Abstract
Water salinization is a recognized growing threat to freshwaters, whose consequences on streams’ function, per se or concomitantly with other stressors, are still far from clear. This microcosm study evaluated the combined effect of salinization (0 and 4 g/l NaCl) and temperature (5, 15, and 20 °C) on microbial-mediated oak leaf litter decomposition, with fungal biomass, sporulation, and microbial respiration as microbial descriptors. Invertebrate consumption was also assessed using the common shredder Sericostoma vittatum (Trichoptera, Sericostomatidae). Mass loss was affected by temperature and interaction between salinity and temperature. Under salt conditions, mass loss was higher at 15 °C and reduced (~ 10%) at 20 °C. Microbial activity was lower at 5 °C and higher at 15 and 20 °C, irrespective of salinity. Fungal biomass was affected by both temperature (5 < 20 < 15 °C) and salinity (4 < 0 g/l NaCl), although the interaction between both was not significant. The interaction of both variables affected the production of spores: salt addition strongly reduced sporulation rates at all temperatures despite a significant increase in conidial production with temperature. Invertebrate leaf consumption was significantly reduced only by salinization. Overall, our results seem to indicate that temperature may modulate the effect of salinization (at least at ≥ 4 g/l NaCl) on stream leaf decomposition. While stronger salinization effects may be observed at higher temperatures, a consistent strong inhibition of shredders’ feeding behavior promoted by salt, regardless of temperature, may anticipate important repercussions on streams’ secondary production throughout the year.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Forested headwater streams are intimately connected with their riparian areas. As a consequence of the shadow promoted by the trees’ canopies, light access to the channel is limited, resulting in stream communities highly dependent on allochthonous sources of organic matter, mainly in the form of senescent leaves (Abelho 2001; Drake et al. 2017; Marks 2019). Leaf litter decomposition is a key ecosystem-level process in these systems, promoting the incorporation of terrestrial detritus into the brown aquatic food chains (Evans-White and Halvorson 2017; Marks 2019). This process integrates physico-chemical leaf litter changes and is executed by microbial (fungi and bacteria) and invertebrate protagonists (Gessner et al. 1999; Marks 2019; Swan et al. 2021), being closely modulated by environmental factors. The interaction between these biotic and abiotic drivers ultimately determines nutrient recycling and the transfer of energy across trophic levels (Perkins et al. 2010; Tiegs et al. 2019).
Several anthropogenic-related stressors presently affect stream ecosystems at an unprecedented level (Reid et al. 2019; Tickner et al. 2020). Among these, temperature increase (Gonçalves et al. 2015; Tiegs et al. 2019; Pérez et al. 2021; Niedrist 2023) and salinization (Cañedo-Argüelles et al. 2016; Kaushal et al. 2021; Thorslund et al. 2021; Carrier-Belleau et al. 2022) are major and intensifying threats at a global scale (Berger et al. 2019; Reid et al. 2019), directly affecting biotic communities composition, their distribution, and freshwater ecosystems processes such as leaf litter decomposition (Follstad Shah et al. 2017; Verberk et al. 2020; Wilmot et al. 2021). Many studies show that elevated temperature accelerates decomposition leading to the stimulation of leaf leaching and metabolic rates of the decomposer communities (Canhoto et al. 2016; Amani et al. 2019; Liu et al. 2022; Monroy et al. 2022), provided that their optimum temperature is not exceeded (Brown et al. 2004; Bonacina et al. 2023). Enhanced fungal sporulation rates, enzymatic activity, and biomass accrual in response to temperature are known to direct and indirectly, i.e., through the stimulation of invertebrate feeding rates, increase leaf processing (e.g., Fernandes et al. 2012; Gonçalves et al. 2013, 2015; Canhoto et al. 2016; Fenoy et al. 2016). A thermotolerant shredder community shows a generally positive response to temperature increase, usually correlated with an increase in respiration, growth, and developmental rates (Bonacina et al. 2023); this response, however, seems to be resource dependent (Landeira-Dabarca et al. 2019). Nonetheless, while thermal stress has been dealt with as a main concern by the scientific community, particularly in the scope of global changes, salinization is still comparatively overlooked and research fragmentary (Cañedo-Argüelles 2020; Canhoto et al 2021). Works assessing the effects of stream secondary salinization, i.e., increase in the concentration of inorganic ions promoted by human activities (Cañedo-Argüelles et al. 2013), have tended to focus on the effects of salt on invertebrates. Results consistently point to a reduction in their abundance (Piscart et al. 2006), diversity (Kefford et al. 2011), functional richness (Zhao et al. 2021), and distribution (Verberk et al. 2020), mainly due to physiological stress promoted by osmotic imbalances. As the stress threshold of invertebrates (namely shredders; Cummins 1974) is usually lower than that of microbial communities (Canhoto et al. 2021), a microbial-dominated depressed organic matter degradation, largely promoted by a halotolerant and impoverished fungal community, seems to dominate in salinized streams [> 3 g/l; Almeida Júnior et al. 2020)]. Fungal tolerance to salt (Canhoto et al. 2017) seems to be species-specific and result from energetic tradeoffs between osmotic adjustments, mycelial growth and, particularly, sporulation (Canhoto et al. 2017, 2021; Gonçalves et al. 2019b; Carrier-Belleau et al. 2022).
