Abstract
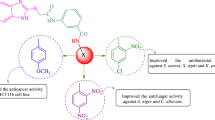
The inability to meet the desired outcomes of anticancer treatment and decrease in treatment success of bacterial and fungal infections accelerated research in these areas. Our research group has conducted numerous studies, especially on benzimidazole ring systems’ antiproliferative and antimicrobial activities. In this study, the antiproliferative activity of benzimidazole compounds was tested against A549, A498, HeLa, A375, and HepG2 cancer cell lines by MTT assay. All compounds exhibited good to potent antiproliferative activity against all tested cancer cell lines. Compounds 6-chloro-2-(4-fluorobenzyl)-1H-benzo[d]imidazole (30) and 6-chloro-2-phenethyl-1H-benzo[d]imidazole (46) were especially active against HeLa and A375 cancer cell lines with IC50 values in the range of 0.02–0.04 µM. In contrast, compounds 6-chloro-2-((p-tolyloxy)methyl)-1H-benzo[d]imidazole (67) and 5(6)-chloro-2-((4-hydroxyphenoxy)methyl)-1H-benzimidazole (68) were active against A549 and A498 cancer cell lines with an IC50 value of 0.08 µM. These compounds (30, 46, 67, and 68) were less toxic to normal human cells than the positive control compound methotrexate, which was screened to determine its toxicity against normal cell lines (HEK293). In the second part of the study, all compounds were tested to demonstrate their antimicrobial properties. All compounds exhibited moderate activity against all tested bacteria and fungi. However, some phenoxy methyl derivatives 5-chloro-2-((4-chlorophenoxy)methyl)-1H-benzo[d]imidazole (69) and 5,6-dichloro-2-((4-chlorophenoxy)methyl)-1H-benzo[d]imidazole and (74) were most active against Candida (<3.90 µg/mL). Molecular docking studies were carried out against certain proteins in order to identify potential targets of the antiproliferative effects of the synthesized compounds. The docking scores of the compounds were found to be significantly compatible with the antiproliferative activity results.

Graphical abstract








Similar content being viewed by others
Data availability
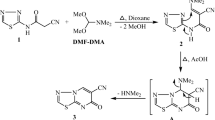
NMR and MS spectra are depicted in the Supplementary Material.
References
Henary M, Kananda C, Rotolo L, Savino B, Owens EA, Cravotto G. Benefits and applications of microwave-assisted synthesis of nitrogen containing heterocycles in medicinal chemistry. RSC Adv. 2020;10:14170–97. https://doi.org/10.1039/d0ra01378a
Ersan RH, Bolelli K, Gonca S, Dogen A, Burmaoglu S, Algul O. Bisbenzimidazole derivatives as potential antimicrobial agents: Design, synthesis, biological evaluation and pharmacophore analysis. Pharm Chem J. 2021;55:149–58. https://doi.org/10.1007/s11094-021-02389-x
Ersan RH, Alagoz MA, Ertan-Bolelli T, Duran N, Burmaoglu S, Algul O. Head-to-head bisbenzazole derivatives as antiproliferative agents: Design, synthesis, in vitro activity, and SAR analysis. Mol Divers. 2021;25:2247–59. https://doi.org/10.1007/s11030-020-10115-0
Algul O, Ersan RH, Alagoz MA, Duran N, Burmaoglu S. An efficient synthesis of novel di-heterocyclic benzazole derivatives and evaluation of their antiproliferative activities. J Biomol Struct Dyn. 2020;39:6926–38. https://doi.org/10.1080/07391102.2020.1803966
