Abstract
Secondary salinization is a growing concern for freshwaters worldwide. The lethal and sub-lethal effects on shredders are known, but not whether these result from direct exposure to contaminated aquatic medium and/or from indirect effects of distinct substrate quality through fungal conditioning in salinized media. Here, chestnut and oak leaves were conditioned for 4 weeks in reference (Cond0, 0 g/l NaCl) or salinized (Cond3, 3 g/l NaCl) media before being offered to the shredder Schizopelex festiva maintained in reference (Inv0) or salinized (Inv3) media. Fungal biomasses associated with leaf litter and consumption, respiration rates, growth, survival, and feeding preference of S. festiva were assessed. We found lower fungal biomass in both leaf species conditioned in Cond3 medium. Consumption rates were higher for oak than chestnut, and in Inv0 than Inv3, but were not affected by conditioning media. Growth was also affected by invertebrate media (Inv0 > Inv3), while Inv3 led to the lowest survival. Schizopelex festiva preferred Cond0 over Cond3 oak leaves only in Inv0. Results strongly suggest that direct exposure to salinized media is a main pathway of salt toxicity to shredders through a generalized reduction in invertebrates’ metabolic rates when facing salt stress. Salt addition to the media may result in an energetic investment in osmotic regulation at the expense of consumption and growth, with consequences for invertebrate survival. Potential negative effects of salt contamination on shredders’ ability to select more nutritious food items may contribute to cascading effects throughout the stream food webs, particularly in streams lined with more recalcitrant leaf litter.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Freshwater salinization is a serious global problem that threatens biodiversity and ecological functions in streams and rivers and, consequently, the services they provide (Berger et al. 2019; Sowa et al. 2020). Its detrimental effects, far from fully measured, are closely related to multiple and intensified human activities and are exacerbated by climate change (Hintz and Relyea 2019; Berger et al. 2019; Iglesias 2020; Sowa et al. 2020). Streams are among the systems most severely impacted by secondary salinization, i.e., anthropogenically induced increases in the concentration of ions dissolved in water (e.g., Na+ e Cl−). This is mainly due to the use of salts as deicing agents (during winter), in agriculture, and in water abstraction often coinciding with flow reductions that are currently becoming more frequent and intense (Arenas-Sánchez et al. 2016).
Forested headwaters are shadowed systems that mainly rely on organic matter, particularly of terrestrial origin, as a source of nutrients and energy. These systems harbor, along with microbial decomposers (namely aquatic hyphomycetes), a diverse and conspicuous community of macroinvertebrates. Among them, leaf consumers (i.e. shredders) exhibit high consumption rates on microbially colonized (Marks 2019) and, consequently, more nutritious leaf litter, strongly contributing to leaf litter decomposition (Graça 2001). Environmental conditions, such as increased salinization, affect this key ecosystem process through changes in community composition and trophic interactions (Cañedo-Argüelles et al. 2014; Le et al. 2021; Williams et al. 2023). Globally more sensitive to salinization than fungi (Blasius and Merritt 2002), invertebrates show reduced abundance and functional/taxonomic diversity (Piscart et al. 2005; Szöcs et al. 2014; Sowa et al. 2020) as a response to increased salt ions, with deleterious consequences for leaf processing. Salinization effects on invertebrates are visible at both lethal (Kefford et al. 2012) and sub-lethal levels (Hassell et al. 2006; Cañedo-Argüelles et al. 2016), namely with changes to their physiology (Szöcs et al. 2014), behavior (Blasius and Merritt 2002; Cañedo-Argüelles et al. 2012), and performance (e.g., consumption, growth, reproduction) (Kefford et al. 2003; Hassell et al. 2006; Entrekin et al. 2017; Martínez et al. 2020). With increasing water salinization, the probable exclusion of leaf-associated macroinvertebrates may result in a predominantly microbial-driven decomposition (Almeida Júnior et al. 2020; Canhoto et al. 2021).
The negative impacts of salinization on shredders may be the result of direct exposure to the contaminated aquatic medium (waterborne contamination), which causes osmoregulatory stress and/or ion toxicity (Kefford et al. 2003; Scheibener et al. 2016; Bal et al. 2021; Ruiz et al. 2022). However, under salinized conditions, shredders are also faced with changes to the quality of the substrate due to altered microbial conditioning (Marks 2019; Martínez et al. 2020)—food-mediated contamination. Recent studies have shown that altered diversity, biomass, and activity of leaf-associated fungi, regulated by inherent leaf litter quality (Foucreau et al. 2013; Santonja et al. 2020) and due to salt contamination, may impair shredder consumption, growth, and survival (Martínez et al. 2020). While both pathways are starting to be considered (Entrekin et al. 2017), the relative importance of leaf species as a modulator of consumer response has been practically ignored.