To date, only limited information exists on the impacts of salt contamination co-occurring with other abiotic factors on stream communities and ecosystem function (Schäfer et al. 2012; Gonçalves et al. 2019b; Orr and Buchwalter 2020; Carrier-Belleau et al. 2022). To our knowledge, no previous approach concomitantly assessed the effects of salinization and temperature increase on litter decomposition dynamics. Nonetheless, a generalized overriding impact of the former over other stressors seems to occur in several previous tested/observed combinations (Velasco et al. 2019; Cuthbert et al. 2019), with some exceptions being reported (e.g., Gonçalves et al. 2019b).
In this study, we aimed to evaluate the combined effect of salinization [control (C; 0 g/l NaCl) or enriched (E; 4 g/l NaCl) media] and temperature (5, 15, and 20 °C) in the decomposition of oak leaves and associated microbial parameters. The effects of both factors on consumption rates by the shredder Sericostoma vittatum (Trichoptera; Sericostomatidae) were also assessed. We expected (1) salinity to have deleterious effects on leaf litter mass loss and related descriptors, particularly at the tested extreme temperatures (i.e., 5 and 20 °C), as fungal species from central Portugal streams have an optimal activity at around 11 °C (Dang et al. 2009; Gonçalves et al. 2015); (2) higher microbial-mediated leaf litter quality at 15 °C to be translated into higher consumption rates by the shredder in both media; (3) salt enrichment to decrease shredder consumption due to osmotic energetic requirements, despite marked increases on invertebrate’s metabolism occurring at 20 °C.
Materials and methods
Study site and experimental procedures
Oak leaves (Quercus robur L.) were collected after abscission and air-dried in the dark at room temperature. Leaves were placed in fine-mesh bags (10 × 15 cm, 0.5 mm mesh) and submerged in a reference oligotrophic stream (Ribeira do Candal, Lousã, central Portugal, 40° 4′ 44″ N, 8° 12′ 10″ W; 620 m asl) to allow microbial colonization. This second-order stream runs through a mixed deciduous forest, mostly composed of Quercus robur L. and Castanea sativa Mill. trees. Mean stream flow was 0.50 ± 0.09 m/s, oxygen concentration 10.80 ± 0.40 mg/l (WTW Oxi 3310, Germany), pH 6.4 ± 0.1 (WTW pH 3110, Germany), and conductivity 198.00 ± 32.30 µS (Hanna HI 98192, Portugal). After 2 weeks, leaves were brought back to the laboratory. Using a cork borer, leaf discs (12 mm diameter) were punched out either randomly or as pairs cut symmetrically relative to the main leaf vein. In this case, discs from the same pair were assumed to have similar initial mass (Bärlocher et al. 2020) and were further used to assess consumption rates by the shredder S. vittatum (see below).
Twenty-four groups of 15 random leaf discs were distributed in Erlenmeyer flasks, containing either 40 ml nutrient medium [75.5 mg CaCl2, 10 mg MgSO4∙7H2O, 0.5 g 3-morpholinopropanesulfonic acid, 5.5 mg K2HPO4, and 100 mg KNO3 per liter of sterile distilled water (Dang et al. 2005)], corresponding to the control treatment (C medium, n = 12), or 40 ml nutrient medium enriched with 4 g/l NaCl (E medium, n = 12). This NaCl concentration, previously known to have an effect on fungal community structure and activity (Gonçalves et al. 2019b), corresponds to realistic conditions induced on streams by agriculture and road de-icing practices (Gonçalves et al. 2019a; Oliveira et al. 2021).