Ayaz F, Ersan RH, Algul O. Symmetric bis-benzoxazole-based chemicals exerted anti-inflammatory effect on danger signal LPS-stimulated macrophages. Monatsh Chem. 2019;150:1137–46. https://doi.org/10.1007/s00706-019-02398-3
Ayaz F, Ersan RH, Kuzu B, Algul O. New-generation benzimidazole-based plasmid delivery reagents with high transfection efficiencies on the mammalian cells. Vitr Cell Dev Biol. 2020;56:34–41. https://doi.org/10.1007/s11626-019-00418-4
Ayaz F, Isse QA, Kheeree R, Ersan RH, Algul O. Bisbenzoxazole derivatives had anti-proliferative effect on human cancer cells. Eskişehir Tech Univ J Sci Tech C – Life Sci Biotech. 2019;8:203211- https://doi.org/10.18036/estubtdc.598863
Ayaz F, Kheeree R, Isse QA, Ersan RH, Algul O. DNA base bioisosteres, bis-benzoxazoles, exert anti-proliferative effect on human prostate and breast cancer cells. J Turkish Chem Soc Sect Chem. 2018;5:1145–52. https://doi.org/10.18596/jotcsa.429504
Ayaz F, Kheeree R, Isse QA, Ersan RH, Algul O. Bisbenzoxazole derivatives had an antiinflammatory effect on in vitro stimulated macrophages. Turk J Chem. 2019;43:963–71. https://doi.org/10.3906/kim-1812-54
Ersan RH, Alagoz MA, Dogen A, Duran N, Burmaoglu S, Algul O. Bisbenzoxazole derivatives: Design, synthesis, in vitro antimicrobial, antiproliferative activity, and molecular docking studies. Polycycl Aromat Compd. 2020; Latest article. https://doi.org/10.1080/10406638.2020.1852589
Ersan RH, Yuksel A, Ertan-Bolelli T, Dogen A, Burmaoglu S, Algul O. One-pot synthesis of novel benzimidazoles with a naphthalene moiety as antimicrobial agents and molecular docking studies. J Chin Chem Soc. 2021;68:374–83. https://doi.org/10.1002/jccs.202000125
Tahlan S, Kumar S, Kakkar S, Narasimhan B. Benzimidazole scaffolds as promising antiproliferative agents: A review. BMC Chem. 2019;13:66 https://doi.org/10.1186/s13065-019-0579-6
Shrivastava N, Naim MJ, Alam MJ, Nawaz F, Ahmed S, Alam O. Benzimidazole scaffold as anticancer agent: Synthetic approaches and structure–activity relationship. Arch Pharm. 2017;350:e201700040 https://doi.org/10.1002/ardp.201700040
Olvera SV, Zamora HS, Cardoso EJ, Aldrete MEC, Gonzalez CP, Hadda TB. In vitro anti-Giardia lamblia activity of 2-aryl-3-hydroxymethyl imidazo[1,2-a]pyridines and pyrimidines, individually and in combination with albendazole. Acta Trop. 2016;155:6–10. https://doi.org/10.1016/j.actatropica.2015.11.013
Koukoula M, Dotsikas Y, Molou E, Schulpis KH, Thodi G, Chatzidaki M, et al. Study of the effect of CYP2C19 polymorphisms on omeprazole pharmacokinetics by utilizing validated LC–MS/MS and Real Time-PCR methods. J Chromatogr B. 2016;1047:173–9. https://doi.org/10.1016/j.jchromb.2016.06.046
Grip G, Svensson BA, Gordh T Jr, Post C, Hartvig P. Histopathology and evaluation of potentiation of morphine-induced antinociception by intrathecal droperidol in the rat. Acta Anaesthesiol Scand. 1992;36:145–52. https://doi.org/10.1111/j.1399-6576.1992.tb03442.x
Akhtar J, Yar MS, Khan AA, Ali Z, Haider R. Recent advances in the synthesis and anticancer activity of some molecules other than nitrogen containing heterocyclic moeities. Mini Rev Med Chem. 2017;17:1602–32. https://doi.org/10.2174/1389557516666161031121639
Keri RS, Hiremathad A, Budagumpi S, Nagaraja BM. Comprehensive review in current developments of benzimidazole-based medicinal chemistry. Chem Biol Drug Des. 2015;86:19–65. https://doi.org/10.1111/cbdd.12462
