Abstract
Objective
In recent years, the trauma mechanisms and fracture types in tibial plateau fractures (TPF) have changed. At the same time, treatment strategies have expanded with the establishment of new classification systems, extension of diagnostics and surgical strategies. Evidence-based recommendations for treatment strategies are rare. The aim of this study is to assess the extent of standardization in the treatment of complex TPF.
Material and methods
For the study, specialists in trauma surgery/orthopaedics were presented thin-slice CT data sets of three complex TPFs including 3D reconstructions. A standardized questionnaire on fracture morphology and planned treatment strategy was then completed.
Results
A total of 23 surgeons from 7 hospitals (Trauma center levels I–III) were included.
All three fractures were most frequently classified as Schatzker type V (fracture I: 52.2%, II: 56.5%, III: 60%). Averaged over all three fractures, 55% of the respondents chose the same patient positioning. The combination of a posteromedial and anterolateral approach was the most frequently chosen approach at 42.7%. Double plating was favored for the surgical treatment of all fractures (70.7%). Preoperative MRI, extended approaches and intraoperative fraturoscopy were significantly more common in level I trauma centres.
Conclusion
There are major differences in the management of complex TPF. 360° treatment is carried out in all departments regardless of the level of care, but without further standardization in terms of preoperative imaging, classification, initial treatment, approach, fixation and intraoperative imaging. There are major differences within the departments with different level of care.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
Tibial plateau fractures (TPF) pose a significant challenge in trauma and orthopedic surgery, primarily due to their complexity. Currently, the preferred diagnostic tool is computed tomography (CT) imaging, recognized as the gold standard [1], while modern techniques like 3D printing and Mixed-Reality (MR) visualization enhance improved comprehension of the fracture [2, 3]. Presently, there is no generally recommended approach for identifying additional soft tissue damage by Magnetic Resonance Imaging (MRI) in complex TPF cases [1]. This holds clinical significance given the rising incidence of TPF in the past decade [5].
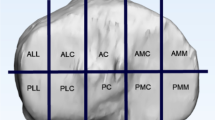
In an ageing society the accident mechanism is shifting towards low-energy trauma with an accompanying change in fracture morphology [1, 4,5,6]. Conventional two-dimensional fracture classifications, such as those by Schatzker, Arbeitsgemeinschaft für Osteosynthesefragen (AO), and Moore [7,8,9], are progressively giving way to three-dimensional assessments like the ten-segment classification or the three-column model [10,11,12,13].
This shift in fracture analysis has significantly impacted the treatment strategies of TPF. New approaches and step-by-step extension of the existing approaches for better visualisation of the articular surface and treatment of posterior fracture fragments have been developed [4, 14,15,16]. Despite significant advances in diagnostics and treatment, the incidence of secondary osteoarthritis after TPF remains high, ranging from 13 to 83% [17,18,19].
In the realm of daily clinical practice, surgeons encounter a diverse range of diagnostic and therapeutic possibilities but evidence-based recommendations are rare [1, 20]. Furthermore, significant disparities in the reliability of different classification systems pose a challenge, raising doubts about the comparability of treatment recommendations and outcome data across studies [3, 21, 22].
Therefore, the purpose of this study was to examine the care strategies utilized for managing complex TPF across different healthcare facilities. We hypothesized that there is no consensus within a cohort of highly experienced surgeons and there would be the need for a more standardized process for the successful treatment of complex TPF.
Methods
Case selection
Three randomly selected complex TPF from a German level I trauma center TPF database were selected for this study. A 3-Dimensional (3D) CT reconstruction was generated from the available CT thin slice dataset (slice thickness < 0.7 mm). The fractures included bicondylar, anterior, and posterior fracture fragments. CT imaging, along with 3D CT reconstruction, was presented to specialists in trauma surgery and/or orthopedics using Visage 7.1.16 software (Visage Imaging, CA, USA), as shown in Figs. 1 and 2. These specialists, attending specialists in trauma surgery and orthopedics, provided expert insights.
