Abstract
Purpose
Sarcopenia may complicate treatment in cancer patients. Herein, we assessed whether sarcopenia measurements derived from radiation planning computed tomography (CT) were associated with complications and tumor progression during radiochemotherapy for glioblastoma.
Methods
Consecutive patients undergoing radiotherapy planning for glioblastoma between 2010 and 2021 were analyzed. Retrocervical muscle cross-sectional area (CSA) was measured via threshold-based semi-automated radiation planning CT analysis. Patients in the lowest sex-specific quartile of muscle measurements were defined as sarcopenic. We abstracted treatment characteristics and tumor progression from the medical records and performed uni- and multivariable time-to-event analyses.
Results
We included 363 patients in our cohort (41.6% female, median age 63 years, median time to progression 7.7 months). Sarcopenic patients were less likely to receive chemotherapy (p < 0.001) and more likely to be treated with hypofractionated radiotherapy (p = 0.005). Despite abbreviated treatment, they more often discontinued radiotherapy (p = 0.023) and were more frequently prescribed corticosteroids (p = 0.014). After treatment, they were more often transferred to inpatient palliative care treatment (p = 0.035). Finally, progression-free survival was substantially shorter in sarcopenic patients in univariable (median 5.1 vs. 8.4 months, p < 0.001) and multivariable modeling (hazard ratio 0.61 [confidence interval 0.46–0.81], p = 0.001).
Conclusion
Sarcopenia is a strong risk factor for treatment discontinuation and reduced progression-free survival in glioblastoma patients. We propose that sarcopenic patients should receive intensified supportive care during radiotherapy and during follow-up as well as expedited access to palliative care.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glioblastoma patients face a limited prognosis despite trimodal therapy regimens (surgical resection followed by chemoradiotherapy) [1, 2]. Early progression is common and median overall survival is 14 months [1]. However, within the cohort, individual prognosis differs significantly [3]. While some patients are transferred to inpatient palliative care weeks after diagnosis, others may survive for years without tumor recurrence [3]. Treatment tolerance similarly varies, with some patients progressing during radiotherapy and/or discontinuing treatment due to deteriorating clinical condition [4].
Sarcopenia refers to reduced skeletal muscle strength or mass and is associated with decreased physical functioning [5]. Underlying mechanisms include aging, malnutrition, and systemic inflammation [6]. In cancer patients, sarcopenia may result in increased treatment- and cancer-related complications [7] as well as deteriorating outcomes [8, 9]. Assessment of sarcopenia on computed tomography (CT) imaging is a key noninvasive approach to identify this at-risk group [10].
We previously established a semi-automated threshold-based algorithm for muscle measurement on cranial glioblastoma radiation planning CT scans [11]. Patients with low muscle measurements showed reduced overall survival (OS) [11]. In this follow-up analysis in a larger cohort, we aimed to assess whether CT-based measurements are helpful to identify patients with more immediate clinically meaningful outcomes, including treatment-related complications and early cancer progression.
Methods
The ethics committee of the Medical Association of Westphalia-Lippe (2021-685-f-S) approved this retrospective analysis.
Patient cohort
We screened consecutive adult patients undergoing radiotherapy planning CT scans for histologically proven primary glioblastoma (isocitrate dehydrogenase 1 [IDH1] wildtype) at our institution between January 1, 2010, and December 31, 2021. Patients with incomplete clinical data and those with incomplete visualization of the C1 vertebra (either due to cutoff or due to streak artifacts) were excluded. We also omitted patients with prior cranial radiotherapy. Only patients with a novel glioblastoma diagnosis were considered, while recurrences were not included. Hence, inclusion criteria were similar to before [11] and the prior cohort from the exploratory study was part of this follow-up analysis. A screening flowchart is demonstrated in Supplementary Fig. 1.
Glioblastoma was defined according to the current definition of the World Health Organization (WHO) [12]. Clinical data collected included patient demographics (age, sex, height, weight, comorbidities according to the Charlson Comorbidity Index [CCI], and postoperative Eastern Cooperative Oncology Group [ECOG] score), tumor characteristics (date of diagnosis, tumor volume, O6-methylguanine-DNA methyltransferase [MGMT] promotor methylation, and IDH1 mutational status), and course of treatment (surgery date, resection status, planned radiotherapy dose and fractionation, applied radiotherapy dose and fractionation, chemotherapy application, prescription of corticosteroids, posttreatment stay, and progression during chemoradiotherapy). Hypofractionation was defined as a daily radiation dose exceeding 2 Gy. ECOG score was determined postoperatively at the onset of chemoradiotherapy, as recommended [13]. We quantified tumor volume on preoperative contrast-enhanced T1-weighted magnetic resonance imaging (MRI). Finally, we also collected data on first-time postoperative progression according to the Response Assessment in Neuro-Oncology Criteria (RANO) criteria [14]. Progression was defined as the time interval from diagnosis to MRI-based progression or death, whichever occurred first, similar to previous studies [15]. Data were partly abstracted from the patient records as well as from a neurosurgical database and the cancer registry of the Western German Cancer Center (Westdeutsches Tumorzentrum, WTZ).
Muscle measurements
Non-contrast radiation planning CT scans were downloaded from the ARIA Oncology Information System (Varian Medical Systems, Inc., Palo Alto, USA). After upload onto 3D Slicer (version 4.13.0), we reformatted the CT scans along the frontal and vertical plains to ensure consistent measurements independent of patient positioning. This way, the nasal septum and the dens axis were both oriented in a vertical plain [11].