Boggu P, Venkateswararao E, Manickam M, Kwak D, Kim Y, Jung SH. Exploration of 2-benzylbenzimidazole scaffold as novel inhibitor of NF-κB. Bioorg Med Chem. 2016;24:1872–8. https://doi.org/10.1016/j.bmc.2016.03.012
Menteşe E, Ülker S, Kahveci B. Synthesis and study of α-glucosidase inhibitory, antimicrobial and antioxidant activities of some benzimidazole derivatives containing triazole, thiadiazole, oxadiazole, and morpholine rings. Chem Heterocycl Compd. 2015;50:1671–82. https://doi.org/10.1007/s10593-015-1637-1
Yildiz-Oren I, Yalcin I, Aki-Sener E, Ucarturk N. Synthesis and structure–activity relationships of new antimicrobial active multisubstituted benzazole derivatives. Eur J Med Chem. 2004;39:291–8. https://doi.org/10.1016/j.ejmech.2003.11.014
Bai YB, Zhang AL, Tang JJ, Gao JM. Synthesis and antifungal activity of 2-Chloromethyl-1H-benzimidazole derivatives against phytopathogenic fungi in vitro. J Agric Food Chem. 2013;61:2789–95. https://doi.org/10.1021/jf3053934
Hasaninejad A, Niknam K, Zare A, Farsimadan E, Shekouhy M. Silphox [POCl3−n (SiO2)n] as a new, efficient, and heterogeneous reagent for the synthesis of benzimidazole derivatives under microwave irradiation. Phosphorus Sulfur Silicon Relat Elem. 2009;184:147–55. https://doi.org/10.1080/10426500802080931
Algul O, Kaessler A, Apcin Y, Yılmaz A, Jose J. Comparative studies on conventional and microwave synthesis of some benzimidazole, benzothiazole and indole derivatives and testing on inhibition of hyaluronidase. Molecules. 2008;13:736–48. https://doi.org/10.3390/molecules13040736
Goud NS, Kumar P, Bharath RD (January 2nd 2020). Recent Developments of Target-Based Benzimidazole Derivatives as Potential Anticancer Agents, Heterocycles - Synthesis and Biological Activities (Eds: Nandeshwarappa BP, Sadashiv SO). IntechOpen. https://doi.org/10.5772/intechopen.90758. Available from: https://www.intechopen.com/chapters/70696
Akhtar W, Khan MF, Verma G, Shaquiquzzaman M, Rizvi MA, Mehdi SH, et al. Therapeutic evolution of benzimidazole derivatives in the last quinquennial period. Eur J Med Chem. 2017;126:705–53. https://doi.org/10.1016/j.ejmech.2016.12.010
Wu L, Jiang Z, Shen J, Yi H, Zhan Y, Sha M, et al. Design, synthesis and biological evaluation of novel benzimidazole-2-substituted phenyl or pyridine propyl ketene derivatives as antitumour agents. Eur J Med Chem. 2016;114:328–36. https://doi.org/10.1016/j.ejmech.2016.03.029
Madabhushi S, Mallu KKR, Vangipuram VS, Kurva S, Poornachandra Y, Kumar CG. Synthesis of novel benzimidazole functionalized chiral thioureas and evaluation of their antibacterial and anticancer activities. Bioorg Med Chem Lett. 2014;24:4822–5. https://doi.org/10.1016/j.bmcl.2014.08.064
Shao KP, Zhang XY, Chen PJ, Xue DQ, He P, Ma LY, et al. Synthesis and biological evaluation of novel pyrimidine–benzimidazol hybrids as potential anticancer agents. Bioorg Med Chem Lett. 2014;24:3877–81. https://doi.org/10.1016/j.bmcl.2014.06.050
Akhtar J, Khan AA, Ali Z, Haider R, Yar MS. Structure-activity relationship (SAR) study and design strategies of nitrogen-containing heterocyclic moieties for their anticancer activities. Eur J Med Chem. 2017;125:143–89. https://doi.org/10.1016/j.ejmech.2016.09.023
