Abstract
Background
The emergence of bacterial resistance is a major public health problem. It is essential to develop and synthesize new therapeutic agents with better activity. The mode of actions of certain newly developed antimicrobial agents, however, exhibited very limited effect in treating life threatening systemic infections. Therefore, the advancement of multi-potent and efficient antimicrobial agents is crucial to overcome the increased multi-drug resistance of bacteria and fungi. Cancer, which remains as one of the primary causes of deaths and is commonly treated by chemotherapeutic agents, is also in need of novel and efficacious agents to treat resistant cases. As such, a sequence of novel substituted benzamides was designed, synthesized and evaluated for their antimicrobial and anticancer activities.
Methodology
All synthesized compounds were characterized by IR, NMR, Mass and elemental analysis followed by in vitro antimicrobial studies against Gram-positive (Staphylococcus aureus), Gram-negative (Salmonella typhi and Klebsiella pneumoniae) bacterial and fungal (Candida albicans and Aspergillus niger) strains by the tube dilution method. The in vitro anticancer evaluation was carried out against the human colorectal carcinoma cell line (HCT116), using the Sulforhodamine B assay.
Results, discussion and conclusion
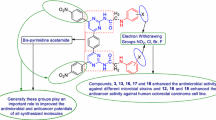
Compound W6 (MICsa, st, kp = 5.19 µM) emerged as a significant antibacterial agent against all tested bacterial strains i.e. Gram-positive (S. aureus), Gram-negative (S. typhi, K. pneumoniae) while compound W1 (MICca, an = 5.08 µM) was most potent against fungal strains (A. niger and C. albicans) and comparable to fluconazole (MIC = 8.16 µM). The anticancer screening demonstrated that compound W17 (IC50 = 4.12 µM) was most potent amongst the synthesized compounds and also more potent than the standard drug 5-FU (IC50 = 7.69 µM).

Similar content being viewed by others
Background
Antibiotics with a wide spectrum of activity are considered to be most potent against Gram positive or Gram-negative microbes. Nevertheless, there is now a frightening increment of resistance against commercially accessible antimicrobials which diminishes the scope of treatment against distinctive irresistible infections. Resistance against β-lactam, macrolides, quinolones, and vancomycin is amongst the most vital medical issues. This has called for the discovery of novel and potent antimicrobials with varying chemical characteristics [1].
Colorectal tumour (CRC) is one of the most widely recognized gastrointestinal malignancies. Changes in way of life, high-fat eating regimen, physical apathy and smoking are related to CRC pathogenesis. About 25% instances of CRC were presented with metastases at early analysis and nearly 50% of CRC patients would suffer from metastasis at some stage of life. To a large extent, the outcomes of treatment for these patients are unsatisfactory because usual regimens consider the probability of homogeneous distribution of tumor mass. It is increasingly recognized that CRC is sustained by a distinctive set of neoplastic cells named “cancer stem cells (CSCs)” which have an intrinsic potential of stemness (protection from treatment) and oncogenesis (malignant growth) [2].
Owing to their diverse potential, heterocyclic scaffolds are of major interest in the pharmaceutical industry. The heterocyclic entities which have a nitrogen group occupying a central position make them highly resemblance in structure with numerous naturally occurring molecules. In terms of improvement of biologically and therapeutically important molecules, benzimidazole nucleus has been proven as the most advantageous pharmacophore [3].
Several benzimidazole motifs with wide spectrum of diversified biological and pharmacological potentials have already been published in literature. They were reported to exhibit essential antimicrobial and antifungal [4,5,6,7], anticancer [8, 9], antiulcer [10], antihistaminic [11], antiviral [12], antihelmentic [13], analgesic [14], antihypertensive [15] and antidepressant activities [16]. Figure 1 shows a profound number of marketed medicines containing benzimidazole as a core moiety. There is now a profoundly large number of benzimidazole at several stages of evaluation in different research associations all over the globe [17].
Benzimidazole is also a vital pharmacophore, a privileged sub-structure in medicinal chemistry which contributes as a key part for different natural activities. The prominent organic applications as demonstrated by molecules related to these cores have incited wide examinations for their synthesis. Extensive biochemical and pharmacological investigations have affirmed that benzimidazole derivatives are effective against different microorganisms. Given the basic resemblance with purine, antibacterial capacity of these scaffolds showed that their opposition with purines would bring about a particular hindrance against the combination of nucleic acids and proteins inside the bacterial cell wall [18, 19]. Figure 2 reviews the biological and therapeutic profile of benzimidazoles as motivated by the structural features. As part of our continued effort in exploring new therapeutic molecules, we hereby report the synthesis, antimicrobial and anticancer evaluation as well as SAR studies of some 3-(2-(1H-benzo[d]imidazol-2-ylthio)acetamido)-N-(substituted phenyl) benzamide derivatives.