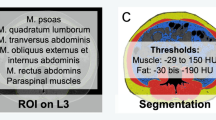
We measured the retrocervical muscle area on a single axial CT image at the level of the first cervical vertebra (C1), as previously established (Fig. 1a; [11]). Threshold-based semi-automated segmentations were performed with established cutoff values of −29 to +150 Hounsfield units [9]. Besides the autochthonous muscles, we also included the trapezoid, the sternocleidomastoid, and the levator scapulae muscles in the analyses. Quantifications resulted in a muscle cross-sectional area value, measured in square centimeters.
Body composition measurement results.
a Illustration of the measurements on an axial cranial CT image at the height of the first cervical vertebra (C1). The retrocervical muscle area is marked in blue and encompasses the autochthonous, the trapezoid, the sternocleidomastoid and the levator scapulae muscles. b, c: Histograms of muscle measurements in male (b) and female (c) patients. Females had lower measurements compared to male patients. Patients in the lowest quartile of their sex were defined as sarcopenic, as marked in grey in the histogram
Statistical analysis
Descriptive statistics were used to analyze patient characteristics. We compared the incidence of bivariate outcomes between sarcopenic and non-sarcopenic patients using the chi2 test. For progression-free survival (PFS), we chose a time-to-event analysis, starting at the time of histopathologic diagnosis. In univariable analyses, we used the Kaplan–Meier method and ran a log-rank test to evaluate statistical significance. In multivariable analyses, Cox proportional hazard regressions were applied. The same model previously developed for OS [11] was used for consistency for PFS and OS. Additionally, we generated a second multivariable PFS model restricted to patients who completed standard chemoradiotherapy (e.g., 60 Gy of radiation with concomitant chemotherapy). Statistics were performed using the STATA software package (version 13.0, StataCorp, College Station, TX). A p-value below 0.05 was considered significant.
Results
Patient cohort and treatment
We screened 372 glioblastoma patients treated at our institution and included 363 in the study (Supplementary Fig. 1). Median age was 63 years, median body mass index (BMI) was 26 kg/m2, and most patients were male (58.4%). Median CCI was 4, indicative of a low comorbidity score. Roughly half of the tumors showed MGMT methylation. Most glioblastomas were fully (44.6%) or partially (40.2%) resected, while the remainder (15.2%) underwent biopsy only (Table 1).
Of 363 patients, 311 (85.7%) received the standard radiation dose of 60 Gy. 90.1% of patients were administered concomitant chemotherapy. Chemotherapy overwhelmingly included temozolomide according to Stupp et al. [16] (81.9%), or temozolomide and CCNU according to Herrlinger et al. [17] (14.1%). 52.6% of patients were prescribed corticosteroids during radiotherapy. 9.4% discontinued radiation. Overall, 303/363 (83.5%) of patients completed chemoradiotherapy (i.e., radiotherapy to a dose of 60 Gy and concomitant chemotherapy). 7.7% progressed during radiotherapy. After treatment completion, 86.3% continued outpatient care while 6.3% were transferred to inpatient palliative care treatment. Median PFS was 7.7 months (95% confidence interval 7.0–8.5 months). 89.5% of patients progressed during follow-up: 259 (71.3%) patients showed progression on follow-up imaging while 66 (18.2%) of patients died with no available prior evidence of progression. Finally, 38 (10.5%) patients did not progress during follow-up.
Muscle measurements
Median muscle measurement was 27.7 cm2 (interquartile range 22.5–33.0 cm2). Men demonstrated a larger muscle area at 31.1 cm2 (interquartile range 27.6–35.2 cm2) compared to females at 22.2 cm2 (interquartile range 19.9–25.4 cm2; p < 0.001). Measurements of less than 27.6 cm2 in males and less than 19.9 cm2 in females were considered sarcopenic as they were in the lowest quartile of their peers (Fig. 1b, c). In sum, 91 patients (38 females and 53 males) were considered sarcopenic while 272 patients (113 females and 159 males) were considered non-sarcopenic.
Sarcopenic patients had a lower BMI (p = 0.006), were older (p < 0.001), and had higher CCI (p < 0.001) and ECOG scores (p < 0.001). Finally, tumors were larger at the time of diagnosis (p = 0.023). There were no differences in tumor characteristics otherwise, and resection status was not different between groups (Table 1).
Radiochemotherapy treatment
Sarcopenic patients were more infrequently treated with a standard dose of 60 Gy (78.0% vs. 88.2% for non-sarcopenic patients, p = 0.016). Concomitant chemotherapy was also more rarely administered (79.1% vs. 93.7%, p < 0.001). Conversely, radiotherapy was more often applied in a hypofractionated treatment regimen (12.1% vs. 4.0%, p = 0.005, Fig. 2a).