Microcosms containing C and E media were randomly and equitably distributed to chambers at three different temperatures: 5, 15, and 20 °C. These temperature levels are common in low-order temperate streams across seasons and may provide insights into the effects of temperature increase in streams experiencing the effects of global warming. All microcosms, regardless of temperature and salinization treatment, were aerated on an orbital shaker (100 rpm) under a 12:12 h light/dark photoperiod; nutrient medium was renewed every 2 days under sterile conditions (Gonçalves et al. 2014), and Erlenmeyer flasks were always closed with sterile cotton plugs (Gonçalves et al. 2014). After 10 days of incubation in the laboratory, random discs were used to assess mass loss, microbial activity, fungal biomass, and sporulation rates. Six additional Erlenmeyer flasks, each with 10 pairs of leaf discs, were prepared, in the same conditions, for consumption tests.
Leaf mass loss
After the initial 14-day colonization period in the stream, 20 sets of 15 random discs obtained from stream-conditioned oak leaves were oven-dried (60 °C, 48 h) and weighed (± 0.1 mg); the average mass was considered as the initial dry mass (DM) of 15 discs after stream colonization. At the end of the experimental period, dry mass loss was estimated as the difference between the initial and final dry mass of the 15 discs from each microcosm, obtained after all microbial analyses (see below).
Microbial activity
Microbial activity was assessed by fluorescein acetate (FDA) hydrolysis (Datry et al. 2011; Simões et al. 2022). From each microcosm, a random subset of five leaf discs was placed in a sterile glass vial with 3 ml phosphate buffer (pH = 7.6) and 100 µl FDA stock solution (0.02 g FDA in 10 ml acetone). After incubation in the dark for 60–100 min, the reaction was stopped with 3 ml acetone. Absorbance (490 nm) was measured using a spectrophotometer (6400 Jenway, Dunmow, Essex, UK). Leaf discs were oven-dried (60 °C, 48 h) and weighed, and microbial activity was expressed as μmol FDA/h/g DM.
Fungal biomass
Another random subset of five discs from each microcosm was frozen, freeze-dried for 24 h (lyophilizer CHRIST, ALPHA 1–2 LD Plus, Germany) and weighed. Ergosterol was extracted by microwave exposure in methanol, separated by pentane (Reis et al. 2018), and quantified by high-performance liquid chromatography (HPLC; Shimadzu Prominence UFLC, Kyoto, Japan) using a HPLC C18 column (Mediterranean sea 18, 250 × 4.6 mm, 5 μm particle size; Teknokroma). Ergosterol concentration was converted into fungal biomass (5.5 μg ergosterol per mg fungal dry mass) (Gessner and Chauvet 1993). Results were expressed as mg fungal biomass/g DM.
Aquatic hyphomycete sporulation
A final subset of five discs from each microcosm was incubated in 25 ml nutrient medium and maintained under the original salinization/temperature treatment. After 48 h, conidial suspensions were collected into 50-ml Falcon tubes and preserved with 2 ml 37% formalin. Subsamples were filtered (Millipore SMWP, 5 μm pore size, Billerica, MA, USA) and the retained spores stained (0.05% cotton blue in lactic acid 60%) and counted under a compound microscope at 250× magnification (Bärlocher et al. 2020). Discs were oven-dried (60 °C, 48 h) and weighed; aquatic hyphomycete sporulation rates were expressed as number of conidia/mg DM/day.
Invertebrate consumption test
Sericostoma vittatum larvae were collected from Ribeira do Candal (Lousã, central Portugal, 40° 4′ 44″ N, 8° 12′ 10″ W; 620 m asl) and brought to the laboratory. Individuals were acclimatized in containers with aerated filtered stream water at 5, 15, or 20 °C for 1 week. Larvae were fed ad libitum with colonized leaves collected from the same stream and exposed to a 12:12 h light/dark photoperiod.