Specialist selection
A personal invitation was sent to specialists in trauma surgery who work at certified german trauma centers. The cases were presented by one of the study authors (M.B.) to the participating surgeons at the participating hospital. Participating surgeons were also asked if they had intraoperative 3D CT imaging and arthroscopy/fracturoscopy available for treatment.
Outcomes
A standardized questionnaire on fracture morphology, classification and recommended treatment strategy was completed by each specialist following the case presentation. This questionnaire (Appendix) was created using the web application SoSci Survey (SoSci Survey GmbH, Munich, Germany). All surveyed surgeons had to assess two fractures. Afterwards they were given the choice of taking time to assess an additional third fracture.
Statistical analysis
The statistical analysis was performed using SPSS Statistics 26.0 software (IBM Corp., Armonk, USA). The statistical test procedures used were the chi-square test and the exact test according to Fischer, with a significance level of p < 0.05. The reliability analysis was used for Fleiss’ Kappa. The graphical representation was carried out using Microsoft Excel 365 MSO Version 2207 (Microsoft Corp., Redmond, USA).
Results
Specialist selection
A total of 23 specialist surgeons from 7 different hospitals with varying trauma levels were included. The distribution of the departments' levels of care and the previous number of surgically treated TPF by the respondents is delineated in Table 1. Trauma center level I represents the highest level. Of the respondents, 69.6% (n = 16) had treated at least 50 TPF in their previous professional experience.
All surgeons reported that intraoperative arthroscopy/fracturoscopy was available in their department. Intraoperative 3D CT imaging was available at all participating level I hospitals. Two level II/III departments did not have the option of intraoperative 3D CT imaging. In total, 20 out of 23 surgeons had the possibility of intraoperative 3D CT imaging.
Thirteen surgeons assessed 2 fractures, while 10 surgeons assessed 3 fractures. Overall, this resulted in 56 cases/assessments. Table 4 in the appendix shows the most frequently selected answer option for each category.
Fracture classification
When using the Schatzker classification, all 3 fracture cases were most frequently classified as Schatzker 5, accounting for 55.4% (n = 31) of cases, on average. The highest agreement in classification was found for case 3, with 60% (Fig. 3). Table 2 represents the interrater reliability for the Schatzker classification with Fleiss’ Kappa going from 0.620 for the second case to 0.643 for the third case.
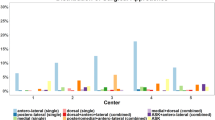
When using the ten-segment classification, Fig. 4 shows the frequency distribution of the selected segments within the ten-segment classification. On average, 8.1 affected segments were chosen for fracture I, 5.5 for fracture II, and 7.2 for fracture III. The highest agreement in the number of selected segments was observed for fracture 3, with 8 segments at 50% (n = 5). On average, respondents selected an identical combination of segments in 16.1% (n = 9) of all cases. The greatest agreement was found for fracture I, at 21.7% (n = 5).
The patient’s age in the first case was 56 years, in the second case 30 years and in the third case 53 years.
Preoperative imaging
In addition to CT, respondents expressed a desire for additional MRI in 42.9% (n = 24) on average. The highest agreement was observed for case 2, with 56.5% (n = 13).
Surgical treatment
The primary treatment for all fractures was most frequently recommended using external fixation, averaging 76.8% (n = 43). Alternatively, initial treatment was recommended using a brace or cast.
None of the respondents opted for Total Knee Arthroplasty (TKA), all favoured osteosynthesis.
Most of the surgeons opted for intraoperative patient re-positioning (prone to supine) for the treatment of case I and III (case I: 56.5% (n = 13), case III: 70% (n = 7)). In contrast, 60.9% (n = 14) of the respondents were planning the case II without re-positioning.
Figure 5 illustrates the selected approaches and fixation techniques. In 75% (n = 42) of cases, fracture treatment is planned via combined approaches. The most common combination is an anterolateral and posteromedial approach, accounting for 41.1% (n = 23). Double plate osteosyntheses were most frequently planned (73.2% (n = 41)). Bone augmentation is planned in 78.6% (n = 44) on average, most frequently with allogenic material (60.7% (n = 34)).