This work aimed to clarify the relative importance of waterborne and/or food-mediated salinization regarding shredders' performance when fed substrates of contrasting quality. For that purpose, chestnut (Castanea sativa) and oak (Quercus robur) leaves were conditioned under reference (Cond0, 0 g/l NaCl) or salinized (Cond3, 3 g/l NaCl) media in the laboratory before being offered to the shredder S. festiva (Sericostomatidae; Trichoptera), also maintained in reference (Inv0) or salinized (Inv3) media, in a full factorial design. After 28 days of fungal conditioning, leaf-associated fungal biomass as well as larval consumption respiration rates, growth, and survival was assessed for each leaf species. Additionally, feeding preference tests were performed: Schizopelex festiva larvae, maintained in Inv0 or Inv3 media, were simultaneously offered Cond0 and Cond3 chestnut or oak leaves to evaluate their capacity to discriminate between leaf conditioning media under the influence of waterborne contamination.
Based on previous tests, we hypothesized that (1) fungal biomass would be lower in Cond3 than Cond0 leaf discs (Gonçalves et al. 2019b); (2) exposure of invertebrates to Inv3 media would lead to lower consumption, respiration, growth, and survival (Entrekin et al. 2017; Martínez et al. 2020); (3) such negative effects would be enhanced when shredders were fed Cond3 discs because of the additional reduced substrate quality; (4) negative salt effects would occur regardless of initial intrinsic leaf quality (Almeida Júnior et al. 2020); (5) invertebrates would prefer Cond0 discs, regardless of medium salt contamination, because of the increased associated fungal biomass.
Materials and methods
Leaf conditioning and fungal biomass
Leaves from chestnut (C. sativa) and oak (Q. robur) were collected after abscission and air-dried in the dark at room temperature. The two leaves differed in their initial chemical and structural characteristics: total phenolics (%) were higher in chestnut (3.66 ± 0.05) than oak (1.97 ± 0.10). Nitrogen content (%) was higher in oak than chestnut (0.7 ± 0.01 and 0.6 ± 0.02, respectively; one-way ANOVA, F = 21.8, p < 0.01); the same pattern was found for leaf toughness (86.0 ± 5.9 g and 55.6 ± 5.7 g, respectively; one-way ANOVA, F = 13.7, p < 0.01) (Almeida Júnior et al. 2020).
Chestnut and oak leaf discs were cut using a cork borer (Ø = 12 mm) and oven dried (60 ℃, 24 h). For consumption and food preference tests (see below), disc pairs were cut symmetrically relative to the main leaf vein and sewn together; disc pairs were assumed to have similar mass (Bärlocher et al. 2020). Discs were autoclaved (121 ℃, 15 min) and distributed into 12 100-mL Erlenmeyer flasks (i.e., microcosms) (20 chestnut/oak discs per microcosm) containing 40 ml nutrient solution (75.5 mg CaCl2, 10 mg MgSO4.7H2O, 0.5 g 3-morpholinopropanesulfonic acid, 5.5 mg K2HPO4, and 100 mg KNO3 per liter of sterile distilled water; pH = 7; Dang et al. 2005) (Cond0 medium). In half of the Erlenmeyer flasks for each leaf species, this nutrient medium was enriched with 3 g/l of NaCl (Cond3 medium).
All microcosms were inoculated with a plug (Ø = 8 mm; 14-day-old pure cultures in malt extract agar) of each of the following aquatic hyphomycete (AH) species: Articulospora tetracladia, Flagellospora curta, Neonectria lugdunensis, Tetrachaetum elegans, and Tetracladium marchalianum. These species were isolated from Ribeira do Candal (central Portugal; 40°4′44″N, 8°12′10″W; 620 m a.s.l.; salinity < 0.01 ± 0.01 g/l; conductivity 26.80 ± 2.1 µS/cm [Gonçalves et al. 2019a]). Microcosms were maintained in an orbital shaker (120 rpm) at 16 ℃ (12:12 h light/dark). After 7 days, fungal plugs were removed, and media were renewed every 2 days for a total fungal conditioning period of 28 days.
After the conditioning period, five discs were removed from each microcosm for ergosterol (proxy of fungal biomass [Gessner and Chauvet 1993]) quantification. Discs were frozen, freeze-dried, and weighed. Ergosterol was extracted as in Reis et al. (2018) and quantified by high-performance liquid chromatography (HPLC; Shimadzu Prominence UFLC, Kyoto, Japan) using a HPLC C18 column (Mediterranea sea18, 250 × 4.6 mm, 5 μm particle size; Teknokroma). Ergosterol concentration was converted into fungal biomass (5.5 μg ergosterol per mg fungal dry mass [DM]; [Gessner and Chauvet 1993]). Results were expressed as mg fungal biomass/g DM (n = 3).