A total of 60 invertebrates were individually allocated into plastic containers (i.e., feeding chambers; 70 mm diameter × 85 mm high), each containing 200 ml filtered (Whatman GF/F 50 mm) and aerated stream water. The bottom of each feeding chamber was covered by a fine layer of sieved (< 2 mm diameter) and ashed stream sediment. In half the containers for each temperature treatment, the stream water was enriched with 4 g/l NaCl. Just before the beginning of the tests, the diameter of the larval case opening (CO; mm) was measured, and individual DM (0.0115 ± 0.0004 g) was estimated using the regression model DM = CO × 0.0136 – 0.0162 (Gonçalves et al. 2011). Individuals were starved for 14 h before the beginning of the tests. For each temperature and salt treatment, 10 replicates were assessed. Each microcosm was supplied with a pair of leaf discs (previously conditioned in C or E medium at the three temperature levels): one oak disc from each pair was offered to the invertebrate by directly placing it in the sediment, while the other symmetrical disc was enclosed in a small fine-mesh bag (0.5 mm mesh), allowing continued microbial decomposition while restricting consumption by the invertebrate (Bärlocher et al. 2020).
Leaf discs were removed from the containers when half of the exposed discs were 50% consumed (visual assessment); the discs from each replicate and treatment were recovered, oven-dried (60 °C, 48 h) and weighed. Invertebrate consumption was estimated as the difference between the final DM of the control and corresponding disc offered to the invertebrate. Individual relative consumption rates (RCR) for each treatment were expressed as g leaf DM consumed/g invertebrate DM/day (Bärlocher et al. 2020).
Statistical analyses
Differences in mass loss, microbial activity, fungal biomass, sporulation, and consumption rates were each analyzed using two-way analysis of variance (ANOVA), with temperature (5, 15, and 20 °C) and salinization (0 and 4 g/l) as categorical variables. Tukey’s HSD tests were applied whenever significant (p < 0.05) statistical differences were found to determine differences between treatments. Normality and homoscedasticity assumptions were met in all cases (assessed through histograms and Bartlett’s test). Statistical analyses were performed with Statistica 7 software (StatSoft, Tulsa, OK, USA).
Results
Microbial-mediated mass loss
Microbial-mediated mass loss was not significantly affected by salinity (2-way ANOVA, p = 0.08) but was affected by temperature and the interaction between both factors (2-way ANOVA, p < 0.001) (Fig. 1). In the C medium, mass loss was almost negligible (< 1%) at 5 °C (Tukey’s test, p < 0.001), while high values (26% and 30%) were observed at 15 and 20 °C, respectively (Tukey’s test, p = 0.84). In the E medium, mass loss was highest (29%) at 15 °C (Tukey’s test, p < 0.001) and significantly lower (ca. 10%) at both 5 and 20 °C (Tukey’s test, p = 0.99).
Microbial activity
Microbial activity was not significantly affected by salinity or the interaction between both factors (2-way ANOVA, p > 0.12). Temperature affected microbial activity (2-way ANOVA, p < 0.001); it was significantly lower at 5 °C (Tukey’s test, p < 0.001), with no differences between the values observed at 15 and 20 °C (Tukey’s test, p = 0.06).
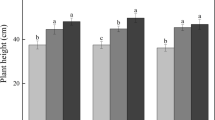
Minimum microbial activity values (1.65 ± 0.30 µmol FDA/h/g DM) were found associated with oak leaf discs exposed to E medium at 5 °C; maximum values (4.42 ± 0.16 µmol FDA/h/g DM) were found in the same salinization treatment at 15 °C (Fig. 2a).
Microbial activity (a), fungal biomass (b), and sporulation rate (c) associated with oak leaf discs exposed to two salinization (C, 0 g/l NaCl; E, 4 g/l NaCl) and three temperature (5, 15, and 20 °C) treatments. Values are means ± SE. Different letters indicate significant (p < 0.05) differences between temperature treatments regardless of salinization for microbial activity; between temperature (lower-case letters) and between salinization treatments (upper-case letters) for fungal biomass; and between all treatments, for sporulation rate
Fungal biomass
Fungal biomass was significantly affected by both temperature (15 > 20 > 5 °C; 2-way ANOVA, p < 0.001, Tukey’s test, p < 0.001) and salinity (E medium < C medium, 2-way ANOVA, p = 0.007); the interaction between both factors was not significant (p < 0.16).
Fungal biomass was highest (103.69 ± 6.01 mg/g DM) in oak leaves exposed to the C medium at 15 °C and lowest (31.62 ± 0.41 mg/g DM; Fig. 2b) in leaves exposed to salt (E medium) at 5 °C (Fig. 2b).