Results and discussion
Chemistry
The new target molecules (W1–W21) had been synthesized by multistep procedure as discussed in Scheme 1. Firstly, the 3-(2-chloroacetamido) benzoic acid (int-I) was synthesized by the reaction of chloroacetyl chloride with m-amino benzoic acid, then it was refluxed with 2-mercaptobenzimidazole which yielded 3-(2-(1H-benzo[d]imidazol-2-ylthio)acetamido)benzoic acid (int-II) which on reaction with thionyl chloride in suitable alcohol resulted in formation of 3-(2-(1H-benzo[d]imidazol-2-ylthio)acetamido)benzoyl chloride (int-III). The reaction of above synthesized benzoyl chloride (int-III) with different substituted anilines in methanolic/ethanolic solvent yielded the title scaffolds (W1–W21) with appreciable yields. The physicochemical properties with Mass spectra and elemental analysis of synthesized compounds are given in experimental section. The synthesized molecules (W1–W21) were also illustrated by FT-IR, proton and carbon-NMR data which are in concurrence with the proposed molecular structures of synthesized compounds. The IR stretching vibration at 3107–3097 cm−1 and ̴ 1600 cm−1 illustrated the occurrence of aromatic C–H and C = C groups, respectively. The IR band in the range of 653 cm−1 to 651 cm−1 corresponds to the C–Br stretching of Ar-Br compounds (W5 and W21). The presence of aromatic nitro group in compounds, W1, W2, W3, W4, W6 and W12 is indicated by the appearance of stretching in the range of 1554–1484 cm−1. IR band appearance around 2832–2820 cm−1 has established the existence of Ar-OCH3 (an arylalkyl ether) in compounds W15, W16 and W17. Moreover, halogen group presence in compounds, W6, W12 and W13 is specified by the presence of stretching vibration at 758–742 cm−1 of Ar–Cl and in compounds W18 and W19 by presence of Ar-F stretching at 1085–1084 cm−1. IR stretching at 2900–2869 cm−1 in the spectral data of synthesized derivatives (W9–W11) depicted the presence of Ar-CH3. Presence of C–S group is indicated by the appearance IR stretching at 708–679 cm−1 in synthesized compound’s spectral data. The appearance of –CONH– group is suggested by the IR stretching at 1670–1662 cm−1 spectral data of synthesized compounds. The presence of –C=N– (3o amine) and –C-NH– (2o amine) groups is suggested by the appearance of IR stretching 1372–1321 cm−1 and 1338–1302 cm−1 of synthesized compound’s spectral data respectively. The structures of N-phenylbenzamide were further confirmed by the corresponding 1H-NMR. The multiplet signals between 7.12 and 10.75 δ ppm in 1H-NMR spectra indicated the aromatic proton of synthesized derivatives. The presence of –CH2– and –CONH– between 2-mercaptobenzimidazole and para amino benzoic acid in all the synthesized derivatives were indicated by appearance of singlet at 2.51–4.34 δ ppm and 7.82–8.15 δ ppm, respectively. Owing to presence of CH3 of Ar-CH3, the compounds W9, W10 and W11 reflected singlet at 2.51–2.53 δ ppm. As a result of presence of –OCH3 of Ar-OCH3, compounds W15, W16 and W17 showed singlet at range of 3.73–3.74 δ ppm. Due to presence of -NH- of benzimidazole, all synthesized compounds reflected singlet at 4.31–4.37 δ ppm. The findings of elemental analysis of synthesized 2-mercaptobenzimidazoles were recorded within theoretical results of ± 0.4%. Conclusively, the 13C-NMR spectra of synthesized benzamides were in DMSO-d6 and their molecular structures were in accordance with the spectral signals. Mass spectra of the synthesized derivatives reflected the characteristic molecular ion peaks.
In vitro antimicrobial and anticancer screening results
The antimicrobial results of synthesized of benzamides (W1–W21) are presented in Table 1, Figs. 3 and 4. The antimicrobial potential of synthesized compounds is comparable to the reference ofloxacin (antibacterial) and fluconazole (antifungal). Antimicrobial screening results revealed that among the synthesized compounds, 4-chloro-2-nitro substituted benzamide i.e. compound W6 (MICsa, st, kp = 5.19 µM) exhibited significant antibacterial potency against all the tested bacterial strains i.e. Gram-positive (S. aureus), Gram-negative (S. typhi, K. pneumoniae) while the antifungal results indicated that the 2, 4-dinitro substituted derivative i.e. compound W1 (MICca, an = 5.08 µM) was found to be most potent against fungal strains (A. niger and C. albicans), even more potent than standard fluconazole (MIC = 8.16 µM). On the other hand, the in vitro anticancer activity of synthesized analogues (W1–W21) were evaluated against the human colorectal carcinoma [HCT116 (ATCC CCL-247)] cancer cell line using the SRB assay and in comparison to 5-FU, the standard drug. Table 1 shows the results in vitro anticancer activity of the synthesized analogues (W1–W21). The anticancer screening results showed that 4-methoxy substituted scaffold i.e. compound W17 (IC50 = 4.12 µM) had the highest anticancer activity amongst the synthesized ones. It was found to be more potent than the standard drug, 5-FU (IC50 = 7.69 µM).
SAR (structure activity relationship) studies
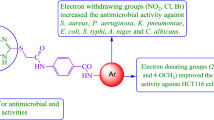
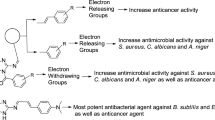
The structure activity relationship for antimicrobial and anticancer screening results of synthesized benzamides (SAR, Fig. 5): substitution of aromatic ring with 2, 4-dinitro substituent (compound W1, MIC = 5.08 µM) considerably improved the antifungal activity of benzamide derivatives against C. albicans and A. niger respectively whereas substitution with 4-chloro-2-nitro group (compound W6, MIC = 5.19 µM) resulted in improved antibacterial activity against both Gram-negative E. coli, K. pneumoniae and Gram-positive S. aureus species, respectively. Presence of methoxy group at para position of compound W17 (IC50 = 4.12 µM) is responsible for improved antiproliferative activity against HCT116 cancer cell line whereas methoxy group at ortho and meta position (compound W15 and W16, IC50 = 23.12 µM) exhibited lesser activity against same cancer cell line. From the analysis of structures of most active antimicrobial compounds, it may be concluded that the introduction of nitro and halo groups to aromatic ring as an electron-withdrawing moiety may increase the antifungal as well as the antibacterial activities of the synthesized scaffolds. The above findings had demonstrated that variation in nature and position of functional groups on aromatic framework caused a considerable change in anticancer potency. The substitution of methoxy group at para position of N-phenylbenzamide enhanced the anticancer potential. These studies reveal that different functional groups are required for different activities.