Invertebrate tests
Experimental conditions
Larvae of S. festiva (Sericostomatidae; Trichoptera)—endemic species of the Iberian Peninsula with a key role in leaf litter decomposition in small order streams (González and Martínez 2011)—were collected from Ribeira de Múceres (central Portugal, 40°32′01'' N, 08°09′15″W; 210 m a.s.l.) and brought to the laboratory. Larvae were kept in aerated stream water and fed ad libitum with leaves collected from the same stream (16 ℃, 12:12 h light/dark photoperiod). Similarly sized larvae were selected; the diameter of the larval case opening (CO; mm) was measured, and individual DMs were estimated using the regression model DM = CO × 0.0136–0.0162 (Gonçalves et al. 2011).
Larvae were individually allocated to plastic cups containing 150 ml aerated reference (Inv0; distilled water) or salinized media (Inv3; distilled water enriched with 3 g/l NaCl) (n = 10 per medium) and a fine layer of sieved (< 2 mm diameter) and ashed (550 °C; 5 h) stream sediment. All cups were maintained at 16 °C with a 12:12 h light/dark photoperiod (Bärlocher et al. 2020).
Consumption and respiration
For leaf consumption tests, one disc from each pair (see above), previously conditioned in Cond0 or Cond3 media for 28 days, was immersed in each cup and offered to each invertebrate; the other disc was enclosed in a small fine-mesh bag (0.5-mm mesh size), immersed in the cup but excluding invertebrate consumption. When half of the discs from half the cups had been consumed (visual assessment), discs were retrieved, oven-dried (60 °C; 48 h) and weighed. Individual consumption was calculated as the difference between the final DM of the control and the offered discs. Consumption rates were expressed as mg leaf DM/mg individual DM/day (Bärlocher et al. 2020) (n = 10 per treatment). After the consumption test, larvae were individually placed in 50-ml Falcon tubes filled with the corresponding (Inv0 or Inv3) O2-saturated medium. Falcon tubes were maintained in the dark at 16 °C. After 24 h, O2 concentration was measured (Jenway 9200 oxygen meter; Jenway, UK). Oxygen consumption was calculated as the difference between the initial and final O2 concentration values, and respiration rates were expressed as mg O2/g DM/day (n = 10 per treatment).
Growth and survival
To assess larval growth and survival, a total of 80 S. festiva individuals were individually maintained in Inv0 and Inv3 media and fed ad libitum with leaf discs previously conditioned in Cond0 or Cond3 media (n = 10 per treatment). Each week, leaf discs, media (Inv0 or Inv3), and sediment were renewed. Survival was registered, and each individual’s DM was assessed as above. Measurements were continued until mortality reached 50% in at least one treatment (i.e., 35 days). Relative growth rates (RGR; mg DM/g DM/day) were calculated as:
DMg/(DMf × t).
Here, DMg is the dry mass gained by the invertebrate during the experiment time (t, 35 days), given by the difference between final and initial dry mass (mg), and DMf if the final dry mass (g) (Ferreira et al. 2010). Survival was expressed as the percentage of invertebrates alive at the end of the experiment in each treatment.
Feeding preference
Feeding preference was assessed separately for chestnut or oak by simultaneously offering two leaf discs to each invertebrate: one leaf disc from Cond0 and the other from Cond3 media (differentiated by colored pins). The two corresponding disc pairs were placed inside the cup in a fine-mesh bag as described above. Tests were performed in reference (Inv0) and salinized (Inv3) media. After 24 h, the remaining leaf material was retrieved, dried, and weighed. Consumption of each disc was estimated and expressed as above (n = 10).
Statistical analysis
Statistical differences in fungal biomass were assessed through a two-way ANOVA, with leaf species (chestnut vs. oak) and conditioning media (Cond0 vs. Cond3) as categorical variables. Consumption, respirometry, and relative growth rates were each evaluated through a three-way ANOVA, with leaf species, conditioning media, and invertebrate media (Inv0 vs. Inv3) as categorical variables. Whenever statistically significant interactions (p < 0.05) were found between variables, one-way ANOVAs were conducted for each factor. Planned comparison tests were applied to compare between specific levels of the factors. Survival was analyzed using chi-square statistics (p < 0.05). Food preference was compared for each leaf species and for each invertebrate medium with a paired-samples t-test. Normality and homoscedasticity assumptions were met. Statistical analyses were performed using Statistica 7 software (StatSoft, Tulsa OK, USA).
Results
Fungal biomass
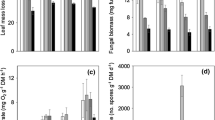
Chestnut leaves conditioned in Cond0 presented the highest fungal biomass (179.96 ± 0.170 mg/g DM), while Cond3 oak leaves showed the lowest values (35.98 ± 4.69 mg/g DM) (Fig. 1). Fungal biomass was significantly affected by leaf species (two-way ANOVA, F1,8 = 162.27, p < 0.05) and conditioning media (two-way ANOVA, F1,8 = 336.93, p < 0.05), and the interaction between both factors was significant (two-way ANOVA, F1,8 = 13.36, p < 0.05). Values were higher in chestnut than oak, regardless of conditioning media (one-way ANOVA, F1,11 = 4.53, p < 0.05). The addition of salt led to a 35 and 43% reduction in fungal biomass associated with oak and chestnut leaves, respectively (one-way ANOVA, F1,11 = 18.35, p < 0.05).