Elshihawy H, Helal MA, Said M, Hammad MA. Design, synthesis, and enzyme kinetics of novel benzimidazole and quinoxaline derivatives as methionine synthase inhibitors. Bioorg Med Chem. 2014;22:550–8. https://doi.org/10.1016/j.bmc.2013.10.052
Li Z, Zhang S, Deng L, Hu J, Li H, Zhao Y, et al. Synthesis and evaluation of 2-(2-((4-substituted-phenoxy)methyl)-1H-benzo[d]imidazol-1-yl)acetohydrazone derivatives as antitumor agent. Med Chem Res. 2014;23:4050–9. https://doi.org/10.1007/s00044-014-0981-5
Munuganti RSN, Leblanc E, Axerio-Cilies P, Labriere C, Frewin K, Singh K, et al. Targeting the Binding Function 3 (BF3) Site of the Androgen Receptor Through Virtual Screening. 2. Development of 2-((2-phenoxyethyl) thio)-1H-benzimidazole Derivatives. J Med Chem. 2013;56:1136–48. https://doi.org/10.1021/jm3015712
Bansal Y, Silakari O. The therapeutic journey of benzimidazoles: A review. Bioorg Med Chem. 2012;20:6208–36. https://doi.org/10.1016/j.bmc.2012.09.013
Iwahi T, Satoh H, Nakao M, Iwasaki T, Yamazaki T, Kubo K, et al. Lansoprazole, a novel benzimidazole proton pump inhibitor, and its related compounds have selective activity against Helicobacter pylori. Antimicrob Agents Chemother. 1991;35:490496- https://doi.org/10.1128/AAC.35.3.490
Masaki M, Yamakawa T, Nomura Y, Matsukura H. U.S. Patent. 1996;5576:341.
Oren İ, Temiz Ö, Yalçın I, Şener E, Altanlar N. Synthesis and antimicrobial activity of some novel 2,5- and/or 6-substituted benzoxazole and benzimidazole derivatives. Eur J Pharm Sci. 1999;7:153–60. https://doi.org/10.1016/S0928-0987(98)00017-7
Goker H, Boykin DW, Yıldız S. Synthesis and potent antimicrobial activity of some novel 2-phenyl or methyl-4H-1-benzopyran-4-ones carrying amidinobenzimidazoles. Bioorg Med Chem. 2005;13:1707–14. https://doi.org/10.1016/j.bmc.2004.12.006
Bansal S, Tawar U, Singh M, Nikravesh A, Good L, Tandon V. Old class but new dimethoxy analogue of benzimidazole: a bacterial topoisomerase I inhibitor. Int J Antimicrob Agents. 2010;35:186–90. https://doi.org/10.1016/j.ijantimicag.2009.07.018
Huigens RW III, Reyes S, Reed CS, Bunders C, Rogers SA, Steinhauer AT, et al. The chemical synthesis and antibiotic activity of a diverse library of 2-aminobenzimidazole small molecules against MRSA and multidrug-resistant A. baumannii. Bioorg Med Chem. 2010;18:663–74. https://doi.org/10.1016/j.bmc.2009.12.003
Milite C, Amendola G, Nocentini A, Bua S, Cipriano A, Barresi E, et al. Novel 2-substituted-benzimidazole-6-sulfonamides as carbonic anhydrase inhibitors: Synthesis, biological evaluation against isoforms I, II, IX and XII and molecular docking studies. J Enzym Inhib Med Chem. 2019;34:1697–710. https://doi.org/10.1080/14756366.2019.1666836
Le Sann C, Baron A, Mann J, Berg H, Gunaratnam M, Neidle S. New mustard-linked 2-aryl-bis-benzimidazoles with anti-proliferative activity. Org Biomol Chem. 2006;4:1305–12. https://doi.org/10.1039/B600567E
Phillips MA. XXV.—The formation of 2-methylbenziminazoles. J Chem Soc. 1928;64:172–7. https://doi.org/10.1039/JR9280000172
Wang Y, Qin Y, Yang N, Zhang Y, Liu C, Zhu H. Synthesis, biological evaluation, and molecular docking studies of novel 1-benzene acyl-2-(1-methylindol-3-yl)-benzimidazole derivatives as potential tubulin polymerization inhibitors. Eur J Med Chem. 2015;99:125–37. https://doi.org/10.1016/j.ejmech.2015.05.021.