Experimental section
The reactants and reagents for syntheses were taken from commercial resource. The microbes (Microbial type cell cultures-MTCC) were acquired from Institute of Microbial Technology and Gene bank (IMTECH), Chandigarh. Reaction steps forward was checked by thin layer chromatography (TLC) using ethyl acetate as mobile phase. The scheme was drawn via ChemDraw 8.03. Melting point of synthesized derivatives was determined by open capillary tube technique. An infrared (IR) spectrum was recorded on Bruker 12060280, Software: OPUS 7.2.139.1294 spectrometer using ATR and results were in cm−1. Bruker Avance III 600 NMR spectrometer was utilized for 1H/13C-NMR (DMSO-d6, δ ppm). Waters Micromass Q-ToF Micro instrument was used for mass spectra. Elemental analysis was performed on Perkin-Elmer 2400 C, H and N analyzer and all synthesized compounds gave C, H and N analysis within ± 0.4% of the theoretical results.
Procedure for synthesized benzamides (W1–W21)
Step a: Synthesis of int-I
A mixture of m-aminobenzoic acid (0.01 mol) and triethylamine (0.01 mol) in ethanol was stirred to get a clear solution. Then the solution was cooled in ice for 30 min followed by dropwise addition of chloroacetylchloride (0.01 mol) with stirring (1 h). The resultant precipitate was strained via filtering, desiccated and recrystallized using alcohol [20].
Step b: Synthesis of int-II
To the reaction mixture of 3-(2-chloroacetamido) benzoic acid (int-I, 0.01 mol) and 2-mercaptobenzimidazole (0.01 mol) in alcohol potassium carbonate (0.01 mol) was added and then refluxed for 5–6 h and cooled to room temperature followed by evaporation to dryness. The resultant residue was washed with water and recrystallized from ethanol [1].
Step c: Synthesis of int-III
A mixture of thionyl chloride (0.3 mol) and 3-(2-(1H-benzo[d]imidazol-2-ylthio)acetamido) benzoic acid (int-II, 0.25 mol) was refluxed for 2 h. The excess of thionyl chloride was removed by distillation [21].
Step d: Synthesis of final (W1–W21) benzamides
The reaction mixture of 3-(2-(1H-benzo[d]imidazol-2-ylthio)acetamido)benzoyl chloride (int-III, 0.01 mol) and substituted aniline (0.01 mol) in suitable solvent was refluxed for appropriate time and thin layer chromatography was used to monitor the reaction. After completion of reaction, it was poured into ice cold water and the resultant precipitate was filtered, desiccated and recrystallized using ethanol [22].
Spectral data elucidation of the synthesized N-substituted phenyl benzamide derivatives.
3-(2-(1H-benzo[d]imidazol-2-ylthio)acetamido)-N-(2,4-dinitrophenyl)benzamide (W1)
M.pt. °C: 200–203; Rf value: 0.70; % yield: 97.55; IR: [3099 (C–H str.), 1600 (C=C str.) of pn (phenyl nucleus)], 1666 (–CONH str.), 1334 (C=N str.), 1304 (C–N str.), 698 (C–S str., CH2-S), 2915 (C–H str., –CH2–), 1520 (C–NO2 str., C6H5NO2); 1H-NMR: 7.16–10.75 (m, 11H, Ar–H), 4.34 (s, 1H, NH of imidazole), 2.53 (s, 2H, CH2), 8.15 (s, 2H, (CONH)2); 13C-NMR: 36.19, 119.67, 119.78, 121.52, 123.06, 123.26, 128.59, 129.00, 129.15, 135.06, 139.015, 149.71, 149.74, 166.38, 167.02; Mol. Formula: C22H16N6O6S; Elem. Anal. Calcd: C, 53.66; H, 3.27; N, 17.07; Found: C, 53.69; H, 3.23; N, 17.03; MS: m/z 493 [M+ +1].
3-(2-(1H-benzo[d]imidazol-2-ylthio)acetamido)-N-(2-nitrophenyl)benzamide (W2)
M.pt. °C: 212–215; Rf value: 0.54; % yield: 67.71; IR: [3097 (C–H str.), 1598 (C=C str.) of pn], 1664 (-CONH str., amide), 1335 (C=N str., N=CH), 1304 (C–N str.), 682 (C–S str., CH2-S), 2915 (C–H str., –CH2–), 1508 (C–NO2 str., C6H5NO2); 1H-NMR: 7.17–8.30 (m, 12H, Ar–H), 4.34 (s, 1H, NH of imidazole), 2.53 (s, 2H, CH2), 7.84 (s, 2H, (CONH)2); 13C-NMR: 36.18, 115.42, 119.71, 119.81, 121.58, 123.15, 124.28, 125.33, 129.06, 131.37, 135.62, 139.03, 146.14, 166.41, 167.04; Mol. Formula: C22H17N5O4S; Elem. Anal. Calcd: C, 59.05; H, 3.83; N, 15.65; Found: C, 53.09; H, 3.87; N, 15.69; MS: m/z 448 [M+ +1].
3-(2-(1H-benzo[d]imidazol-2-ylthio)acetamido)-N-(3-nitrophenyl)benzamide (W3)
M.pt. °C: 213–216; Rf value: 0.57; % yield: 71.07; IR: [3100 (C–H str.), 1598 (C=C str.) of pn], 1665 (–CONH str., amide), 1335 (C=N str., N=CH), 1304 (C–N str.), 681 (C–S str., CH2-S), 2915 (C–H str., –CH2–), 1506 (C–NO2 str., C6H5NO2); 1H-NMR: 7.16–8.32 (m, 12H, Ar–H), 4.36 (s, 1H, NH of imidazole), 2.53 (s, 2H, CH2), 7.85 (s, 2H, (CONH)2); 13C-NMR: 36.20, 113.84, 113.86, 113.87, 121.63, 123.17, 124.29, 129.05, 131.38, 139.03, 149.73, 166.39, 167.05; Mol. Formula: C22H17N5O4S; Elem. Anal. Calcd: C, 59.05; H, 3.83; N, 15.65; Found: C, 59.01; H, 3.79; N, 15.61 MS: m/z 448 [M+ +1].