Differences in treatment application and tolerance between sarcopenic and non-sarcopenic patients. a Sarcopenic patients were less likely to receive the standard dose of 60 Gy and concomitant chemotherapy treatment. They were more likely to be treated with hypofractionated radiotherapy and more likely to be prescribed corticosteroids. b Sarcopenic patients more often progressed during therapy and discontinued treatment. They were less likely to be discharged home after treatment, but more likely to be transferred to in-patient palliative care
During treatment, sarcopenic patients were more often prescribed corticosteroids due to intracranial pressure symptoms (63.7% vs. 48.9%, p = 0.014). Sarcopenic patients showed a higher rate of progression during therapy (13.2% vs. 5.9%, p = 0.024) and radiotherapy was more often discontinued before reaching the prescribed dose (15.4% vs. 7.4%, p = 0.023). In sum, 72.5% of sarcopenic patients received standard chemoradiotherapy (i.e., radiation to a dose of 60 Gy in 2‑Gy fractions with concomitant chemotherapy), compared to 87.1% of non-sarcopenic patients (p = 0.001). Among those patients treated with chemotherapy, there was no difference in the choice of agent (temozolomide, temozolomide plus CCNU, or other) between the groups (p = 0.2, Supplementary Table 1). 11.0% of sarcopenic and 4.8% of non-sarcopenic patients were transferred to inpatient palliative care (p = 0.035). Conversely, sarcopenic patients were less likely to remain in outpatient care (as opposed to inpatient treatment) after completion of radiotherapy (80.2% vs. 88.2%, p = 0.055, Fig. 2b).
Progression-free survival
PFS was markedly reduced in sarcopenic patients in univariable analyses in Kaplan–Meier assessment (Fig. 3a) and log rank test (p < 0.001). Median time to progression was 5.1 months (95% confidence interval 4.0–7.0 months) in sarcopenic patients and 8.4 months in the non-sarcopenic group (95% confidence interval 7.5–9.9 months). When assessing all muscle measurement quartiles separately, no significant differences in PFS were seen between the upper three quartiles (median time to progression was 9.1, 8.0, and 8.7 months for the second, third, and fourth quartile, respectively; Fig. 3b). We then performed multivariable modeling, describing hazard ratios (HR) and confidence intervals (CI) for the association between patient, tumor, or treatment parameters (including sarcopenia measurements) and PFS (Table 2). When including predefined risk factors, sarcopenia remained independently associated with PFS (HR 0.61 [CI 0.46–0.81] relative to non-sarcopenic patients, p = 0.001). Additionally, BMI, CCI, and bilateral tumors showed a negative, while extent of resection and methylated MGMT status showed a positive association with PFS (Table 2). We also tested continuous muscle measurements in a multivariable model, finding that increased muscle measurements (per cm2) were independently associated with prolonged PFS (HR 0.96 [CI 0.94–0.99], p = 0.002, Table 2). In a final analysis, we restricted the multivariable model to patients completing standard chemoradiotherapy treatment (i.e., treatment with 60 Gy in 2 Gy fractions and concomitant chemotherapy) to assess independence of muscle-related associations from treatment completion. The significance of muscle measurements remained unchanged both in analyses for sarcopenic/non-sarcopenic groups (HR 0.65 [CI 0.47–0.89], p = 0.008) and for continuous measurements (HR 0.97 [CI 0.94–0.99], p = 0.007) in this subgroup (Supplementary Table 2).
Progression-free survival (PFS) in sarcopenic and non-sarcopenic patients in univariable analyses. a Sarcopenic patients showed a reduced PFS compared to non-sarcopenic patients. b There was no difference in PFS within the non-sarcopenic group (e.g., between patients in the second, third, and fourth quartile of muscle measurements)
Our novel sarcopenia definition was also strongly associated with OS in univariable log-rank analyses (median survival 9.1 vs 15.2 months, p < 0.001, Supplementary Fig. 2) as well as in multivariable modeling (HR 0.64, CI 0.49–0.85, p = 0.002, Supplementary Table 3). This extends and reconfirms previous exploratory results [11].
Discussion
In this study, we found that sarcopenia is a strong risk factor for reduced treatment regimens, treatment discontinuation, and diminished PFS in glioblastoma patients. We suggest that sarcopenic patients may profit from enhanced follow-up and expedited palliative care access.
Our work is based on a prior proof-of-concept study in glioblastoma patients. It demonstrated that radiation planning CTs could be used for body composition measures at C1, that these measures were representative of whole-body measurements, and that muscle measures were associated with OS in glioblastoma patients [11].
In this follow-up study in an enlarged and updated patient cohort, we investigated whether body composition parameters were clinically valuable for identifying patients at risk for adverse treatment courses. Here, we a priori defined patients in the lowest quartile of muscle measurements as sarcopenic. While predefined cutoffs for sarcopenic patients are available at multiple levels in the trunk [10], no data exist regarding the neck. Subsequently, we defined a cohort-based cutoff, similar to previous studies [18]. Based on this setup, we drew multiple key conclusions, which are discussed in the following sections.
Sarcopenic patients undergo abbreviated postoperative treatment regimens
The American Society for Radiation Oncology (ASTRO) guideline [19] suggests age-stratified glioblastoma treatment with hypofractionated radiotherapy recommended for patients older than 70 years. The guideline’s authors note that age cutoff values may differ substantially between studies and many subgroup analyses are small and retrospective in nature [19]. Age also remains an imperfect variable given variation in physical functioning, quality of life, and prognosis between patients of similar age [20, 21]. Elderly patients with good physical functioning may indeed profit from chemoradiotherapy as opposed to radiotherapy alone [22]. Thus, the authors also suggest stratifying by “performance status” to better individualize treatment recommendations [19]. However, Karnofsky index and ECOG score are prone to high interrater variability [23, 24].