Intraoperative imaging is more often done by image intensifier, less frequently with arthroscopy/fracturoscopy.
Table 3 below provides an overview of the differences in treatment strategy between Trauma centers level I and other levels.
Surgeons in a maximum care setting (level I) are significantly (p < 0.001) more likely to request additional preoperative MRI. In these hospitals intraoperative repositioning and extended approaches are significantly more frequent (p < 0.001 respectively p = 0.019). Further, the number of fracturoscopies is also significantly (p = 0.019) higher in these hospitals.
Discussion
The most important finding of this study was that there were significant differences in the management of TPF among highly experienced surgeons, with these differences partly attributed to the level of care of the trauma center. However, whether the different methods and strategies lead to different outcomes and whether this justifies treatment of complex fractures exclusively in maximum care centres has not yet been clarified and remains the subject of further research.
This study calls for further research on different treatment strategies to establish a more standardized approach in the management of TPF to improve outcomes, reduce future complications, and lower conversion rates to TKA.
In the beginning, the classification system should exhibit high validity and reliability, facilitate the derivation of a treatment algorithm, and encompass expected patient outcomes [23]. Miler et al. highlighted the existence of 38 different classification systems for TPF in the literature, with only a few meeting these criteria [22]. Even within the less complex Schatzker et al. classification system, the data from this study demonstrates a maximum agreement of only 60%. According to the interpretation of Cohen’s Kappa the observers show only a moderate concordance [24]. However, in the more intricate ten-segment system, agreement sharply declines, with only 16.1% of respondents reporting a matching segment combination. Similarly low values have been documented in the existing literature [13, 22, 25, 26].
Nevertheless, the literature suggests that both inter- and intrarater reliability of classification systems can be enhanced through three-dimensional fracture visualization of CT data sets. Techniques such as 3D printing and/or MR visualization have demonstrated the potential for further improvement [2, 3]. It is advisable to leverage the most advanced visualization technology available in routine clinical practice.
Notably, additional MRI imaging has been shown to augment classification reliability [27]. In this study, the specialists sought additional preoperative MRI imaging in 42.9% of cases. Additional meniscal and/or ligamentous injuries by TPF are identified as independent factors for early secondary total TKA [28]. The incidence of these injuries is reported to be as high as 90% [29,30,31] and postoperative instability can be detected in the majority of patients [32]. But still there is no current clear recommendation for preoperative MRI [1].
Remarkably, this study reveals that a high percentage (76.8%) of primary external fixators was recommended solely based on the fracture pattern. The participants were not given information on soft tissue conditions, peripheral blood circulation, motor function, or sensitivity.
Moreover, primary TKA was not chosen in any of the cases. This might be attributed, among other factors, to the absence of information on patient age. However, it also underscores that osteosynthetic treatment continues to be considered the standard procedure, e.g. in this kind of fracture types.
Focusing on the treatment strategy, all respondents aim for comprehensive 360° treatment; however, variations arise in its implementation. These differences start with the patient’s positioning, while the complexity deepens when considering the choice of approach and plate position. The most common combination is a posteromedial and anterolateral approach with plate position, yet surgeons may opt to perform this with or without repositioning. The anterolateral approach emerges as the most frequently chosen, aligning with findings in the literature for treating TPF [20, 33, 34].
Despite the recognition of the importance of addressing posterior fracture fragments for improved patient outcomes [16, 35], the majority of respondents combine the anterolateral approach with a posterior, usually posteromedial, approach. However, studies indicate limitations in the visibility of the tibial plateau through standardized approaches [36,37,38], such as approximately 36% visibility of the joint surface with the anterolateral approach [36]. Frosch proposed “the concept of direct approach to the fractures and stepwise extension as needed” [39], with lateral condylar osteotomy capable of achieving an additional joint space opening of 5–7 mm [40]. Interestingly, this study reveals that extended approaches are not intended in the majority of cases. The German S2k guideline recommends extending the approach if the fracture is difficult to assess, but it does not specify the preferred method of extension [41]. Consequently, a standard extension of the approach is not commonly performed, possibly due to the absence of recommendations regarding whether a condylar or fibular osteotomy should be preferred.