Fungal sporulation
Sporulation rates were affected by both salinity and temperature (2-way ANOVA, p < 0.001) and the interaction between both factors (p < 0.001; Fig. 2c). In the C medium, sporulation was significantly reduced at 5 °C (2.30 ± 0.09 no./mg DM/day, Tukey’s test, p < 0.001); a 54% reduction in sporulation rates was found from 15 to 20 °C (Tukey’s test, p = 0.02; 579.05 ± 9.75 and 311.98 ± 47.58 no./mg DM/day, respectively). In the E medium, sporulation rates were strongly inhibited at all tested temperatures; even so, conidial production was higher with the increase in temperature in the order 20 > 15 > 5 °C (Tukey’s test, p < 0.001).
Consumption rates
Consumption rates were significantly affected by salinity (2-way ANOVA, p < 0.001) but not by temperature or the interaction between both factors (p = 0.176). Regardless of the temperature, consumption rates were significantly reduced (> 35%) in the E medium (Tukey’s test, p < 0.001; Fig. 3).
Relative consumption rate by Sericostoma vittatum of oak leaf discs exposed to two salinization (C, 0 g/l NaCl; E, 4 g/l NaCl) and three temperature (5, 15, and 20 °C) treatments. Values are mean ± SE. Different uppercase letters indicate significant (p < 0.05) differences between salinity treatments
Discussion
The results from this study confirm that the process of leaf decomposition is affected by temperature and salinization; more importantly, they show that the combined effect of both stressors may differentially affect leaf mass loss, particularly at higher temperatures. In addition, salinization was deleterious to detritivore consumption, while the thermal conditions did not affect consumption in either treatment.
Microbial-mediated mass loss was globally not significantly impacted by the addition of salt by itself. This result is in line with previous laboratory (Gonçalves et al. 2019b; Martínez et al. 2020) and field (Berger et al. 2019; Vander Vorste et al. 2019) works, although contradictory results have also been found (Cañedo-Argüelles et al. 2014; Gómez et al. 2016; Sauer et al. 2016; Canhoto et al. 2017, 2022). This disparity may be explained by the short exposure to salinization in this work (i.e., 10 days) but also by potential changes in fungal community composition and/or interactions among fungal species (Gonçalves et al. 2019a), frequently occurring at the salt contamination level (4–6 g/l) used in these tests (Gonçalves et al. 2019a; Canhoto et al. 2021). As fungal biomass decreased globally with salt addition, it seems likely that, under salt stress, fewer tolerant fungal species, less expressive in terms of biomass, may dominate and guarantee oak leaf degradation (Canhoto et al. 2021) eventually through functional redundancy (Dang et al. 2005; Allison and Martiny 2009; Gonçalves et al. 2013, 2015). Also, the negative effects of salinization seem to be less evident in low (i.e., oak; present study) than high (i.e., alder) quality leaf litter (Oliveira et al. 2021), strengthening the idea that intrinsic leaf physico-chemical characteristics are central determinants of decomposition in salt-contaminated freshwaters (Stoler et al. 2017) and streams (Almeida Júnior et al. 2020; Monroy et al. 2022).
Previous works suggest 11 °C (e.g., autumn/spring) to be the optimum temperature for leaf decomposition promoted by most aquatic hyphomycete species isolated from Central Portuguese streams (Gonçalves et al. 2015b; Simões et al. 2022). According to our hypothesis, it seems that the negative impact of salinization on microbial-mediated litter decomposition tends to be buffered when water temperatures are close to this optimum, probably as a result of thermal metabolic stimulation, suggested by the increased microbial activity and fungal biomass. In fact, decomposition efficiency was higher at 15 °C regardless of salinization treatment. On the other hand, at both temperature extremes tested, the effect of salinization on oak mass loss depended on temperature: compared to the control media, salt addition resulted in 15-times higher mass loss at 5 °C but 3-times lower at 20 °C. The first result, although not significant, cannot be ignored, as it mimics what commonly happens during winter when salt is used as deicer agent leading to the salinization of mountain streams. The potential high ability of aquatic hyphomycetes to sequester Na (Kaspari et al. 2009), associated with physiological mechanisms to enhance tolerance to cold temperatures [e.g., accumulation/increase of the disaccharide trehalose, polyols, antifreeze proteins, and/or unsaturated lipids (Robinson 2001; Senik et al. 2019)], may have facilitated the maintenance of hyphal integrity, increasing the degradative capacity in salt-rich medium compared to the control at 5 °C, although at a cost of lower biomass accrual and vestigial sporulation rates.