Invertebrate leaf consumption and O 2 consumption
Consumption rates were the highest (0.14 ± 0.02 mg DM/mg individual/day) for invertebrates maintained in Inv0 media fed with Cond3 oak leaves and lowest (0.02 ± 0.00 mg DM/mg individual/day) for invertebrates in Inv3 media fed with Cond3 chestnut leaves (Fig. 2a). Consumption was significantly affected by leaf species (chestnut < oak; three-way ANOVA, F1,52 = 72.06, p < 0.01) and invertebrate media (Inv3 < Inv0; three-way ANOVA, F1,52 = 16.99, p < 0.01) but not by conditioning media (three-way ANOVA, F1,52 = 0.21, p = 0.65); no interaction between factors was significant (three-way ANOVA, F1,52 = 3.48, 0.81, 0.11, and 0.00; all p > 0.06).
Consumption (a) and respiration (b) rates of Schizopelex festiva larvae in reference (Inv0) or salinized (Inv3) media fed with chestnut or oak leaves conditioned in reference (Cond0) or salinized (Cond3) media. Results are means ± SE (n = 10). Different letters represent significant differences between treatments (p < 0.05)
O2 consumption was the highest (9.82 ± 0.30 mg O2/g DM/day) when invertebrates in Inv0 media were fed with Cond0 oak leaves and lowest (4.32 ± 0.19 mg O2/g DM/day) in invertebrates in Inv3 media fed with Cond0 chestnut leaves (Fig. 2b). O2 consumption was significantly affected by leaf species, conditioning media, and invertebrate media (three-way ANOVA, F1,37 = 13.07, 9.87, 256.50, respectively, p < 0.01); all interactions were significant (p < 0.03). Higher values were found in Inv0 than Inv3 regardless of leaf species and conditioning media (one-way ANOVA, F1,44 = 80.99, p < 0.05). For invertebrates maintained in Inv3 media, there were no differences in O2 consumption between leaf species or conditioning media (planned comparisons, p > 0.99); for invertebrates maintained in Inv0 media, O2 consumption was the lowest when they were fed Cond3 chestnut leaves (planned comparisons, p < 0.01).
Invertebrate growth and survival
Growth was the lowest (1.68 ± 0.31 mg DM/g DM/day) in invertebrates in Inv3 media fed with Cond3 oak leaves and highest (4.51 ± 0.78) in invertebrates in Inv0 media fed with Cond3 oak leaves (Fig. 3a). Relative growth rates were only affected by invertebrate media (Inv3 < Inv0; three-way ANOVA, F1,36 = 6.03, p = 0.02). After 35 days, survival was consistently lower (c.a. 40%) in invertebrates maintained in Inv3 (vs. Inv0) media (p < 0.001), regardless of the offered leaf species (χ2 = 0,215; d.f. = 3; p > 0.05) (Fig. 3b).
Relative growth rate (a) and survival (b) of Schizopelex festiva larvae in reference (Inv0) or salinized (Inv3) media, fed with chestnut or oak leaves conditioned in reference (Cond0) or salinized (Cond3) media. Results are mean ± SE (n = 10). Different letters represent significant differences between invertebrate media (p < 0.05)
Feeding preference test
When simultaneously offered Cond0 and Cond3 oak leaves, S. festiva in Inv0 media consumed more Cond0 leaves (paired-samples t-test, M = 0.11, SD = 0.00) than Cond3 leaves (M = 0.04, SD = 0.00) [t(6) = = 2.99, p = 0.02] (Fig. 4). No differences were found in consumption of Cond0 and Cond3 oak leaves when the invertebrates were in Inv3 media [t(4) = 1.75, p = 0.16] or when invertebrates were fed chestnut leaves in either Inv0 [t(4) = 0.96, p = 0.39] or Inv3 media [t(5) = − 0.61, p = 0.57).
Feeding preference test measured as consumption (mean ± SE) of chestnut or oak discs. Schizopelex festiva larvae maintained in reference (Inv0) or salinized (Inv3) media were simultaneously offered discs conditioned in reference (Cond0) or salinized (Cond3) media. *Significant differences between Cond0 and Cond3 media for each leaf species (p < 0.05)
Discussion
Water salinization significantly reduced leaf-associated fungal biomass and consumption, metabolic activity, growth, and survival in S. festiva. Oak was consumed at a higher rate than chestnut, regardless of conditioning media and despite presenting lower fungal biomass. The addition of salt to the invertebrate media suppressed S. festiva's capacity to discriminate between distinct oak qualities determined by the conditioning media.
