, hybridity was < 85 percent in several combinations. Genome re-sequencing of five fertility restorers, mapping sequencing reads to B. juncea reference and synteny analysis with Raphanus sativus D81Rfo genomic region (AJ550021.2) helped to detect ~ 108 Kb of radish chromosome (R) fragment substitution in all fertility restorers. This radish segment substitution was itself located amidst a large C genome translocation on the terminal region of chromosome A09 of B. juncea. The size of alien segment substitution varied from 11.3 (NTCN-R9) to 22.0 Mb (NAJR-102B-R). We also developed an in silico SSR map for chromosome A09 and identified many homoeologous A to the C genome exchanges in the introgressed region. A to the R genome exchanges were rare. Annotation of the substituted fragment showed the gain of many novel genes from R and C genomes and the loss of B. juncea genes from the corresponding region. We have developed a KASPar marker for marker-aided transfer of Rfo and testing hybridity levels in seed production lots.




Similar content being viewed by others
References
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410
Banga SS, Labana KS (1983) Heterosis in Indian mustard (Brassica juncea L. Coss.). Z Pflanzenzuecht 92:61–70
Bannerot H, Boulidard L, Cauderon Y, Tempe J (1974) Transfer of cytoplasmic male sterility from Raphanus sativus to Brassica oleracea. Proc Eucarpia Meet Cruciferae 25:52–54
Bonhomme S, Budar F, Lancelin D, Small I, Defrance MC, Pelletier G (1992) Sequence and transcript analysis of Nco2.5 Ogura specific fragment correlated with cytoplasmic male sterility in Brassica cybrids. Mol Gen Genet 235:240–248
Delannoy E, Stanley WA, Bond CS, Small ID (2007) Pentatricopeptide repeat (PPR) proteins as sequence-specificity factors in post-transcriptional processes in organelles. Biochem Soc Trans 35(6):1643–1648
Delourme R, Eber F (1992) Linkage between an isozyme marker and a restorer gene in radish cytoplasmic male sterility of rapeseed (Brassica napus L.). Theor Appl Genet 85:222–228
Delourme R, Foisset N, Horvais R, Barret P, Champagne G, Cheung WY, Renard M (1998) Characterisation of the radish introgression carrying the Rfo restorer gene for the Ogu-INRA cytoplasmic male sterility in rapeseed (Brassica napus L.). Theor Appl Genet 97:129–134
Desloire S, Gherbi H, Laloui W, Marhadour S, Clouet V, Cattolico L, Falentin C, Giancola S, Renard M, Budar F, Small I (2003) Identification of the fertility restoration locus, Rfo, in radish, as a member of the pentatricopeptide-repeat protein family. EMBO Rep 4(6):588–594
Doyle JJ, Doyle JL (1987) A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem Bulletin 19:11–15
Gaborieau L, Brown GG (2016) Comparative genomic analysis of the compound Brassica napus Rf locus. BMC Genomics 17:834. https://doi.org/10.1186/s12864-016-3117-0
Gaborieau L, Brown GG, Mireau H (2016) The propensity of pentatricopeptide repeat genes to evolve into restorers of cytoplasmic male sterility. Front Plant Sci 7:1816
Giancola S, Marhadour S, Desloire S, Clouet V, Falentin-Guyomarc'h H, Laloui W, Falentin C, Pelletier G, Renard M, Bendahmane A, Delourme R, Budar F (2003) Characterization of a radish introgression carrying the Ogura fertility restorer gene Rfo in rapeseed, using the Arabidopsis genome sequence and radish genetic mapping. Theor Appl Genet 107:1442–1451
Gray MW, Lang BF, Cedergren R, Golding GB, Lemieux C, Sankoff D, Turmel M, Brossard N, Delage E, Littlejohn TG, Plante I, Rioux P, Saint-Louis D, Zhu Y, Burger G (1998) Genome structure and gene content in protist mitochondrial DNAs. Nucl Acids Res 26:865–878