There is little agreement on the methods of intraoperative imaging, reaching a maximum of 40% for case III. In all three cases, the image intensifier is used most commonly, supplemented in two out of three cases by an intraoperative 3D scan. Studies indicate that unsatisfactory reduction results are missed when using image intensifier controls only [42, 43]. Improved anatomical reduction can be achieved with additional intraoperative fracturoscopy [42]. Nanoscopy appears promising for enhancing visibility in the posterior-lateral-central region, even though it may still face challenges [44]. Despite the highest radiation exposure, intraoperative CT scans are the most accurate method for detecting malreduction and malpositioning of implants [40]. In complex fractures, such as those presented in this paper, it may not be sufficient to control the reduction of the image intensifier alone. It remains unclear whether the infrequent use of fracturoscopy and/or 3D scanning was due to a lack of knowledge of the technique or the unavailability of equipment.
The treatment strategy appears to be influenced by the hospitals level of care. Level II and III hospitals are significantly less likely to request a preoperative MRI, possibly due to the limited MRI capacity in their infrastructure. Investigating other notable differences between care levels, such as positioning, surgical approach and control of reduction, emphasises the importance of respondents' professional experience. Interestingly, there is no significant difference in professional experience between hospitals in level I and care levels II + III, indicating that the variations cannot be attributed to the number of operations performed by the individual surgeon.
This study has several limitations. Firstly, the small number of fracture cases introduces a selection bias, and the limited number of participants further compounds this constraint. Additionally, no information on patient age, trauma history, or clinical examination was provided during the interviews. This makes it difficult to decide whether a plate osteosynthesis or an endoprosthetic therapy is the right choice. The inconsistency in fracture classification also contributes to the variation in treatment methods, as the classification serves as a foundation for following decisions. Besides, surgeons were not asked whether they have practical experience in using an extended approach. Therefore, the present study cannot clarify whether the low use of extended approaches is due to surgeons’ lack of surgical knowledge or to the inconclusive data on the use of these approaches. Moreover, the hospitals and surgeons were not randomly chosen. It would be valuable to explore differences among a larger and more diverse group of specialists, not just from a single region but also from various countries.
Despite the limitations, the data from this study show that even among highly experienced surgeons, significant variations exist in the management of TPF. Although guidelines have been recently published [1], there is a lack of standardization concerning the overall management of TPF. As such, the authors of this paper advocate for the establishment of a national register to compile comprehensive and standardized data in the surgical management of TPF.
Conclusion
Significant variations exist in management of TPF. While a comprehensive 360° treatment approach is universally implemented across specialists, there is a lack of standardization concerning preoperative imaging, classification, initial treatment, approaches, osteosynthesis, and reduction control. Subsequently, the current lack of standardization underscores the ongoing need for uniform data in the surgical treatment of TPF, aiming to improve and reducing complications.
Data availability
No datasets were generated or analysed during the current study.
References
Deutsche Gesellschaft für Orthopädie und Unfallchirurgie e.V. (DGOU). [Tibial head fractures. Version 1.0 (29.10.2021)] 2022. https://www.awmf.org/uploads/tx_szleitlinien/187-042l_S2k_Tibiakopffrakturen_2022-07.pdf (accessed October 3, 2022).
Bitschi D, Fürmetz J, Gilbert F, Jörgens M, Watrinet J, Pätzold R, et al. Preoperative mixed-reality visualization of complex tibial plateau fractures and its benefit compared to CT and 3D printing. JCM. 2023;12:1785. https://doi.org/10.3390/jcm12051785.
Dust T, Hartel MJ, Henneberg J-E, Korthaus A, Ballhause TM, Keller J, et al. The influence of 3D printing on inter- and intrarater reliability on the classification of tibial plateau fractures. Eur J Trauma Emerg Surg. 2023;49:189–99. https://doi.org/10.1007/s00068-022-02055-1.