Despite some recognized tolerance of mycelia (and, in particular, of our used species) to stream salinization (Canhoto et al. 2017; Pereira da Silva et al. 2021), fungal biomass was 35 and 43% lower following salt addition to the media in oak and chestnut leaves, respectively. This agrees with previous reports that mentioned negative effects at ≥ 4 g/l NaCl in oak, chestnut, and poplar leaves (Gonçalves et al. 2019b, a; Almeida Júnior et al. 2020). This may be due to an obligatory investment in the production of osmoprotective secondary compounds (Overy et al. 2017; Martínez et al. 2020) at the expense of biomass accrual. Even though we have no data on conidia identification, we would expect to find distinct community compositions in both substrata and conditioning media (Ramos et al. 2021; Oliveira et al. 2021) after the conditioning period. Leaf intrinsic quality is known to modulate the composition of fungal communities and even microbial performance in the face of several stressors, salinization included (Gonçalves et al. 2013; Zhang et al. 2019; Oliveira et al. 2021). Considering that different fungal species may present distinct ergosterol concentrations (proxy of fungal biomass; Gessner and Chauvet 1993), the observed biomass decrease in salinized media may also reflect such differences in fungal assemblages.
Consumption rates were negatively affected by salt addition to the media (Inv3) in both leaf species. A combined effect of lower fungal-mediated food quality and physiological stress from osmoregulatory adjustments has been observed and may explain these results (Entrekin et al. 2017; Martínez et al. 2020). Overall, oak was significantly more consumed than chestnut by S. festiva maintained in either medium. This was unexpected, since lower invertebrate consumption rates are usually associated with higher leaf toughness and lower fungal conditioning (Canhoto and Graça 2008; Biasi et al. 2019; Marks 2019; Swan et al. 2021). It is plausible that, in the presence of a more recalcitrant substrate such as oak, invertebrates presented a compensatory feeding mechanism (Gessner et al. 2010; Correa-Araneda et al. 2015; Landeira-Dabarca et al. 2019). As previously reported (Flores et al. 2014; Siders et al. 2018), such increased consumption, eventually also stimulated by higher N levels of these tougher leaves, did not result in increased growth.
Even though invertebrate growth was not affected by leaf species or leaf conditioning treatment, it was significantly reduced by waterborne salinization (Inv3 < Inv0). This seems to relate to the lower consumption rates and a generalized reduction in invertebrate metabolic rates. Most energetic investments were probably channeled into osmotic regulation (Scheibener et al. 2016), likely involving chloride cells to absorb chloride and balance hypotonic pressure (Tiwari and Rachlin 2018), at the expense of growth and also survival. These results agree with the “subsidy-stress gradient,” in which increased exposure to any toxic substance results in performance reduction (Odum et al. 1979). Similar effects on survival (≥ 3 g/l, S. festiva; Martínez et al. 2020) and growth [> 6 g/l NaCl, Cloeon sp., Centroptilum sp. (Ephemeroptera), and Chironomus sp. (Diptera); Hassell et al. 2006] have been previously detected; a 12% reduction in growth of Lirceus sp. was even found at lower salt levels (0.14 g/l NaCl) (Tyree et al. 2016) (vs. approximately 40%, in our case, at 3 g/l NaCl). Such negative effects of a wide range of salinization values, possibly being invertebrate species specific (Castillo et al. 2018), suggests that alterations in ion concentrations may have important implications for aquatic ecosystem functioning through reduction of shredder biomass. Here, we reinforce the need to evaluate long-term effects of freshwater salinization (Canhoto et al. 2023) particularly on key leaf consumers with distinct tolerance to salinity; our results were obtained with S. festiva, a case-building shredder, described as potentially presenting higher tolerance to salinity and other pollutants than non-case forming species (Castillo et al. 2018).
The distinct leaf quality induced by Cond0 or Cond3 conditioning led to selective feeding: S. festiva in Inv0 media preferred Cond0 leaves, although results were only significant in the case of oak. This could be related to S. festiva’s preference and ability to select either fully/better conditioned leaves (as Cond0 presented higher fungal biomass than Cond3) or leaves conditioned by preferred fungal species potentially expressed in Cond0 but not Cond3 leaves (Gonçalves et al. 2014; Canhoto et al. 2017; Martínez et al. 2020). Nonetheless, while chestnut’s lower leaf toughness and higher fungal biomass levels may “dilute” a preferential behavior for any of these food items, the reduced biomass concentrations in oak Cond3 and its toughness seem to lead to a clear positive preference for oak Cond0 leaves in Inv0. However, such food preference behavior was lost under Inv3 media, possibly related to either (1) waterborne metabolic alterations inhibiting larval ability to perceive and discriminate between more subtle leaf quality differences or (2) salt absorption from the media to the leaf surface (personal observation) that may inhibit consumption and conceal any finer differences in substrate quality. These results require further attention, as they suggest that a salinization imprint on detritus may result in invertebrates’ altered processing capacity and/or biased interactions between shredders and their preferred detritus. If confirmed in field tests and with other shredder species, this effect of salinization on food selection and consumption may have important consequences on nutrient recycling and energy flows in stream ecosystems.