A more deleterious effect of salinization was observed under warmer water temperatures (20 °C). Despite a similar starting fungal community due to the leaves’ stream incubation, this could translate a fungal community with a higher proportion of dormant species (Fenoy et al. 2022). However, as microbial activity was only slightly (and non-significantly) reduced from 15 to 20 °C, we suggest that the fungal community was restricted to active thermotolerant species that presented lower degradative capacity because of physiological strategies to cope with thermal stress (del Campo et al. 2021). Also, as happens with other stressors, higher temperatures may determine increased salt toxicity (Orr and Buchwalter 2020). This is particularly worrisome as salinization is expected to increase in most persistent and intermittent streams during warmer seasons as a result of reduced dilution ability of aquatic systems due to lower precipitation and runoff, increased evaporation and water abstraction (Jeppesen et al. 2020). However, a subtle increment in conidial production paralleled the temperature increase in salt-rich media. This may suggest a thermal metabolic stimulation that leads to the production and release of conidia as a potential strategy to overcome unfavorable ionic conditions (Bärlocher et al. 2008; Canhoto et al. 2016).
The overpowering effect of salinization in relation to temperature, particularly visible in shredder feeding behavior, confirmed previous studies suggesting Trichoptera to be more sensitive to salt than temperature (Fenoy et al. 2020; Verberk et al. 2020). Consumption rates were consistently reduced in salinized treatments regardless of temperature. This may have occurred directly through waterborne toxicity, indirectly due to the reduced quality of offered food (leaves conditioned by less palatable, salinity-tolerant fungal assemblages), or a combination of both. In fact, elevated ion concentrations may determine osmoregulatory stress on several insect groups, caddisflies included, even at levels below the ones used in our tests (Tyree et al. 2016; Dowse et al. 2017; Jackson and Funk 2019; Feld et al. 2023). Although our experimental design does not allow a causal discrimination (i.e., food vs. waterborne effect), we foresee that reduced food intake under salinized conditions will likely compromise larval performance and consequently litter decomposition, particularly if conditions persist. It was interesting to notice that, although non-significant, consumption rates in salt-enriched medium increased with temperature (Orr and Buchwalter 2020). This shredder species is known to tolerate our tested temperature levels (Dunlop et al. 2008); nonetheless, the increased metabolism at higher temperatures and the maintenance of an osmotic balance occur at an energetic cost that may require a compensatory food intake, particularly when facing leaves of poorer quality (Swan and Palmer 2006; Tyree et al. 2016; Landeira-Dabarca et al. 2019; Orr and Buchwalter 2020). If confirmed, this supports previous findings that indicate lower effects of salinity at lower temperatures (Jackson and Funk 2019; Velasco et al. 2019) and litter quality as an important modulator of leaf processing in streams affected by salinity per se or co-occurring with other stressors (Landeira-Dabarca et al. 2019; Almeida Júnior et al. 2020; Oliveira et al. 2021).
Our results globally confirm a dominant negative effect of salinity over temperature on microbial and detritivore-mediated decomposition of oak leaves, while also strengthening the crucial role of fungi, over invertebrates, on leaf litter processing in salinized streams. Based on our results, we expect a higher resilience and activity of the microbial communities to salt stress at intermediate temperatures (15 °C), frequent in autumn when the organic matter is more abundant in temperate freshwaters. Increased salt concentration occurring at higher temperatures seems to be more deleterious to microbial leaf degradation than at lower ones, while potentially resulting in a frail stimulation of S. vittatum larvae (reduced) consumption rates, as a compensatory feeding behavior on lower-quality oak detritus. Salt effects on leaf consumers were obvious but need to be amplified and further scrutinized; invertebrates’ response to salinization under different thermal scenarios is likely species specific and occurs via the still not disentangled direct waterborne toxicity and/or food contamination pathways, with consequences propagating through the aquatic food webs.