Khairat SHM, Omar MA, Ragab FAF, Roy S, Turab Naqvi AA, Abdelsamie AS, et al. Design, synthesis, and biological evaluation of novel benzimidazole derivatives as sphingosine kinase 1 inhibitor. Arch Pharm. 2021;354:e2100080 https://doi.org/10.1002/ardp.202100080
El-Meguid EAA, El-Deen EMM, Nael MA, Anwar MM. Novel benzimidazole derivatives as anti-cervical cancer agents of potential multi-targeting kinase inhibitory activity. Arab J Chem. 2020;13:9179–95. https://doi.org/10.1016/j.arabjc.2020.10.041
Serafini M, Torre E, Aprile S, Del Grosso E, Gesù A, Griglio A, et al. Discovery of Highly Potent Benzimidazole Derivatives as Indoleamine 2,3-Dioxygenase-1 (IDO1) Inhibitors: From structure-based virtual screening to in vivo pharmacodynamic activity. J Med Chem. 2020;63:3047–65. https://doi.org/10.1021/acs.jmedchem.9b01809
McGahon AJ, Martin SJ, Bissonnette RP, Mahboubi A, Shi Y, Mogil RJ, et al. Chapter 9 The End of the (Cell) Line: Methods for the Study of Apoptosis in Vitro. Methods Cell Biol. 1995;46:153–85. https://doi.org/10.1016/S0091-679X(08)61929-9
Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. https://doi.org/10.1016/0022-1759(83)90303-4
Sabina XJ, Karthikeyan J, Velmurugan G, Tamizh MM, Shetty AN. Design and in vitro biological evaluation of substituted chalcones synthesized from nitrogen mustards as potent microtubule targeted anticancer agents. N. J Chem. 2017;41:4096–109. https://doi.org/10.1039/C7NJ00265C
Jorgensen JH, Ferraro MJ. Antimicrobial susceptibility testing: General principles and contemporary practices. Clin Infect Dis. 1998;26:973–80. https://doi.org/10.1086/513938
Duran N, Polat MF, Aktas DA, Alagoz MA, Ay E, Cimen F, et al. New chalcone derivatives as effective against SARS-CoV-2 agent. Int J Clin Pr. 2021;75:e14846 https://doi.org/10.1111/ijcp.14846
Kuzu B, Hepokur C, Alagoz MA, Burmaoglu S, Algul O. Synthesis, biological evaluation and in silico studies of some 2-substituted benzoxazole derivatives as potential anticancer agents to breast cancer. ChemistrySelect. 2022;7:e202103559 https://doi.org/10.1002/slct.202103559
Algul O, Ersan RH, Alagoz MA, Duran N, Burmaoglu S. An efficient synthesis of novel di-heterocyclic benzazole derivatives and evaluation of their antiproliferative activities. J Biomol Struct Dyn. 2021;39:6926–38. https://doi.org/10.1080/07391102.2020.1803966
Acknowledgements
We thank Mersin University for their financial support (BAP-SBE-2018-1-TP3-2911).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
About this article
Cite this article
Ersan, R.H., Kuzu, B., Yetkin, D. et al. 2-Phenyl substituted Benzimidazole derivatives: Design, synthesis, and evaluation of their antiproliferative and antimicrobial activities. Med Chem Res 31, 1192–1208 (2022). https://doi.org/10.1007/s00044-022-02900-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-022-02900-3