As a quantitative, non-rater-dependent biomarker, sarcopenia measurements incorporate both age and performance status [25]. Sarcopenia becomes more prevalent with age [25] and is negatively associated with physical functioning [5]. Some studies refer to body composition parameters as measures of “biologic age” as they encompass nutritional factors, functioning, inflammation, and chronological age [26]. This is reflected in our findings, as sarcopenia was associated with these factors in our cohort: Significant associations were found for chronological age, BMI, comorbidities, and ECOG score. The strongest signal was found for ECOG, demonstrating a close association between sarcopenia and (reduced) physical functioning, as expected [27]. Notably, whether sarcopenia is the result of a limited physical status or vice versa remains unclear, as the retrospective nature of our study allows for tests regarding association, but not causation. In any case, sarcopenia is a quantitative marker related to numerous otherwise difficult-to-quantify parameters.
Importantly, as discussed above, sarcopenia is associated with key treatment stratification parameters from the ASTRO guideline. Consistent with this, sarcopenic patients were less likely to receive the standard chemoradiotherapy treatment and more likely to undergo hypofractionated irradiation without chemotherapy in our study. Interestingly, despite abbreviated and de-escalated treatment prescription, patients still discontinued radiotherapy at a higher rate. This underlines the need for reduced treatment paradigms in sarcopenic patients. Hence, sarcopenia may be a potential prospective marker that helps guide treatment decision-making.
Treatment tolerance is reduced in sarcopenic patients
It is well known that elderly patients are at risk for increased treatment toxicities, most prominently from chemotherapy [28]. Treating younger patients with poor performance status also remains a major concern, yet data are more limited [29].
Sarcopenic patients were more often prescribed corticosteroids during treatment (64% vs. 49%). Steroids may change body composition measures in the long term [30] and aggravate sarcopenia. However, this is unlikely to have affected our measurements, as radiation planning CTs are commonly performed within weeks after primary diagnosis and neurosurgical evaluation, and before application of radiotherapy. Our data indicate that sarcopenic patients may be more at risk for brain edema symptoms, as these trigger corticosteroid therapy during radiation.
While chemoradiotherapy discontinuation was increased among sarcopenic patients, 85% of sarcopenic patients still completed the prescribed therapy. We believe our data suggest that sarcopenic patients may profit from more intensive medical care during therapy. However, the relatively high completion rate of radiotherapy among sarcopenic patients may support continued use of postoperative radiotherapy (with the potential omission of chemotherapy) in this group, similar to findings from the ASTRO guideline for elderly patients or those with low performance status [19]. However, our study is retrospective in nature and this question may only be definitively answered in prospective trials.
Upon treatment completion, a small subpopulation of patients were directly transferred to in-patient palliative care, likely indicative of limited outcomes. While these patients were overrepresented among sarcopenic patients (11% vs. 5%), the vast majority of sarcopenic patients, 80%, returned home after treatment. Sarcopenia may nonetheless help to identify patients who could profit from intensified, and early, palliative care interventions.
Progression-free survival is substantially reduced in sarcopenic patients
The associations between sarcopenia and OS [31, 32] or complications [33,34,35] have been well described in numerous malignancies. Most studies imply that reduced physical functioning (commonly associated with sarcopenia [5]) is likely to make patients more susceptible to developing complications, limiting survival [36].
In our study, sarcopenic glioblastoma patients were more likely to show early progression. This is an intriguing finding, as PFS (different from OS) is likely not directly associated with reduced physical functioning. We initially hypothesized that treatment discontinuation in sarcopenic patients may induce early progression, similar to other malignancies [37]. However, while treatment was more limited in some sarcopenic patients, nearly three quarters of sarcopenic patients received full standard chemoradiotherapy treatment. Thus, we considered differences in treatment completion or chemotherapy application as unlikely to account for the drastic difference in PFS across the entire sarcopenic population (median PFS 5.1 vs. 8.4 months). When assessing this hypothesis, we were reluctant to include chemoradiotherapy treatment completion in our multivariable PFS model as we aimed to only include parameters available at the onset of postoperative treatment. Chemotherapy discontinuation may be advised during treatment depending on blood test results [38]. Hence, we generated a second, separate multivariable model and only included patients who completed standard chemoradiotherapy. In this homogeneously treated cohort, the strong association between sarcopenia and PFS remained unchanged. Consequently, we concluded that the link between sarcopenia and PFS was not mediated via treatment discontinuation.
Similarly, sarcopenic patients did not show differences in tumor characteristics or resection status, making it unlikely that these factors confounded our findings. Notably, tumor volume at diagnosis was slightly increased in sarcopenic patients, raising questions regarding the interplay between increased tumor growth and a rising incidence of sarcopenia. This association has been confirmed in different tumor entities [39]. However, despite inclusion of tumor volume in our multivariable model, sarcopenia was independently associated with PFS. Interestingly, residual tumor volume was not significantly different between the groups after resection.
Thus, after careful consideration of potential clinical confounders, we believe that sarcopenia is instead likely to be associated with PFS via biological factors. In other tumor entities, these include sarcopenia-related immune senescence [40] and proinflammatory signaling [41]. In glioblastoma, immunosuppression increases treatment resistance [42, 43] and induces a distinct neuroinflammatory microenvironment that promotes tumor growth and invasion [44]. In lung cancer, immunotherapy (which is not part of standard treatment in glioblastoma patients) showed a substantially reduced efficacy in sarcopenic patients, potentially indicative of an altered baseline immune system in this group [45]. Prospective studies have linked sarcopenia to systemic inflammation and a distinct disbalance in the immune system [46].