Krause M, Frosch K-H. Change in the treatment of tibial plateau fractures. Unfallchirurgie (Heidelb). 2022;125:527–34. https://doi.org/10.1007/s00113-022-01165-0.
Bormann M, Neidlein C, Gassner C, Keppler AM, Bogner-Flatz V, Ehrnthaller C, et al. Changing patterns in the epidemiology of tibial plateau fractures: a 10-year review at a level-I trauma center. Eur J Trauma Emerg Surg. 2022. https://doi.org/10.1007/s00068-022-02076-w.
Rudran B, Little C, Wiik A, Logishetty K. Tibial plateau fracture: anatomy, diagnosis and management. Br J Hosp Med (Lond). 2020;81:1–9. https://doi.org/10.12968/hmed.2020.0339.
Schatzker J, McBroom R, Bruce D. The tibial plateau fracture. The Toronto experience 1968--1975. Clin Orthop Relat Res. 1979;94–104.
Marsh JL, Slongo TF, Agel J, Broderick JS, Creevey W, DeCoster TA, et al. Fracture and dislocation classification compendium—2007: Orthopaedic Trauma Association classification, database and outcomes committee. J Orthop Trauma. 2007;21:S1-133. https://doi.org/10.1097/00005131-200711101-00001.
Moore TM, Patzakis MJ, Harvey JP. Tibial plateau fractures: definition, demographics, treatment rationale, and long-term results of closed traction management or operative reduction. J Orthop Trauma. 1987;1:97–119.
Krause M, Preiss A, Müller G, Madert J, Fehske K, Neumann MV, et al. Intra-articular tibial plateau fracture characteristics according to the “Ten segment classification.” Injury. 2016;47:2551–7. https://doi.org/10.1016/j.injury.2016.09.014.
Luo C-F, Sun H, Zhang B, Zeng B-F. Three-column fixation for complex tibial plateau fractures. J Orthop Trauma. 2010;24:683–92. https://doi.org/10.1097/BOT.0b013e3181d436f3.
Wang Y, Luo C, Zhu Y, Zhai Q, Zhan Y, Qiu W, et al. Updated three-column concept in surgical treatment for tibial plateau fractures—a prospective cohort study of 287 patients. Injury. 2016;47:1488–96. https://doi.org/10.1016/j.injury.2016.04.026.
Schröter S, Schreiner AJ. Klassifikationen der tibiaplateaufraktur: CT-basierte Klassifikationen für eine umfassende frakturbeurteilung und gezielte operationsplanung. Knie J. 2020;2:67–75. https://doi.org/10.1007/s43205-020-00037-0.
Krause M, Müller G, Frosch K-H. Surgical approaches to tibial plateau fractures. Unfallchirurg. 2018;121:569–82. https://doi.org/10.1007/s00113-018-0515-6.
Kraus TM, Freude T, Stöckle U, Stuby FM. Pearls and pitfalls for the treatment of tibial head fractures. Orthopade. 2016;45:24–31. https://doi.org/10.1007/s00132-015-3206-9.
Van den Berg JD, Quintens L, Zhan Y, Hoekstra H. Why address posterior tibial plateau fractures? Injury. 2020;51:2779–85. https://doi.org/10.1016/j.injury.2020.09.011.
van Dreumel RLM, van Wunnik BPW, Janssen L, Simons PCG, Janzing HMJ. Mid- to long-term functional outcome after open reduction and internal fixation of tibial plateau fractures. Injury. 2015;46:1608–12. https://doi.org/10.1016/j.injury.2015.05.035.
Elsoe R, Johansen MB, Larsen P. Tibial plateau fractures are associated with a long-lasting increased risk of total knee arthroplasty a matched cohort study of 7,950 tibial plateau fractures. Osteoarthritis Cartilage. 2019;27:805–9. https://doi.org/10.1016/j.joca.2018.12.020.