3-(2-(1H-benzo[d]imidazol-2-ylthio)acetamido)-N-(4-nitrophenyl)benzamide (W4)
M.pt. °C: 206–209; Rf value: 0.69; % yield: 78.25; IR: [3100 (C–H str.), 1595 (C=C str.) of pn], 1662 (–CONH str., amide), 1331 (C=N str., N=CH), 1302 (C–N str.), 679 (C–S str., CH2-S), 2913 (C–H str., –CH2–), 1554 (C–NO2 str., C6H5NO2); 1H-NMR: 7.19–8.33 (m, 12H, Ar–H), 4.37 (s, 1H, NH of imidazole), 2.54 (s, 2H, CH2), 8.01 (s, 2H, (CONH)2); 13C-NMR: 36.21, 113.94, 119.84, 121.67, 123.17, 124.31, 126.33, 129.05, 139.03, 139.12, 139.14, 149.73, 166.39, 167.06; Mol. Formula: C22H17N5O4S; Elem. Anal. Calcd: C, 59.05; H, 3.83; N, 15.65; Found: C, 59.08; H, 3.87; N, 15.61; MS: m/z 448 [M+ +1].
3-(2-(1H-benzo[d]imidazol-2-ylthio)acetamido)-N-(4-bromophenyl)benzamide (W5)
M.pt. °C: 202–205; Rf value: 0.72; % yield: 73.96; IR: [3107 (C–H str.), 1597 (C=C str.) of pn], 1665 (–CONH str., amide), 1334 (C=N str., N=CH), 1304 (C–N str.), 681 (C–S str., CH2-S), 2915 (C–H str., –CH2–), 651 (C–Br str., C6H5Br); 1H-NMR: 7.18–8.33 (m, 12H, Ar–H), 4.36 (s, 1H, NH of imidazole), 4.34 (s, 2H, CH2), 7.86 (s, 2H, (CONH)2); 13C-NMR: 36.20, 119.84, 121.60, 123.17, 124.30, 129.05, 131.38, 139.03, 149.73, 166.41, 1667.06; Mol. Formula: C22H17N4O2SBr; Elem. Anal. Calcd: C, 54.89; H, 3.56; N, 11.64; Found: C, 54.84; H, 3.52; N, 11.60; MS: m/z 482 [M+ +1].
3-(2-(1H-benzo[d]imidazol-2-ylthio)acetamido)-N-(2-nitro-4-chlorophenyl)benzamide (W6)
M.pt. °C: 215–218; Rf value: 0.76; % yield: 97.71; IR: [3100 (C–H str.), 1599 (C=C str.) of pn], 1670 (–CONH str., amide), 1367 (C=N str., N=CH), 1338 (C–N str.), 681 (C–S str., CH2-S), 2915 (C–H str., –CH2–),758 (C–Cl str., C6H5Cl), 1503 (C–NO2 str., C6H5NO2); 1H-NMR: 7.16–8.29 (m, 11H, Ar–H), 4.33 (s, 1H, NH of imidazole), 7.96 (s, 2H, (CONH)2); 13C-NMR: 36.18, 118.30, 121.15, 123.13, 124.00, 124.26, 129.97, 131.36, 139.03, 145.03, 166.40, 167.02; Mol. Formula: C22H16N5O4SCl; Elem. Anal. Calcd: C, 54.83; H, 3.35; N, 14.53; Found: C, 54.87; H, 3.39; N, 14.57; MS: m/z 482 [M+ +1].
3-(2-(1H-benzo[d]imidazol-2-ylthio)acetamido)-N-(2-chlorophenyl)benzamide (W7)
M.pt. °C: 217–220; Rf value: 0.44; % yield: 89.98; IR: [3098 (C–H str.), 1598 (C=C str.) of pn], 1664(-CONH str., amide), 1335 (C=N str., N=CH), 1304 (C–N str.), 681 (C–S str., CH2-S), 2915 (C–H str., –CH2-), 742 (C–Cl str., C6H5Cl); 1H-NMR: 7.14–8.33 (m, 12H, Ar–H), 4.33 (s, 1H, NH of imidazole), 7.84 (s, 2H, (CONH)2); 13C-NMR: 36.18, 119.80, 119.78, 121.50, 123.15, 124.28, 129.06, 131.37, 139.04, 149.71, 166.42, 167.04; Mol. Formula: C22H17N4O2SCl; Elem. Anal. Calcd: C, 60.48; H, 3.92; N, 12.82; Found: C, 60.44; H, 3.96; N, 12.86; MS: m/z 437 [M+ +1].
3-(2-(1H-benzo[d]imidazol-2-ylthio)acetamido)-N-(3-chlorophenyl)benzamide (W8)
M.pt. °C: 219–222; Rf value: 0.66; % yield: 77.47; IR: [3097 (C–H str.), 1608 (C=C str.) of pn], 1665 (–CONH str., amide), 1369 (C=N str., N=CH), 1338 (C–N str.), 688 (C–S str., CH2-S), 2945 (C–H str., –CH2–), 745 (C–Cl str., C6H5Cl); 1H-NMR: 7.15–8.31 (m, 12H, Ar–H), 4.35 (s, 1H, NH of imidazole), 7.85 (s, 2H, (CONH)2); 13C-NMR: 36.18, 119.81, 119.78, 121.51, 123.16, 124.29, 129.06, 131.38, 139.04, 149.71, 166.43, 167.05; Mol. Formula: C22H17N4O2SCl; Elem. Anal. Calcd: C, 60.48; H, 3.92; N, 12.82; Found: C, 60.52; H, 3.88; N, 12.85; MS: m/z 437 [M+ +1].
3-(2-(1H-benzo[d]imidazol-2-ylthio)acetamido)-N-(2,6-dimethylphenyl)benzamide (W9)
M.pt. °C: 218–221; Rf value: 0.51; % yield: 77.62; IR: [3100 (C–H str.), 1599 (C=C str.) of pn], 1665 (–CONH str., amide), 1336 (C=N str., N=CH), 1304 (C–N str.), 704 (C–S str., CH2-S), 2915 (C–H str., –CH2–), 2886 (C–H str., CH3); 1H-NMR: 7.14–8.30 (m, 11H, Ar–H), 4.33 (s, 1H, NH of imidazole), 3.87 (s, 2H, CH2), 7.84 (s, 2H, (CONH)2), 2.52 (s, 6H, (CH3)2); 13C-NMR: 36.17, 119.80, 121.50, 123.15, 124.27, 129.07, 131.37, 139.04, 149.71, 166.42, 167.04; Mol. Formula: C24H22N4O2S; Elem. Anal. Calcd: C, 66.96; H, 5.15; N, 13.01; Found: C, 66.92; H, 5.15; N, 13.01; MS: m/z 431 [M+ +1].