Heyn FW (1976) Transfer of restorer genes from Raphanus to cytoplasmic male sterile Brassica napus. Cruciferae Newslett 1:15–16
Hu X, Sullivan-Gilbert M, Kubik T, Danielson J, Hnatiuk N, Marchione W, Greene T, Thompson S (2008) Mapping of the Ogura fertility restorer gene Rfo and development of Rfo allele-specific markers in canola (Brassica napus L.). Mol Breed 22:663–674
Jain A, Bhatia S, Banga SS, Prakash S, Lakshmikumaran M (1994) Potential use of random amplified polymorphic DNA (RAPD) to study the genetic diversity in Indian mustard (Brassica juncea L. Czern and Coss.) and its relationship with heterosis. Theor Appl Genet 88:116–122
Kaminski P, Podwyszyńska M, Starzycki M, Starzycka-Korbas E (2016) Interspecific hybridisation of cytoplasmic male-sterile rapeseed with Ogura cytoplasm and Brassica rapa var. pekinensis as a method to obtain male-sterile Chinese cabbage inbred lines. Euphytica 208:519–534
Kearse M, Moir R, Wilson A (2012) Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28:1647–1649
Kirti PB, Mohapatra T, Prakash S, Chopra VL (1995) Development of a stable cytoplasmic male sterile line of Brassica juncea from the somatic hybrid Trachystoma balli + Brassica juncea. Plant Breed 113:21–24
Labana KS, Banga SS (1984) Floral biology in Indian mustard (Brassica juncea L. coss). Genetica Agraria 38(2):131–138
Labana KS, Banga SK (1989) Transfer of Ogura cytoplasmic male sterility of Brassica napus into genetic background of Brassica juncea. Crop Improv 16:82–83
Lodhi MA, Ye GN, Weeden NF, Reisch BI (1994) A simple and efficient method for DNA extractions from grapevine cultivars and Vitis species. Pl Mol Biol Rep 12:6–13
Maliyakal EJ (1992) An efficient method for isolation of RNA and DNA from plants containing polyphenolics. Nucleic Acids Res 20:2381
Niemela T, Seppanen M, Badakshi F, Rokka VM, Heslop-Harrison JP (2012) Size and location of radish chromosome regions carrying the fertility restorer Rfk1 gene in spring turnip rape. Chromosome Res 20:353–361
Ogura H (1968) Studies on the new male sterility in Japanese radish, with special references on the utilization of this sterility towards the practical raising of hybrid seeds. Mem Fac Agric Kagoshima Univ 6:39–78
Pelletier G, Budar F (2015) Brassica Ogu-INRA cytoplasmic male sterility: An example of successful plant somatic fusion for hybrid seed production. In: Li XQ, Donnelly D, Jensen T (eds) Somatic Genome Manipulation. Springer, New York, NY, pp 199–216
Pelletier G, Primard C, Vedel F, Chétrit P, Remy R, Rousselle P, Renard M (1983) Intergeneric cytoplasmic hybridization in Cruciferae by protoplast fusion. Mol Gen Genet 191:244–250
Pradhan AK, Sodhi YS, Mukhopadhyay A (1993) Heterosis breeding in Indian mustard (Brassica juncea L. Czern & Coss): analysis of component characters contributing to heterosis for yield. Euphytica 69:219–229
Primard-Brisset C, Poupard JP, Horvais R, Eber F, Pelletier G, Renard M, Delourme R (2005) A new recombined double low restorer line for the Ogu-INRA CMS in rapeseed (Brassica napus L.). Theor Appl Genet 111:736–746
Sodhi YS, Chandra A, Arumugam N, Mukhopadhyay A, Gupta V, Pental D, Pradhan AK (2006) A new cytoplasmic male sterility system for hybrid seed production in Indian oilseed mustard Brassica juncea. Theor Appl Genet 114:93–99
Stam P (1993) Construction of integrated genetic linkage maps by means of a new computer package: Join Map. Pl J 3:739–744