To the best of our knowledge, this is the first study assessing the concomitant effect of salinization (waterborne and food mediated) and litter quality on freshwater leaf consumers’ feeding behavior and performance. Globally, based on our results and confirming previous works (Entrekin et al. 2017), we expect sub-lethal effects to occur primarily through direct waterborne toxicity, regulated by leaf litter inherent and microbially acquired quality. Nonetheless, results also suggest that media salinization may impair invertebrate capacity to evaluate fine microbial-mediated leaf quality differences, particularly in the presence of more recalcitrant leaf litter, with consequences for larval fitness (Canhoto and Graça 2008). Further examination of shredder-detritus relationships and their drivers is needed, particularly considering that salinization of forested riparian soils is usually associated with changes in the diversity and quality (Entrekin et al. 2019; Kaspari 2020; Oliveira et al. 2021) of leaf litter, which sustains freshwater food webs.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Almeida Júnior ES, Martínez A, Gonçalves AL, Canhoto C (2020) Combined effects of freshwater salinization and leaf traits on litter decomposition. Hydrobiologia 847:3427–3435
Arenas-Sánchez A, Rico A, Vighi M (2016) Effects of water scarcity and chemical pollution in aquatic ecosystems: state of the art. Sci Total Environ 572:390–403
Bal A, Panda F, Pati SG et al (2021) Modulation of physiological oxidative stress and antioxidant status by abiotic factors especially salinity in aquatic organisms: Redox regulation under salinity stress. Comp Biochem Physiol Part - C Toxicol Pharmacol 241:108971. https://doi.org/10.1016/j.cbpc.2020.108971
Bärlocher F, Gessner MO, Graça MAS (2020) Methods to study litter decomposition. Springer International Publishing, Cham
Berger E, Frör O, Schäfer RB (2019) Salinity impacts on river ecosystem processes: a critical mini-review. Philos Trans R Soc B Biol Sci 374:20180010. https://doi.org/10.1098/rstb.2018.0010
Biasi C, Cogo GB, Hepp LU, Santos S (2019) Shredders prefer soft and fungal-conditioned leaves, regardless of their initial chemical traits. Iheringia Série Zool 109:1–7
Blasius BJ, Merritt RW (2002) Field and laboratory investigations on the effects of road salt (NaCl) on stream macroinvertebrate communities. Environ Pollut 120:219–231
Cañedo-Argüelles M, Grantham TE, Perrée I et al (2012) Response of stream invertebrates to short-term salinization: a mesocosm approach. Environ Pollut 166:144–151
Cañedo-Argüelles M, Bundschuh M, Gutiérrez-Cánovas C et al (2014) Effects of repeated salt pulses on ecosystem structure and functions in a stream mesocosm. Sci Total Environ 476–477:634–642. https://doi.org/10.1016/j.scitotenv.2013.12.067
Cañedo-Argüelles M, Sala M, Peixoto G et al (2016) Can salinity trigger cascade effects on streams? A Mesocosm Approach Sci Total Environ 540:3–10
Canhoto C, Graça MAS (2008) Interactions between fungi and invertebrates: back to the future. In: Sridhar KR, Bärlocher F, Hyde KD (eds) Novel techniques and ideas in mycology. University of Hong Kong
Canhoto C, Simões S, Gonçalves AL et al (2017) Stream salinization and fungal-mediated leaf decomposition: a microcosm study. Sci Total Environ 599–600:1638–1645. https://doi.org/10.1016/j.scitotenv.2017.05.101
Canhoto C, Bärlocher F, Cañedo-Argüelles M et al (2021) Salt modulates plant litter decomposition in stream ecosystems. The ecology of plant litter decomposition in stream ecosystems. Springer International Publishing, Cham, pp 323–345
Canhoto C, Oliveira R, Martínez A, Gonçalves AL (2023) Pulsed vs chronic salinization effects on microbial-mediated leaf litter decomposition in fresh waters. Hydrobiologia. https://doi.org/10.1007/s10750-022-04991-w
Castillo AM, Sharpe DMT, Ghalambor CK, De León LF (2018) Exploring the effects of salinization on trophic diversity in freshwater ecosystems: a quantitative review. Hydrobiologia 807:1–17. https://doi.org/10.1007/s10750-017-3403-0
Correa-Araneda F, Boyero L, Figueroa R et al (2015) Joint effects of climate warming and exotic litter (Eucalyptus globulus Labill.) on stream detritivore fitness and litter breakdown. Aquat Sci 77:197–205
Dang CK, Chauvet E, Gessner MO (2005) Magnitude and variability of process rates in fungal diversity-litter decomposition relationships. Ecol Lett 8:1129–1137. https://doi.org/10.1111/j.1461-0248.2005.00815.x