3-(2-(1H-benzo[d]imidazol-2-ylthio)acetamido)-N-(2,4-dimethylphenyl)benzamide (W10)
M.pt. °C: 220–223; Rf value: 0.58; % yield: 55.02; IR: [3098 (C–H str.), 1598 (C=C str.) of pn], 1664 (-CONH str., amide), 1336 (C=N str., N=CH), 1302 (C–N str.), 704 (C–S str., CH2-S), 2912 (C–H str., –CH2–), 2884 (C–H str., CH3); 1H-NMR: 7.13–8.27 (m, 11H, Ar–H), 4.31 (s, 1H, NH of imidazole), 2.52 (s, 2H, CH2), 7.82 (s, 2H, (CONH)2), 2.51 (s, 6H, (CH3)2); 13C-NMR: 36.16, 119.80, 119.78, 121.49, 123.14, 124.26, 124.32, 129.07, 131.36, 139.04, 149.70, 166.40, 167.02; Mol. Formula: C24H22N4O2S; Elem. Anal. Calcd: C, 66.96; H, 5.15; N, 13.01; Found: C, 66.99; H, 5.11; N, 13.04; MS: m/z 431 [M+ +1].
3-(2-(1H-benzo[d]imidazol-2-ylthio)acetamido)-N-(3-methylphenyl)benzamide (W11)
M.pt. °C: 210–213; Rf value: 0.50; % yield: 91.05; IR: [3105 (C–H str.), 1608 (C=C str.) of pn], 1663 (-CONH str., amide), 1372 (C=N str., N=CH), 1334 (C–N str.), 708 (C–S str., CH2-S), 2913 (C–H str., –CH2–), 2869 (C–H str., CH3); 1H-NMR: 7.16–8.33 (m, 12H, Ar–H), 4.37 (s, 1H, NH of imidazole), 7.86 (s, 2H, (CONH)2); 13C-NMR: 36.19, 113.80, 113.82, 119.83, 121.59, 123.17, 124.30, 129.05, 131.38, 139.04, 149.73, 166.42, 167.06; Mol. Formula: C23H20N4O2S; Elem. Anal. Calcd: C, 66.33; H, 4.84; N, 13.45; Found: C, 66.36; H, 4.88; N, 13.49; MS: m/z 417 [M+ +1].
3-(2-(1H-benzo[d]imidazol-2-ylthio)acetamido)-N-(2-chloro-4-nitrophenyl)benzamide (W12)
M.pt. °C: 207–210; Rf value: 0.56; % yield: 93.92; IR: [3098 (C–H str.), 1598 (C=C str.) of pn], 1665 (-CONH str., amide), 1321 (C=N str., N=CH), 1305 (C–N str.), 681 (C–S str., CH2-S), 2842 (C–H str., –CH2–), 743 (C–Cl str., C6H5Cl), 1484 (C–NO2 str., C6H5NO2); 1H-NMR: 7.19–8.31 (m, 11H, Ar–H), 4.37 (s, 1H, NH of imidazole), 7.99 (s, 2H, (CONH)2); 13C-NMR: 36.22, 113.81, 115.51, 119.83, 123.16, 124.29, 124.56, 125.58, 129.03, 131.37, 135.90, 139.01, 149.75, 151.29, 166.32, 167.04; Mol. Formula: C22H16N5O4SCl; Elem. Anal. Calcd C, 54.83; H, 3.35; N, 14.53; Found: C, 54.87; H, 3.38; N, 14.57; MS: m/z 482 [M+ +1].
3-(2-(1H-benzo[d]imidazol-2-ylthio)acetamido)-N-(4-chlorophenyl)benzamide (W13)
M.pt. °C: 206–209; Rf value: 0.45; % yield: 79.12; IR: [3105 (C–H str.), 1598 (C=C str.) of pn], 1665 (-CONH str., amide), 1334 (C=N str., N=CH), 1304 (C–N str.), 681 (C–S str., CH2-S), 2911 (C–H str., –CH2–), 742 (C–Cl str., C6H5Cl); 1H-NMR: 7.18–8.34 (m, 12H, Ar–H), 4.37 (s, 1H, NH of imidazole), 4.35 (s, 2H, CH2), 7.87 (s, 2H, (CONH)2); 13C-NMR: 36.21, 113.90, 119.84, 123.12, 123.17, 124.31, 128.68, 129.05, 137.75, 139.03, 139.10, 149.73, 166.27, 167.06; Mol. Formula: C22H17N4O2SCl; Elem. Anal. Calcd. C, 60.48; H, 3.92; N, 12.82; Found: C, 60.44; H, 3.96; N, 12.86; MS: m/z 437 [M+ +1].
3-(2-(1H-benzo[d]imidazol-2-ylthio)acetamido)-N-(phenyl)benzamide (W14)
M.pt. °C: 203–206; Rf value: 0.45; % yield: 99.18; IR: [3093 (C–H str.), 1598 (C=C str.) of pn], 1663 (-CONH str., amide), 1334 (C=N str., N=CH), 1304 (C–N str.), 703 (C–S str., CH2-S), 2910 (C–H str., –CH2–); 1H-NMR: 7.13–8.29 (m, 13H, Ar–H), 4.32 (s, 1H, NH of imidazole), 7.83 (s, 2H, (CONH)2); 13C-NMR: 36.19, 119.82, 121.52, 123.16, 124.29, 129.06, 131.38, 139.04, 149.72, 166.44, 167.06; Mol. Formula: C22H18N4O2S; Elem. Anal. Calcd. C, 65.65; H, 4.51; N, 13.92; Found: C, 65.69; H, 4.55; N, 13.96; MS: m/z 403 [M+ +1].