We suspect that sarcopenic glioblastoma patients are a subgroup of patients with an unfavorable inflammatory and immune status. This may potentially promote tumor progression, leading to the reduction in PFS we see in the sarcopenic subgroup.
Clinically, this finding is meaningful, as the optimal follow-up intervals for glioblastoma patients remain unclear [47]. In the absence of specific evidence, MRIs are commonly scheduled every 12 weeks following intra-institutional pragmatic considerations [47, 48]. Novel computational studies try to predict individualized time to progression to optimize this decision [49]. Here, sarcopenia may be a valuable parameter. Our study indicates that it may be reasonable for sarcopenic patients to undergo more frequent follow-up imaging, e.g., every 8 weeks. However, given moderate tolerance of primary treatment and aggressive tumor growth in sarcopenic patients, the clinical benefit of sometimes intensive retreatment [50] in this vulnerable subgroup might be debatable and should be considered with care. Notably, our measurements—which become available only postsurgically—are neither designed nor intended to change the role of up-front surgical resection, which remains the gold standard for virtually all glioblastoma patients.
As expected, based on the previous exploratory study [11], our increased cohort again shows a convincing association with overall survival, both in univariable and multivariable modeling. Different from the previous study, we used our novel sarcopenia definition in this analysis (as opposed to simply dividing a cohort by the median, as done in the exploratory stage). The strong difference in median survival of roughly 6 months demonstrates that our sarcopenic measurements have identified an at-risk cohort with good selectivity.
CT-based measurements are universally attainable in radiotherapy patients. The diversification of the glioma diagnosis based on methylation profiling and gene expression [51, 52] will increase the need for commonly available parameters to identify populations in which treatment escalation or de-escalation is advisable. This was also reflected in a recent post-hoc analysis of the CATNON trial [53], questioning the concurrent use of TMZ in adjuvant glioblastoma radiotherapy, and the lack of a clear treatment standard for patients with relapsed disease. Identifying sarcopenia early might help to guide treatment decisions in different prognostic groups and stages of treatment. End-to-end pipelines for automation of measurements have been built and may facilitate clinical implementation [54]. Automated scoring results (e.g., percentiles based on the overall patient cohort) may then be considered for therapeutic decision-making. However, prospective data collection and validation needs to precede any assessment of clinical application.
Sarcopenia may be modifiable to some degree, mainly through changes in nutrition and physical activity [55]. However, whether these interventions improve outcomes remains unclear. Our study indicates that patients with very low muscle measurements would potentially profit most from interventions. However, these patients may suffer from neurologic symptoms, such as hemiplegia, limiting the potential for physical exercise. Nonetheless, we believe that past and ongoing physical exercise [56,57,58] and nutritional investigations [59] in glioblastoma patients have merit.
Some study limitations should be noted. First, this is a retrospective monocentric analysis with the corresponding risk of bias. However, we included a large cohort of patients and patient characteristics were largely representative of the overall glioblastoma population. Nonetheless, we remain unable to address prospective questions such as the modifiability of sarcopenia. Second, multivariable modeling was not possible for treatment application and tolerance outcomes due to high treatment adherence and low complication rates. However, multivariable modeling was performed for PFS. Third, PFS was not available in all patients. However, a large majority of patients, more than 70%, had available PFS. Fourth, semi-automated segmentations required some manual correction, potentially limiting clinical application. However, body composition measurements have been shown to be automatable thanks to novel computational methods [60]. Finally, MRI-based radiation planning may alleviate the need for cranial planning CTs in the future, preventing opportunistic sarcopenia measurements. However, while technically more challenging, sarcopenia measures are also feasible on MRI imaging [61].
Conclusion
In conclusion, we found that sarcopenia is a strong risk factor for abbreviated treatment regimens, treatment discontinuation, and reduced progression-free survival in glioblastoma patients. As a distinct at-risk group, sarcopenic patients may profit from intensified care during treatment, enhanced follow-up after treatment completion, and expedited access to palliative care. Prospective trials are needed to further investigate the potential relevance of sarcopenia for therapeutic decision-making.