Parkkinen M, Lindahl J, Mäkinen TJ, Koskinen SK, Mustonen A, Madanat R. Predictors of osteoarthritis following operative treatment of medial tibial plateau fractures. Injury. 2018;49:370–5. https://doi.org/10.1016/j.injury.2017.11.014.
Rossmann M, Fensky F, Ozga A-K, Rueger JM, Märdian S, Russow G, et al. Tibial plateau fracture: does fracture classification influence the choice of surgical approach? A retrospective multicenter analysis. Eur J Trauma Emerg Surg. 2022;48:3635–41. https://doi.org/10.1007/s00068-020-01388-z.
Doornberg JN, Rademakers MV, Van Den Bekerom MP, Kerkhoffs GM, Ahn J, Steller EPh, et al. Two-dimensional and three-dimensional computed tomography for the classification and characterisation of tibial plateau fractures. Injury. 2011;42:1416–25. https://doi.org/10.1016/j.injury.2011.03.025.
Millar SC, Arnold JB, Thewlis D, Fraysse F, Solomon LB. A systematic literature review of tibial plateau fractures: What classifications are used and how reliable and useful are they? Injury. 2018;49:473–90. https://doi.org/10.1016/j.injury.2018.01.025.
Audigé L, Bhandari M, Hanson B, Kellam J. A concept for the validation of fracture classifications. J Orthop Trauma. 2005;19:404–9. https://doi.org/10.1097/01.bot.0000155310.04886.37.
McHugh ML. Interrater reliability: the kappa statistic. Biochem Med (Zagreb). 2012;22:276–82.
Alvi F, Charalambous CP, Tryfonidis M, Samarji R, Hirst P. Inter- and intra-observer variation of the Schatzker and AO/OTA classifications of tibial plateau fractures and a proposal of a new classification system. Injury Extra. 2008;39:178. https://doi.org/10.1016/j.injury.2007.11.344.
Harish S, Roberts C, Blundell C, Walton NP. AO or Schatzker? How reliable is classification of tibial plateau fractures? Arch Orthop Trauma Surg. 2003;123:396–8. https://doi.org/10.1007/s00402-003-0573-1.
Voss EE, Goode RD, Cook JL, Crist BD. Survey of orthopaedic trauma providers: is MRI superior to CT scan for evaluating and preoperative planning for tibial plateau fractures? Mo Med. 2022;119:261–5.
Hansen L, Larsen P, Elsoe R. Characteristics of patients requiring early total knee replacement after surgically treated lateral tibial plateau fractures-a comparative cohort study. Eur J Orthop Surg Traumatol. 2022;32:1097–103. https://doi.org/10.1007/s00590-021-03083-0.
Adams JD, Loeffler MF. Soft tissue injury considerations in the treatment of tibial plateau fractures. Orthop Clin North Am. 2020;51:471–9. https://doi.org/10.1016/j.ocl.2020.06.003.
Figueroa F, Figueroa D, Putnis S, Guiloff R, Caro P, Espregueira-Mendes J. Posterolateral corner knee injuries: a narrative review. EFORT Open Rev. 2021;6:676–85. https://doi.org/10.1302/2058-5241.6.200096.
Stannard JP, Lopez R, Volgas D. Soft tissue injury of the knee after tibial plateau fractures. J Knee Surg. 2010;23:187–92. https://doi.org/10.1055/s-0030-1268694.
Bormann M, Neidlein C, Neidlein N, Ehrl D, Jörgens M, Berthold DP, et al. high prevalence of persistent measurable postoperative knee joint laxity in patients with tibial plateau fractures treated by open reduction and internal fixation (ORIF). JCM. 2023;12:5580. https://doi.org/10.3390/jcm12175580.
Kandemir U, Maclean J. Surgical approaches for tibial plateau fractures. J Knee Surg. 2013;27:021–30. https://doi.org/10.1055/s-0033-1363519.
Krause M, Frosch K-H. Wandel in der Behandlung der Tibiakopffraktur. Unfallchirurgie. 2022;125:527–34. https://doi.org/10.1007/s00113-022-01165-0.