3-(2-(1H-benzo[d]imidazol-2-ylthio)acetamido)-N-(4-methoxyphenyl)benzamide (W15)
M.pt. °C: 204–207; Rf value: 0.70; % yield: 84.45; IR: [3088 (C–H str.), 1598 (C=C str.) of pn], 1664 (-CONH str., amide), 1335 (C=N str., N=CH), 1303 (C–N str.), 681 (C–S str., CH2-S), 2914 (C–H str., –CH2–), 1200 (C–O–C str., phenyl ether), 2832 (C–H str., O-CH3); 1H-NMR: 7 7.15–8.32 (m, 12H, Ar–H), 4.35 (s, 1H, NH of imidazole), 7.85 (s, 2H, (CONH)2), 2.53 (s, 3H, OCH3); 13C-NMR: 36.18, 119.82, 121.52, 123.16, 124.29, 129.06, 131.38, 139.04, 149.72, 166.44, 167.06; Mol. Formula: C23H20N4O3S; Elem. Anal. Calcd. C, 63.87; H, 4.66; N, 12.95; Found: C, 63.85; H, 4.69; N, 12.98; MS: m/z 433 [M+ +1].
3-(2-(1H-benzo[d]imidazol-2-ylthio)acetamido)-N-(3-methoxyphenyl)benzamide (W16)
M.pt. °C: 209–212; Rf value: 0.72; % yield: 81.90; IR: [3100 (C–H str.), 1598 (C=C str.) of pn], 1665 (-CONH str., amide), 1336 (C=N str., N=CH), 1304 (C–N str.), 704 (C–S str., CH2-S), 2913 (C–H str., –CH2–), 1201 (C–O–C str., phenyl ether), 2820 (C–H str., O-CH3); 1H-NMR: 7.16–8.33 (m, 12H, Ar–H), 4.36 (s, 1H, NH of imidazole), 7.86 (s, 2H, (CONH)2); 13C-NMR: 36.18, 113.75, 119.82, 121.15, 123.16, 124.29, 129.06, 131.38, 139.04, 149.72, 166.44, 167.06; Mol. Formula: C23H20N4O3S; Elem. Anal. Calcd. C, 63.87; H, 4.66; N, 12.95; Found: C, 63.85; H, 4.62; N, 12.99; MS: m/z 433 [M+ +1].
3-2-(1H-benzo[d]imidazol-2-ylthio)acetamido)-N-(4-methoxyphenyl)benzamide (W17)
M.pt. °C: 214–217; Rf value: 0.45; % yield: 79.81; IR: [3100 (C–H str.), 1598 (C=C str.) of pn], 1664 (-CONH str., amide), 1336 (C=N str., N=CH), 1304 (C–N str.), 703 (C–S str., CH2-S), 2914 (C–H str., –CH2–), 1201 (C–O–C str., phenyl ether), 2830 (C–H str., O-CH3); 1H-NMR: 7.14–8.30 (m, 12H, Ar–H), 4.34 (s, 1H, NH of imidazole), 2.52 (s, 2H, CH2), 7.84 (s, 2H, (CONH)2), 3.73 (s, 3H, OCH3); 13C-NMR: 36.08, 55.10, 113.90, 114.60, 119.80, 121.49, 123.15, 124.28, 129.06, 131.37, 131.97, 139.04, 139.23, 149.71, 155.34, 165.61, 167.04; Mol. Formula C23H20N4O3S; Elem. Anal. Calcd. C, 63.87; H, 4.66; N, 12.95; Found: C, 63.91; H, 4.69; N, 12.91; MS: m/z 433 [M+ +1].
3-(2-(1H-benzo[d]imidazol-2-ylthio)acetamido)-N-(2-florophenyl)benzamide (W18)
M.pt. °C: 216–219; Rf value: 0.42; % yield: 80.86; IR: [3105 (C–H str.), 1599 (C=C str.) of pn], 1665 (–CONH str., amide), 1335 (C=N str., N=CH), 1304 (C–N str.), 681 (C–S str., CH2-S), 2916 (C–H str., –CH2–), 1085 (C-F str., C6H5F); 1H-NMR: 7.12–8.26 (m, 12H, Ar–H), 4.31 (s, 1H, NH of imidazole), 2.51 (s, 2H, CH2), 7.84 (s, 2H, (CONH)2); 13C-NMR: 36.16, 119.77, 121.51, 123.13, 124.25, 129.07, 131.36, 139.03, 149.70, 166.39, 167.01; Mol. Formula C22H17N4O2SF; Elem. Anal. Calcd. C, 62.84; H, 4.08; N, 13.33; Found: C, 62.88; H, 4.04; N, 13.29; MS: m/z 421 [M+ +1].
3-(2-(1H-benzo[d]imidazol-2-ylthio)acetamido)-N-(4-florophenyl)benzamide (W19)
M.pt. °C: 207–210; Rf value: 0.51; % yield: 70.64; IR: [3094 (C–H str.), 1598 (C=C str.) of pn], 1665 (–CONH str., amide), 1334 (C=N str., N=CH), 1304 (C–N str.), 704 (C–S str., CH2-S), 2915 (C–H str., –CH2–), 1084 (C-F str., C6H5F); 1H-NMR: 7.16–8.32 (m, 12H, Ar–H), 4.36 (s, 1H, NH of imidazole), 7.86 (s, 2H, (CONH)2); 13C-NMR: 36.18, 119.82, 121.60, 123.16, 124.29, 129.05, 131.37, 139.02, 149.72, 166.40, 167.04; Mol. Formula C22H17N4O2SF; Elem. Anal. Calcd. C, 62.84; H, 4.08; N, 13.33; Found: C, 62.81; H, 4.12; N, 13.37; MS: m/z 421 [M+ +1].