Entrekin SA, Clay NA, Mogilevski A et al (2019) Multiple riparian–stream connections are predicted to change in response to salinization. Philos Trans R Soc B Biol Sci 374:1–10. https://doi.org/10.1098/rstb.2018.0042
Entrekin S, Howard-parker B, Evans-white M, Clay N (2017) Biological and ecological consequences of sub-lethal ion concentrations on microbial and macroinvertebrate detritivores. Ark Bull Water Res
Ferreira V, Gonçalves AL, Godbold DL, Canhoto C (2010) Effect of increased atmospheric CO2 on the performance of an aquatic detritivore through changes in water temperature and litter quality. Glob Chang Biol 16:3284–3296
Flores L, Larrañaga A, Elosegi A (2014) Compensatory feeding of a stream detritivore alleviates the effects of poor food quality when enough food is supplied. Freshw Sci 33:134–141. https://doi.org/10.1086/674578
Foucreau N, Puijalon S, Hervant F, Piscart C (2013) Effect of leaf litter characteristics on leaf conditioning and on consumption by Gammarus pulex. Freshw Biol 58:1672–1681
Gessner MO, Chauvet E (1993) Ergosterol-to-biomass conversion factors for aquatic hyphomycetes. Appl Environ Microbiol 59:502–507
Gessner MO, Swan CM, Dang CK et al (2010) Diversity meets decomposition. Trends Ecol Evol 25:372–380
Gonçalves AL, Lírio AV, Pratas J, Canhoto C (2011) Uranium contaminated water does not affect microbial activity but decreases feeding by the shredder Sericostoma vittatum. Fundam Appl Limnol Arch Für Hydrobiol 179:17–25
Gonçalves AL, Graça MAS, Canhoto C (2013) The effect of temperature on leaf decomposition and diversity of associated aquatic hyphomycetes depends on the substrate. Fungal Ecol 6:546–553
Gonçalves AL, Chauvet E, Bärlocher F et al (2014) Top-down and bottom-up control of litter decomposers in streams. Freshw Biol 59:2172–2182
Gonçalves AL, Carvalho A, Bärlocher F, Canhoto C (2019a) Are fungal strains from salinized streams adapted to salt-rich conditions? Philos. Trans r Soc B Biol Sci 374:20180018
Gonçalves AL, Simões S, Bärlocher F, Canhoto C (2019b) Leaf litter microbial decomposition in salinized streams under intermittency. Sci Total Environ 653:1204–1212
González MA, Martínez J (2011) Checklist of the caddisflies of the Iberian Peninsula and Balearic Islands (Trichoptera). Zoosymposia 5:115–135. https://doi.org/10.11646/zoosymposia.5.1.10
Graça MAS (2001) The role of invertebrates on leaf litter decomposition in streams - a review. Int Rev Hydrobiol 86:383–393. https://doi.org/10.1002/1522-2632(200107)86:4/5%3c383::AID-IROH383%3e3.0.CO;2-D
Hassell KL, Kefford BJ, Nugegoda D (2006) Sub-lethal and chronic salinity tolerances of three freshwater insects: Cloeon sp. and Centroptilum sp. (Ephemeroptera: Baetidae) and Chironomus sp. (Diptera: Chironomidae). J Exp Biol 209:4024–4032
Hintz WD, Relyea RA (2019) A review of the species, community, and ecosystem impacts of road salt salinisation in fresh waters. Freshw Biol 64:1081–1097. https://doi.org/10.1111/fwb.13286
Iglesias MCA (2020) A review of recent advances and future challenges in freshwater salinization. Limnetica 39:185–211. https://doi.org/10.23818/limn.39.13
Kaspari M (2020) The seventh macronutrient: how sodium shortfall ramifies through populations, food webs and ecosystems. Ecol Lett 23:1153–1168. https://doi.org/10.1111/ele.13517
Kefford BJ, Papas PJ, Nugegoda D (2003) Relative salinity tolerance of macroinvertebrates from the Barwon River, Victoria. Australia Mar Freshw Res 54:755. https://doi.org/10.1071/MF02081
Kefford BJ, Hickey GL, Gasith A et al (2012) Global scale variation in the salinity sensitivity of riverine macroinvertebrates: Eastern Australia, France, Israel and South Africa. PLoS ONE 7:1–12
Landeira-Dabarca A, Pérez J, Graça MAS, Boyero L (2019) Joint effects of temperature and litter quality on detritivore-mediated breakdown in streams. Aquat Sci 81:1. https://doi.org/10.1007/s00027-018-0598-8
Le TDH, Schreiner VC, Kattwinkel M, Schäfer RB (2021) Invertebrate turnover along gradients of anthropogenic salinisation in rivers of two German regions. Sci Total Environ 753:141986
Marks JC (2019) Revisiting the fates of dead leaves that fall into streams. Annu Rev Ecol Evol Syst 50:547–568
Martínez A, Barros J, Gonçalves AL, Canhoto C (2020) Salinisation effects on leaf litter decomposition in fresh waters: Does the ionic composition of salt matter? Freshw Biol 65:1475–1483. https://doi.org/10.1111/fwb.13514