References
Tan AC, Ashley DM, López GY et al (2020) Management of glioblastoma: state of the art and future directions. CA Cancer J Clin 70:299–312. https://doi.org/10.3322/caac.21613
Pepper NB, Stummer W, Eich HT (2022) The use of radiosensitizing agents in the therapy of glioblastoma multiforme—a comprehensive review. Strahlenther Onkol 198:507–526
Park CK, Bae JM, Park SH (2020) Long-term survivors of glioblastoma are a unique group of patients lacking universal characteristic features. Neurooncol Adv 2:1–2. https://doi.org/10.1093/noajnl/vdz056
Burton E, Yusuf M, Gilbert MR et al (2020) Failure to complete standard radiation therapy in glioblastoma patients: patterns from a national database with implications for survival and therapeutic decision making in older glioblastoma patients. J Geriatr Oncol 11:680–687. https://doi.org/10.1016/j.jgo.2019.08.014
Santilli V, Bernetti A, Mangone M, Paoloni M (2014) Clinical definition of sarcopenia. Clin Cases Miner Bone Metab 11:177–180. https://doi.org/10.11138/ccmbm/2014.11.3.177
Ligibel JA, Schmitz KH, Berger NA (2020) Sarcopenia in aging, obesity, and cancer. Transl Cancer Res 9:5760–5771. https://doi.org/10.21037/tcr-2019-eaoc-05
Fintelmann FJ, Troschel FM, Mario J et al (2018) Thoracic skeletal muscle is associated with adverse outcomes after lobectomy for lung cancer. Ann Thorac Surg 105:1507–1515. https://doi.org/10.1016/j.athoracsur.2018.01.013
Au PC‑M, Li H‑L, Lee GK‑Y et al (2021) Sarcopenia and mortality in cancer: a meta-analysis. Osteoporos Sarcopenia 7:S28–S33. https://doi.org/10.1016/j.afos.2021.03.002
Troschel FM, Jin Q, Eichhorn F et al (2021) Sarcopenia on preoperative chest computed tomography predicts cancer-specific and all-cause mortality following pneumonectomy for lung cancer: a multicenter analysis. Cancer Med 10:6677–6686. https://doi.org/10.1002/cam4.4207
Derstine BA, Holcombe SA, Ross BE et al (2018) Skeletal muscle cutoff values for sarcopenia diagnosis using T10 to L5 measurements in a healthy US population. Sci Rep 8:1–8. https://doi.org/10.1038/s41598-018-29825-5
Troschel FM, Troschel BO, Kloss M et al (2023) Cervical body composition on radiotherapy planning computed tomography scans predicts overall survival in glioblastoma patients. Clin Transl Radiat Oncol 40:100621. https://doi.org/10.1016/j.ctro.2023.100621
Louis DN, Perry A, Wesseling P et al (2021) The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol 23:1231–1251. https://doi.org/10.1093/neuonc/noab106
Chambless LB, Kistka HM, Parker SL et al (2015) The relative value of postoperative versus preoperative karnofsky performance scale scores as a predictor of survival after surgical resection of glioblastoma multiforme. J Neurooncol 121:359–364. https://doi.org/10.1007/s11060-014-1640-x
Wen PY, Macdonald DR, Reardon DA et al (2010) Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol 28:1963–1972. https://doi.org/10.1200/JCO.2009.26.3541
Tsien CI, Pugh SL, Dicker AP et al (2023) NRG oncology/RTOG1205: a randomized phase II trial of concurrent bevacizumab and reirradiation versus bevacizumab alone as treatment for recurrent glioblastoma. J Clin Oncol 41:1285–1295. https://doi.org/10.1200/JCO.22.00164
Stupp R, Hegi ME, Mason WP et al (2009) Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5‑year analysis of the EORTC-NCIC trial. Lancet Oncol 10:459–466. https://doi.org/10.1016/S1470-2045(09)70025-7
Herrlinger U, Tzaridis T, Mack F et al (2019) Lomustine-temozolomide combination therapy versus standard temozolomide therapy in patients with newly diagnosed glioblastoma with methylated MGMT promoter (CeTeG/NOA-09): a randomised, open-label, phase 3 trial. Lancet 393:678–688. https://doi.org/10.1016/S0140-6736(18)31791-4
Dufour AB, Hannan MT, Murabito JM et al (2013) Sarcopenia definitions considering body size and fat mass are associated with mobility limitations: the framingham study. J Gerontol A Biol Sci Med Sci 68:168–174. https://doi.org/10.1093/gerona/gls109
Cabrera AR, Kirkpatrick JP, Fiveash JB et al (2016) Radiation therapy for glioblastoma: executive summary of an American society for radiation oncology evidence-based clinical practice guideline. Pract Radiat Oncol 6:217–225. https://doi.org/10.1016/j.prro.2016.03.007
Levine ME (2013) Modeling the rate of senescence: can estimated biological age predict mortality more accurately than chronological age? J Gerontol A Biol Sci Med Sci 68:667–674. https://doi.org/10.1093/gerona/gls233
Foo H, Mather KA, Thalamuthu A, Sachdev PS (2019) The many ages of man: diverse approaches to assessing ageing-related biological and psychological measures and their relationship to chronological age. Curr Opin Psychiatry 32:130–137. https://doi.org/10.1097/YCO.0000000000000473
Hanna C, Lawrie TA, Rogozińska E et al (2020) Treatment of newly diagnosed glioblastoma in the elderly: a network meta-analysis. Cochrane Database Syst Rev 3:CD13261. https://doi.org/10.1002/14651858.CD013261.pub2
Chow R, Chiu N, Bruera E et al (2016) Inter-rater reliability in performance status assessment among health care professionals: a systematic review. Ann Palliat Med 5:83–92. https://doi.org/10.21037/apm.2016.03.02