3-(2-(1H-benzo[d]imidazol-2-ylthio)acetamido)-N-ethyl-N-phenylbenzamide (W20)
M.pt. °C: 216–219; Rf value: 0.42; % yield: 83.22; IR: [3096 (C–H str.), 1598 (C=C str.) of pn], 1664 (–CONH str., amide), 1336 (C=N str., N=CH), 1304 (C–N str.), 701 (C–S str., CH2-S), 2915 (C–H str., –CH2–), 2932 (C–H str., CH3), 2826 (C–H str., N-CH3); 1H-NMR: 7.12–8.33 (m, 13H, Ar–H), 4.31 (s, 1H, NH of imidazole), 7.82 (s, 2H, (CONH)2), 2.51 (q, 2H, CH2); 13C-NMR: 36.16, 119.78, 119.78, 121.49, 123.14, 124.26, 129.07, 131.37, 139.04, 149.70, 166.40, 167.02; Mol. Formula C24H22N4O2S; Elem. Anal. Calcd. C, 66.96; H, 5.15; N, 13.01; Found C, 66.93; H, 5.19; N, 13.04; MS: m/z 431 [M+ +1].
3-(2-(1H-benzo[d]imidazol-2-ylthio)acetamido)-N-(3-bromophenyl)benzamide (W21)
M.pt. °C: 221–223; Rf value: 0.47; % yield: 70; IR: [3099 (C–H str.), 1598 (C=C str.) of pn], 1665 (–CONH str., amide), 1336 (C=N str., N=CH), 1304 (C–N str.), 704 (C–S str., CH2-S), 2915 (C–H str., –CH2–), 653 (C–Br str., Br); 1H-NMR: 7.15–8.29 (m, 12H, Ar–H), 4.33 (s, 1H, NH of imidazole), 2.52 (s, 2H, CH2), 7.83 (s, 2H, (CONH)2); 13C-NMR: 36.17, 119.80, 119.80, 121.50, 123.15, 124.27, 129.06, 131.37, 139.04, 149.70, 166.41, 167.03; Mol. Formula C22H17N4O2SBr; Elem. Anal. Calcd. C, 54.89; H, 3.56; N, 11.64; Found C, 54.85; H, 3.59; N, 11.68; MS: m/z 482 [M+ +1].
Biological studies
Antimicrobial evaluation
The in vitro antimicrobial potential of the synthesized benzamides (W1–W21) in μM was determined against Gram-positive bacteria (Staphylococcus aureus- MTCC 3160), Gram-negative bacterium (Klebsiella pneumoniae- MTCC 9024, Salmonella typhi- MTCC 3231) and fungal strains (Aspergillus niger- MTCC 281 and Candida albicans-MTCC 227) by tube dilution method [23] using ofloxacin (antibacterial) and fluconazole (antifungal) as standard. DMSO was used to dissolve the reference and experimental molecules (W1–W21). Dilutions were set up in nutrient broth (I.P.) for bacterial (incubated at 37 ± 1 °C for 24 h) and Sabouraud dextrose broth (I.P.) for fungal species (25 ± 1 °C for 7 days for A. niger) and (37 ± 1 °C for 48 h for C. albicans) [24].
Anticancer evaluation
The in vitro anticancer potential of the synthesized substituted benzamides was determined against the human colorectal cancer cell line using the SRB assay [25]. HCT116 cells were seeded onto the wells of 96-mL plates at 2500 cells/well for 24 h. The reference and test drug molecules were dissolved in DMSO subjected to serial dilutions. They were then incubated with monolayer cells at 37 °C for 72 h. the treated cells were then fixed with trichloroacetic acid for and then stained with 0.4% w/v of SRB in acetic acid. The unbound stain was removed by washing with 1% acetic acid. Bound SRB was solubilised in 10 mM Tris base solution. Absorbance was measured by a computer-interfaced 96-well plate spectrophotometer at 570 nm.
Conclusion
The synthetic work was conducted under appropriate experimental conditions and the expected compounds had been obtained. The biological studies were carried out to observe the effect of substituents on the antimicrobial and anticancer activities. From the outcomes of antimicrobial and anticancer studies, it is concluded that the introduction of electron-withdrawing groups such as nitro and halo substituents increased the antimicrobial potential of the synthesized scaffolds i.e. compounds W1 (3-(2-(1H-benzo[d]imidazol-2-ylthio)acetamido)-N-(2,4-dinitrophenyl)benzamide) and W6 (3-(2-(1H-benzo[d]imidazol-2-ylthio)acetamido)-N-(2-nitro-4-chlorophenyl)benzamide) while the introduction of para-methoxy substituent to phenyl ring as an electron-donating group i.e. compound W17 (3-(2-(1H-benzo[d]imidazol-2-ylthio)acetamido)-N-(4-methoxyphenyl)benzamide) exhibited the most promising anticancer activity amongst all tested compounds.