Odum EP, Finn JT, Franz EH (1979) Perturbation theory and the subsidy-stress gradient. Bioscience 29:349–352. https://doi.org/10.2307/1307690
Oliveira R, Martínez A, Gonçalves AL et al (2021) Salt pulses effects on in-stream litter processing and recovery capacity depend on substrata quality. Sci Total Environ 783:147013. https://doi.org/10.1016/j.scitotenv.2021.147013
Overy D, Correa H, Roullier C et al (2017) Does osmotic stress affect natural product expression in fungi? Mar Drugs 15:254
Pereira da Silva J, Martínez A, Gonçalves AL et al (2021) Fungal richness does not buffer the effects of streams salinization on litter decomposition. Ann Limnol - Int J Limnol 57:5
Piscart C, Moreteau JC, Beisel JN (2005) Biodiversity and structure of macroinvertebrate communities along a small permanent salinity gradient (Meurthe River, France). Hydrobiologia 551:227–236
Ramos SM, Graça MAS, Ferreira V (2021) A comparison of decomposition rates and biological colonization of leaf litter from tropical and temperate origins. Aquat Ecol 5:925–940
Reis F, Nascimento E, Castro H et al (2018) Land management impacts on the feeding preferences of the woodlouse Porcellio dilatatus (Isopoda: Oniscidea) via changes in plant litter quality. Appl Soil Ecol 132:45–52. https://doi.org/10.1016/j.apsoil.2018.08.018
Ruiz T, Koussoroplis AM, Felten V, Bec A (2022) Nutritional context modulates the salinity tolerance of freshwater invertebrates. Aquat Ecol 56:905–915. https://doi.org/10.1007/s10452-022-09975-5
Santonja M, Rodríguez-Pérez H, Le Bris N, Piscart C (2020) Leaf nutrients and macroinvertebrates control litter mixing effects on decomposition in temperate streams. Ecosystems 23:400–416. https://doi.org/10.1007/s10021-019-00410-9
Scheibener SA, Richardi VS, Buchwalter DB (2016) Comparative sodium transport patterns provide clues for understanding salinity and metal responses in aquatic insects. Aquat Toxicol 171:20–29. https://doi.org/10.1016/j.aquatox.2015.12.006
Siders AC, Compson ZG, Hungate BA et al (2018) Litter identity affects assimilation of carbon and nitrogen by a shredding caddisfly. Ecosphere 9:1–14. https://doi.org/10.1002/ecs2.2340
Sowa A, Krodkiewska M, Halabowski D (2020) How does mining salinisation gradient affect the structure and functioning of macroinvertebrate communities? Water Air Soil Pollut 231:453
Swan CM, Boyero L, Canhoto C (2021) The ecology of plant litter decomposition in stream ecosystems. Springer International Publishing, Cham
Szöcs E, Coring E, Bäthe J, Schäfer RB (2014) Effects of anthropogenic salinization on biological traits and community composition of stream macroinvertebrates. Sci Total Environ 468–469:943–949
Tiwari A, Rachlin JW (2018) A review of road salt ecological impacts. Northeast Nat 25:123–142
Tyree M, Clay N, Polaskey S, Entrekin S (2016) Salt in our streams: even small sodium additions can have negative effects on detritivores. Hydrobiologia 775:109–122. https://doi.org/10.1007/s10750-016-2718-6
Williams CJ, Frost PC, Ginn BK et al (2023) Add a dash of salt? effects of road de-icing salt (NaCl) on benthic respiration and nutrient fluxes in freshwater sediments. Limnetica 42:1. https://doi.org/10.23818/limn.42.17
Zhang M, Cheng X, Geng Q et al (2019) Leaf litter traits predominantly control litter decomposition in streams worldwide. Glob Ecol Biogeogr 28:1469–1486. https://doi.org/10.1111/geb.12966
Acknowledgements
In memoriam of Ana Lúcia Gonçalves. We gratefully acknowledge Prof. Felix Bärlocher for the English revision and helpful comments on the manuscript. This work was carried out at the R&D Unit Center for Functional Ecology—Science for People and the Planet (CFE), with reference UIDB/04004/2020, financed by FCT/MCTES through national funds (PIDDAC) and by TERRA (LA/P/0092/2020).
Funding
Open access funding provided by FCT|FCCN (b-on).
Author information
Authors and Affiliations
Contributions
SS and CC contributed to the study conception and design. Material preparation, data collection, and analysis were performed by EAJ, AM, and RO. The first draft of the manuscript was written by SS, CC, and EAJ. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Simões, S., Almeida, E., Martínez, A. et al. Effects of water salinization and substrata quality on the performance of the shredder Schizopelex festiva (Trichoptera; Sericostomatidae). Aquat Sci 86, 60 (2024). https://doi.org/10.1007/s00027-024-01077-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00027-024-01077-8