Stein A, Coriton O, Rousseau-Gueutin M, Samans B, Schiessl SV, Obermeier C, Parkin IAP, Chèvre AM, Snowdon RJ (2017) Mapping of homoeologous chromosome exchanges influencing quantitative trait variation in Brassica napus. Plant Biotechnol J 15:1478–1489
Sun F, Fan G, Hu Q, Zhou Y, Guan M, Tong C, Li J, Du D, Qi C, Jiang L, Liu W, Huang S, Chen W, Yu J, Mei D, Meng J, Zeng P, Shi J, Liu K, Wang X, Wang X, Long Y, Liang X, Hu Z, Huang G, Dong C, Zhang H, Li J, Zhang Y, Li L, Shi C, Wang J, Lee SMY, Guan C, Xu X, Liu S, Liu X, Chalhoub B, Hua W, Wang H (2017) The high quality genome of Brassica napus cultivar ‘ZS11’ reveals the introgression history in semiwinter morphotype. Plant J 92:452–468
Tanaka Y, Tsuda M, Yasumoto K, Yamagishi H, Terachi T (2012) A complete mitochondrial genome sequence of Ogura-type male-sterile cytoplasm and its comparative analysis with that of normal cytoplasm in radish (Raphanus sativus L.). BMC Genomics 13:352. https://doi.org/10.1186/1471-2164-13-352
Tian E, Roslinsky V, Cheng B (2014) Molecular marker-assisted breeding for improved Ogura CMS restorer line (Rfo) and mapping of the restorer gene (Rfo) in Brassica juncea. Mol Breed 34:1361–1371
Uyttewaal MN, Arnal M, Quadrado A, Martin-Canadell N, Vrielynck S, Hiard H, Gherbi A, Bendahmane F, Budar MH (2008) Characterization of Raphanus sativus pentatricopeptide repeat proteins encoded by the fertility restorer locus for Ogura cytoplasmic male sterility. Plant Cell 12:3331–3345
Wang X, Lu P, Luo Z (2013) GMATA: A novel tool for the identification and analysis of microsatellites in large genomes. Bioinformation 9(10):541–544
Wang X, Chen L, Ma J (2019) Genomic introgression through interspecific hybridization counteracts genetic bottleneck during soybean domestication. Genome Biol 20:22. https://doi.org/10.1186/s13059-019-1631-5
Yamagishi H, Bhat SR (2014) Cytoplasmic male sterility in Brassicaceae crops. Breed Sci 64(1):38–47
Acknowledgments
Researches were conducted with financial support from National Professor Project “Broadening the genetic base of Indian mustard (Brassica juncea) through alien introgressions and germplasm enhancement” awarded to SSB. Sequencing of fertility restorers was carried out with the financial support from Department of Biotechnology, Government of India, in the form of Centre of Excellence and Innovation in Biotechnology “Germplasm enhancement for crop architecture and defensive traits in Brassica juncea L. Czern. and Coss.”
Funding
This article was funded by Indian Council of Agricultural Research, Government of India (F.No.27(12)/2009-HRD-PartII) (grant number: ICAR45), Department of Biotechnology, Government of India (BT/01/CEIB/12/1/03).
Author information
Authors and Affiliations
Contributions
SG conducted field experiments. SSB and CA developed the fertility restorers and analyzed field data. JA, NK and SG carried out the bioinformatic data analysis. AG assisted in field and laboratory studies. MM helped with annotation. GK developed KASPar marker. CA and SG wrote the manuscript. SSB planned the experiments, supervised the study and edited the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Communicated by Carlos F. Quiros.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gudi, S., Atri, C., Goyal, A. et al. Physical mapping of introgressed chromosome fragment carrying the fertility restoring (Rfo) gene for Ogura CMS in Brassica juncea L. Czern & Coss. Theor Appl Genet 133, 2949–2959 (2020). https://doi.org/10.1007/s00122-020-03648-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-020-03648-3