References
Turan-Zitouni G, Kaplancikli ZA, Ozdemir A, Revial G, Guven K (2007) Synthesis and antimicrobial activity of some 2-(benzo[d]oxazol/benzo[d]imidazol-2-ylthio)-N-(9H-fluoren-9-yl)acetamide derivatives. Phosphorus Sulfur Silicon 182:639–646
Manhas J, Bhattacharya A, Agrawal SK, Gupta B, Das P, Deo SVS, Pal S, Sen S (2016) Characterization of cancer stem cells from different grades of human colorectal cancer. Tumor Biol. https://doi.org/10.1007/s13277-016-5232-6
Gaba M, Mohan C (2016) Development of drugs based on imidazole and benzimidazole bioactive heterocycles: recent advances and future directions. Med Chem Res 25:173–210
Ozden S, Atabey D, Yildiz S, Goker H (2005) Synthesis and potent antimicrobial activity of some novel methyl or ethyl 1H-benzimidazole-5-carboxylates derivatives carrying amide or amidine groups. Bioorg Med Chem 13:1587–1597
Moreira JB, Mann J, Neidle S, McHugh TD, Taylor PW (2013) Antibacterial activity of head-to-head bis-benzimidazoles. Int J Antimicrob Agents 42:361–366
Noolvi M, Agrawal S, Patel H, Badiger A, Gaba M, Zambre A (2014) Synthesis, antimicrobial and cytotoxic activity of novel azetidine-2-one derivatives of 1H-benzimidazole. Arabian J Chem 7:219–226
Kalinowska-Lis U, Felczak A, Checinska L, Lisowska K, Ochocki J (2014) Synthesis, characterization and antimicrobial activity of silver (I) complexes of hydroxymethyl derivatives of pyridine and benzimidazole. J Organomet Chem 749:394–399
Wang YT, Qin YJ, Yang N, Zhang YL, Liu CH, Zhu HL (2015) Synthesis, biological evaluation, and molecular docking studies of novel 1-benzene acyl-2-(1-methylindol-3-yl)-benzimidazole derivatives as potential tubulin polymerization inhibitors. Eur J Med Chem 99:125–137
Yang YH, Cheng MS, Wang QH, Nie H, Liao N, Wang J, Chen H (2009) Design, synthesis, and antitumor evaluation of novel symmetrical bis-benzimidazoles. Eur J Med Chem 44:1808–1812
Loriga M, Paglietti G, Piras S, Sparatore F, Anania V, Demontis MP, Varoni MV, Fattaccio MC (1992) Synthesis and evaluation of gastroprotective and antiulcer activity of some 2-substituted-1H-imidazo[4,5-b] pyridines and -1H-benzimidazoles. Farmaco 47(3):287–303
Wang XJ, Xi MY, Fu JH, Zhang FR, Cheng GF, Yin DL, You QD (2012) Synthesis, biological evaluation and SAR studies of benzimidazole derivatives as H1-antihistamine agents. Chin Chem Lett 23:707–710
Luo Y, Yao JP, Yang L, Feng CL, Tang W, Wang GF, Zuo JP, Lu W (2010) Design and synthesis of novel benzimidazole derivatives as inhibitors of hepatitis B virus. Bioorg Med Chem 18:5048–5055
Velazquez-Lopez JM, Hernandez-Campos A, Yepez-Mulia L, Tellez-Valencia A, Flores-Carillo P, Nieto-Meneses R, Castillo R (2015) Synthesis and trypanocidal activity of novel benzimidazole derivatives. Bioorg Med Chem Lett 26(17):4377–4381
El-Feky SA, Thabet HK, Ubeid MT (2014) Synthesis, molecular modeling and anti-inflammatory screening of novel fluorinated quinoline incorporated benzimidazole derivatives using the Pfitzinger reaction. J Fluorine Chem 161:87–94
Zhu W, Da Y, Wu D, Zheng H, Zhu L, Wang L, Yan Y, Chen Z (2014) Design, synthesis and biological evaluation of new 5-nitrobenzimidazole derivatives as AT1 antagonists with anti-hypertension activities. Bioorg Med Chem 22:2294–2302
Mathew B, Jerad Suresh J, Anbazhagan S (2016) Development of novel (1-H) benzimidazole bearing pyrimidine-trione based MAO-A inhibitors: synthesis, docking studies and antidepressant activity. J Saudi Chem Soc 20:S132–S139
Akula G, Srinivas B, Vidyasagar M, Kandikonda S (2011) Synthesis of 3-(1H-benzimidazol-2-ylamino)2-phenyl-1,3-thiazolidin-4-one as potential CNS depressant. Int J Pharmtech Res 3(1):360–364
Bandyopadhyay P, Sathe M, Ponmariappan S, Sharma A, Sharma P, Srivastava AK, Kaushik MP (2011) Exploration of in vitro time point quantitative evaluation of newly synthesized benzimidazole and benzothiazole derivatives as potential antibacterial agents. Bioorg Med Chem Lett 21:7306–7309
Spasov AA, Yozhitsa IN, Bugaeva LI, Anisimova VA (1999) Benzimidazole derivatives: spectrum of pharmacological activity and toxicological properties (a review). Pharm Chem J 33(5):1–12
Turan-Zitouni G, Ozdemir A, Kaplancikli ZA, Cevikbas A, Gurbuz B, Gurer US (2009) Studies on some new pyrazolo[3,4-c]pyridine derivatives as antimicrobial agents. Turk J Pharm Sci 6(2):63–72
Kumar H, Kumar P, Narasimhan B, Ramasamy K, Mani V, Mishra RK, Majeed ABA (2013) Synthesis, in vitro antimicrobial, antiproliferative, and QSAR studies of N-(substituted phenyl)-2/4-(1H-indol-3-ylazo)-benzamides. Med Chem Res 22:1957–1971
Kumar S, Lim SM, Ramasamy K, Vasudevan M, Shah SAA Narasimhan B (2017) Bis-pyrimidine acetamides: design, synthesis and biological evaluation
Cappuccino JG, Sherman N (1999) In microbiology-a laboratory manual, 4th edn. Addison Wesley Longman, Inc, California, p 263
Pharmacopoeia of India, vol Ӏ (2007) Controller of publication, Ministry of Health Department, Govt. of India, New Delhi, pp. 37
Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D, Warren JT, Bokesch H, Kenney S, Boyd MR (1990) New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst 82:1107–1112
Author’s contributions
Authors BN and ST have designed, synthesized and carried out the antimicrobial activity and KR, SML, SAAS and VM have carried out the spectral analysis, interpretation and anticancer evaluation of synthesized compounds. All authors read and approved the final manuscript.
Acknowledgements
The authors are thankful to Head, Department of Pharmaceutical Sciences, Maharshi Dayanand University, Rohtak, for providing necessary facilities to carry out this research work.
Competing interests
The authors declare that they have no competing interests.
Ethics approval and consent to participate
Not applicable
Funding
Not applicable.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Tahlan, S., Ramasamy, K., Lim, S.M. et al. Design, synthesis and therapeutic potential of 3-(2-(1H-benzo[d]imidazol-2-ylthio)acetamido)-N-(substituted phenyl)benzamide analogues. Chemistry Central Journal 12, 139 (2018). https://doi.org/10.1186/s13065-018-0513-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13065-018-0513-3