Kim YJ, Hui D, Zhang Y et al (2015) Differences in performance status assessment among palliative care specialists, nurses, and medical oncologists. J Pain Symptom Manage 49:1050–1058.e2. https://doi.org/10.1016/j.jpainsymman.2014.10.015
Anjanappa M, Corden M, Green A et al (2020) Sarcopenia in cancer: risking more than muscle loss. Tech Innov Patient Support Radiat Oncol 16:50–57. https://doi.org/10.1016/j.tipsro.2020.10.001
Lebrasseur NK, de Cabo R, Fielding R et al (2021) Identifying biomarkers for biological age: geroscience and the ICFSR task force. J Frailty Aging 10:196–201. https://doi.org/10.14283/jfa.2021.5
Singh H, Kim D, Kim E et al (2014) Jump test performance and sarcopenia status in men and women, 55 to 75 years of age. J Geriatr Phys Ther 37:76–82. https://doi.org/10.1519/JPT.0b013e3182a51b11
Saito K, Mukasa A, Narita Y et al (2014) Toxicity and outcome of radiotherapy with concomitant and adjuvant temozolomide in elderly patients with glioblastoma: a retrospective study. Neurol Med Chir 54:272–279. https://doi.org/10.2176/nmc.oa2012-0441
Gállego Pérez-Larraya J, Ducray F (2014) Treating glioblastoma patients with poor performance status: where do we go from here? CNS Oncol 3:231–241. https://doi.org/10.2217/cns.14.20
Hong N, Lee J, Ku CR et al (2019) Changes of computed tomography-based body composition after adrenalectomy in patients with endogenous hypercortisolism. Clin Endocrinol 90:267–276. https://doi.org/10.1111/cen.13902
Troschel FM, Kuklinski MW, Knoll SJ et al (2019) Preoperative thoracic muscle area on computed tomography predicts long-term survival following pneumonectomy for lung cancer. Interact CardioVasc Thorac Surg 28:542–549. https://doi.org/10.1093/icvts/ivy280
Brown JC, Caan BJ, Meyerhardt JA et al (2018) The deterioration of muscle mass and radiodensity is prognostic of poor survival in stage I–III colorectal cancer: a population-based cohort study (C-SCANS). J cachexia sarcopenia muscle 9:664–672. https://doi.org/10.1002/jcsm.12305
Madariaga MLL, Troschel FM, Best TD et al (2020) Low thoracic skeletal muscle area predicts morbidity after pneumonectomy for lung cancer. Ann Thorac Surg 109:907–913. https://doi.org/10.1016/j.athoracsur.2019.10.041
DeFilipp Z, Troschel FM, Qualls DA et al (2018) Evolution of body composition following autologous and allogeneic hematopoietic cell transplantation: incidence of sarcopenia and association with clinical outcomes. Biol Blood Marrow Transplant 24:1741–1747. https://doi.org/10.1016/j.bbmt.2018.02.016
Troschel AS, Troschel FM, Best TD et al (2020) Computed tomography-based body composition analysis and its role in lung cancer care. J Thorac Imaging 35:91–100. https://doi.org/10.1097/RTI.0000000000000428
Muhandiramge J, Orchard SG, Warner ET et al (2022) Functional decline in the cancer patient: a review. Cancers 14:1–20. https://doi.org/10.3390/cancers14061368
Prado CMM, Baracos VE, McCargar LJ et al (2009) Sarcopenia as a determinant of chemotherapy toxicity and time to tumor progression in metastatic breast cancer patients receiving capecitabine treatment. Clin Cancer Res 15:2920–2926. https://doi.org/10.1158/1078-0432.CCR-08-2242
Mapelli R, Julita C, Bianchi SP et al (2022) Association between treatment-related lymphopenia and survival in glioblastoma patients following postoperative chemoradiotherapy. Strahlenther Onkol 198:448–457. https://doi.org/10.1007/s00066-021-01855-5
Makino T, Izumi K, Iwamoto H et al (2023) Sarcopenia is associated with aggressive clinicopathological outcomes and is a poor prognostic indicator for non-metastatic renal cell carcinoma. In Vivo 37:1304–1311. https://doi.org/10.21873/invivo.13209
Nelke C, Dziewas R, Minnerup J et al (2019) Skeletal muscle as potential central link between sarcopenia and immune senescence. EBioMedicine 49:381–388. https://doi.org/10.1016/j.ebiom.2019.10.034
Neto NIP, de Murari ASP, Oyama LM et al (2018) Peritumoural adipose tissue pro-inflammatory cytokines are associated with tumoural growth factors in cancer cachexia patients. J Cachexia Sarcopenia Muscle 9:1101–1108. https://doi.org/10.1002/jcsm.12345
Pearson JRD, Cuzzubbo S, McArthur S et al (2020) Immune escape in glioblastoma multiforme and the adaptation of immunotherapies for treatment. Front Immunol. https://doi.org/10.3389/fimmu.2020.582106
Schatz J, Ladinig A, Fietkau R et al (2022) Normofractionated irradiation and not temozolomide modulates the immunogenic and oncogenic phenotype of human glioblastoma cell lines. Strahlenther Onkol. https://doi.org/10.1007/s00066-022-02028-8
Alghamri MS, McClellan BL, Hartlage MS et al (2021) Targeting neuroinflammation in brain cancer: uncovering mechanisms, pharmacological targets, and neuropharmaceutical developments. Front Pharmacol 12:1–20. https://doi.org/10.3389/fphar.2021.680021
Wang J, Cao L, Xu S (2020) Sarcopenia affects clinical efficacy of immune checkpoint inhibitors in non-small cell lung cancer patients: a systematic review and meta-analysis. Int Immunopharmacol 88:1–6. https://doi.org/10.1016/j.intimp.2020.106907