Çağlar C, Akcaalan S, Özaslan Hİ, Bozer M, Emre F, Uğurlu M. Comparative analysis of single lateral locked plate and double locked plate application in the treatment of bicondylar tibial plateau fractures. Cureus. 2021. https://doi.org/10.7759/cureus.19298.
Krause M, Krüger S, Müller G, Püschel K, Frosch K-H. How can the articular surface of the tibial plateau be best exposed? A comparison of specific surgical approaches. Arch Orthop Trauma Surg. 2019;139:1369–77. https://doi.org/10.1007/s00402-019-03200-z.
Solomon LB, Stevenson AW, Lee YC, Baird RPV, Howie DW. Posterolateral and anterolateral approaches to unicondylar posterolateral tibial plateau fractures: a comparative study. Injury. 2013;44:1561–8. https://doi.org/10.1016/j.injury.2013.04.024.
Korthaus A, Ballhause TM, Kolb J-P, Krause M, Frosch K-H, Hartel MJ. Extended approach to the lateral tibial plateau with central meniscal subluxation in fracture repair: feasibility and first clinical and radiographic results. Eur J Trauma Emerg Surg. 2020;46:1221–6. https://doi.org/10.1007/s00068-020-01467-1.
Frosch K-H, Korthaus A, Thiesen D, Frings J, Krause M. The concept of direct approach to lateral tibial plateau fractures and stepwise extension as needed. Eur J Trauma Emerg Surg. 2020;46:1211–9. https://doi.org/10.1007/s00068-020-01422-0.
Krause M, Müller G, Frosch K-H. Chirurgische Zugänge bei Tibiakopffrakturen. Unfallchirurg. 2018;121:569–82. https://doi.org/10.1007/s00113-018-0515-6.
Berninger MT, Schüttrumpf JP, Barzen S, Domnick C, Eggeling L, Fehske K, et al. S2k-leitlinie tibiakopffraktur—klassifikation diagnostik und therapie. Z Orthop Unfall. 2023. https://doi.org/10.1055/a-2121-6538.
Beisemann N, Keil H, Swartman B, Schnetzke M, Franke J, Grützner PA, et al. Intraoperative 3D imaging leads to substantial revision rate in management of tibial plateau fractures in 559 cases. J Orthop Surg Res. 2019;14:236. https://doi.org/10.1186/s13018-019-1286-7.
Krause M, Preiss A, Meenen NM, Madert J, Frosch K-H. “Fracturoscopy” is superior to fluoroscopy in the articular reconstruction of complex tibial plateau fractures-an arthroscopy assisted fracture reduction technique. J Orthop Trauma. 2016;30:437–44. https://doi.org/10.1097/BOT.0000000000000569.
Behrendt P, Berninger MT, Thürig G, Dehoust J, Christensen J, Frosch K-H, et al. Nanoscopy and an extended lateral approach can improve the management of latero-central segments in tibial plateau fractures: a cadaveric study. Eur J Trauma Emerg Surg. 2023;49:1433–9. https://doi.org/10.1007/s00068-022-02188-3.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
Conceptualization: M.B. and C.H. Methodology: M.B., C.N., D.P.B. and J.F. Software: D.B. and C.H. Validation, M.B., D.P.B. and J.F. Formal analysis: C.H., C.N. and D.B. Data curation: C.N., J.W., L.S. Writing—original draft preparation: C.N., M.B., D.P.B. Writing—review and editing: J.F., R.P., B.H. and W.B. Visualization: C.H. Supervision: M.B. Project administration: M.B. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All Authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patentlicensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript. The authors declare no competing interests.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendix Table 4
Appendix Table 4
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hörmandinger, C., Bitschi, D., Berthold, D.P. et al. Lack of standardisation in the management of complex tibial plateau fractures: a multicentre experience. Eur J Trauma Emerg Surg (2024). https://doi.org/10.1007/s00068-024-02616-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00068-024-02616-6