Zhao M, Duan X, Han X et al (2022) Sarcopenia and systemic inflammation response index predict response to systemic therapy for hepatocellular carcinoma and are associated with immune cells. Front Oncol 12:1–11. https://doi.org/10.3389/fonc.2022.854096
Thompson G, Lawrie TA, Kernohan A, Jenkinson MD (2019) Interval brain imaging for adults with cerebral glioma. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD013137.pub2
Booth TC, Luis A, Brazil L et al (2021) Glioblastoma post-operative imaging in neuro-oncology: current UK practice (GIN CUP study). Eur Radiol 31:2933–2943. https://doi.org/10.1007/s00330-020-07387-3
Ji SY, Lee J, Lee JH et al (2021) Radiological assessment schedule for high-grade glioma patients during the surveillance period using parametric modeling. Neuro Oncol 23:837–847. https://doi.org/10.1093/neuonc/noaa250
Nieder C, Willmann J, Andratschke NH (2023) Prospective randomized clinical studies involving reirradiation: update of a systematic review. Strahlenther Onkol 199:787–797. https://doi.org/10.1007/s00066-023-02118-1
Dumke R, Dumke C, Eberle F et al (2022) Monocentric evaluation of Ki-67 labeling index in combination with a modified RPA score as a prognostic factor for survival in IDH-wildtype glioblastoma patients treated with radiochemotherapy. Strahlenther Onkol 198:892–906. https://doi.org/10.1007/s00066-022-01959-6
Frosina G (2023) Most recent update of preclinical and clinical data on radioresistance and radiosensitivity of high-grade gliomas—a radiation oncologist’s perspective. Strahlenther Onkol 199:1–21. https://doi.org/10.1007/s00066-022-02020-2
van den Bent MJ, Tesileanu CMS, Wick W et al (2021) Adjuvant and concurrent temozolomide for 1p/19q non-co-deleted anaplastic glioma (CATNON; EORTC study 26053-22054): second interim analysis of a randomised, open-label, phase 3 study. Lancet Oncol 22:813–823. https://doi.org/10.1016/S1470-2045(21)00090-5
Nowak S, Theis M, Wichtmann BD et al (2022) End-to-end automated body composition analyses with integrated quality control for opportunistic assessment of sarcopenia in CT. Eur Radiol 32:3142–3151. https://doi.org/10.1007/s00330-021-08313-x
Murphy CH, Roche HM (2018) Nutrition and physical activity countermeasures for sarcopenia: time to get personal? Nutr Bull 43:374–387. https://doi.org/10.1111/nbu.12351
Troschel FM, Brandt R, Wiewrodt R et al (2019) High-intensity physical exercise in a glioblastoma patient under multimodal treatment. Med Sci Sports Exerc 51:2429–2433. https://doi.org/10.1249/MSS.0000000000002067
Troschel FM, Ramroth C, Lemcke L et al (2020) Feasibility, safety and effects of a one-week, ski-based exercise intervention in brain tumor patients and their relatives: a pilot study. J Clin Med 9:1006. https://doi.org/10.3390/jcm9041006
Keats MR, Grandy SA, Blanchard C et al (2022) The impact of resistance exercise on muscle mass in glioblastoma in survivors (RESIST): protocol for a randomized controlled trial. JMIR Res Protoc 11:1–12. https://doi.org/10.2196/37709
Huq S, Khalafallah AM, Botros D et al (2021) The prognostic impact of nutritional status on postoperative outcomes in glioblastoma. World Neurosurg 146:e865–e875. https://doi.org/10.1016/j.wneu.2020.11.033
Magudia K, Bridge CP, Bay CP et al (2021) Population-scale CT-based body composition analysis of a large outpatient population using deep learning to derive age-, sex-, and race-specific reference curves. Radiology 298:319–329. https://doi.org/10.1148/RADIOL.2020201640
Chianca V, Albano D, Messina C et al (2022) Sarcopenia: imaging assessment and clinical application. Abdom Radiol 47:3205–3216. https://doi.org/10.1007/s00261-021-03294-3
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
F.M. Troschel, B.O. Troschel, M. Kloss, J. Jost, N.B. Pepper, A.S. Völk-Troschel, R.G. Wiewrodt, W. Stummer, D. Wiewrodt, and H.T. Eich declare that they have no competing interests.
Ethical standards
This retrospective study was performed in accordance with the principles of the Declaration of Helsinki. Ethics approval was obtained from the Medical Association of Westphalia-Lippe (2021-685-f-S).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
66_2024_2225_MOESM1_ESM.pdf
Supplementary Figure 1: Inclusion and exclusion criteria for the study population. Supplementary Figure 2: Overall survival (OS) in sarcopenic and non-sarcopenic patients in univariable analyses. Supplementary Table 1: Choice of agent among patients treated with concomitant chemotherapy. Supplementary Table 2: Multivariable Cox proportional hazard regression of progression-free survival (PFS) in glioblastoma patients, restricted to patients that completed standard chemoradiotherapy. Supplementary Table 3: Multivariable Cox proportional hazard regression of overall survival (OS) in glioblastoma patients
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Troschel, F.M., Troschel, B.O., Kloss, M. et al. Sarcopenia is associated with chemoradiotherapy discontinuation and reduced progression-free survival in glioblastoma patients. Strahlenther Onkol (2024). https://doi.org/10.1007/s00066-024-02225-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00066-024-02225-7