Salinization and increased water temperature are abiotic stressors closely linked with global change, able to alter, per se and in combination, key processes and services of stream ecosystems. These consequences are far from elucidated, despite the urgency to adopt legislative measures and specific environmental guidelines to protect and manage these vulnerable ecosystems.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Abelho M (2001) From litterfall to breakdown in streams: a review. Sci World J 1:656–680. https://doi.org/10.1100/tsw.2001.103
Allison SD, Martiny JBH (2009) Resistance, resilience, and redundancy in microbial communities. Proc Natl Acad Sci U S A 2:149–166
Almeida Júnior ES, Martínez A, Gonçalves AL, Canhoto C (2020) Combined effects of freshwater salinization and leaf traits on litter decomposition. Hydrobiologia 847:3427–3435
Amani M, Graça MAS, Ferreira V (2019) Effects of elevated atmospheric CO2 concentration and temperature on litter decomposition in streams: a meta-analysis. Int Rev Hydrobiol 104:14–25. https://doi.org/10.1002/iroh.201801965
Bärlocher F, Seena S, Wilson KP, Dudley Williams D (2008) Raised water temperature lowers diversity of hyporheic aquatic hyphomycetes. Freshw Biol 53:368–379
Bärlocher F, Gessner MO, Graça MAS (2020) Methods to study litter decomposition. Springer, Cham
Berger E, Frör O, Schäfer RB (2019) Salinity impacts on river ecosystem processes: a critical mini-review. Philos Trans R Soc B Biol Sci 374:20180010. https://doi.org/10.1098/rstb.2018.0010
Bonacina L, Fasano F, Mezzanotte V, Fornaroli R (2023) Effects of water temperature on freshwater macroinvertebrates: a systematic review. Biol Rev 98:191–221. https://doi.org/10.1111/brv.12903
Brown JH, Gillooly JF, Allen AP et al (2004) Toward a metabolic theory of ecology. Ecology 85:1771–1789. https://doi.org/10.1890/03-9000
Cañedo-Argüelles M (2020) A review of recent advances and future challenges in freshwater salinization. Limnetica 39:185–211. https://doi.org/10.23818/limn.39.13
Cañedo-Argüelles M, Kefford BJ, Piscart C et al (2013) Salinisation of rivers: an urgent ecological issue. Environ Pollut 173:157–167. https://doi.org/10.1016/j.envpol.2012.10.011
Cañedo-Argüelles M, Bundschuh M, Gutiérrez-Cánovas C et al (2014) Effects of repeated salt pulses on ecosystem structure and functions in a stream mesocosm. Sci Total Environ 476–477:634–642. https://doi.org/10.1016/j.scitotenv.2013.12.067
Cañedo-Argüelles M, Hawkins CP, Kefford BJ et al (2016) Saving freshwater from salts. Science 351:914–916. https://doi.org/10.1126/science.aad3488
Canhoto C, Gonçalves AL, Bärlocher F (2016) Biology and ecological functions of aquatic hyphomycetes in a warming climate. Fungal Ecol 19:201–218. https://doi.org/10.1016/j.funeco.2015.09.011
Canhoto C, Simões S, Gonçalves AL et al (2017) Stream salinization and fungal-mediated leaf decomposition: a microcosm study. Sci Total Environ 599–600:1638–1645. https://doi.org/10.1016/j.scitotenv.2017.05.101
Canhoto C, Bärlocher F, Cañedo-Argüelles M et al (2021) Salt modulates plant litter decomposition in stream ecosystems. The ecology of plant litter decomposition in stream ecosystems. Springer, Cham, pp 323–345
Canhoto C, Oliveira R, Martínez A, Gonçalves AL (2022) Pulsed vs. chronic salinization effects on microbial-mediated leaf litter decomposition in fresh waters. Hydrobiologia. https://doi.org/10.1007/s10750-022-04991-w
Carrier-Belleau C, Pascal L, Tiegs SD et al (2022) From organismal physiology to ecological processes: effects of nutrient enrichment and salinity variation in a freshwater ecosystem. Limnol Oceanogr. https://doi.org/10.1002/lno.12269
Cummins W (1974) Function of stream ecosystems. Bioscience 24:631–641
Cuthbert RN, Weyl OLF, Wasserman RJ et al (2019) Combined impacts of warming and salinisation on trophic interactions and mortality of a specialist ephemeral wetland predator. Freshw Biol 64:1584–1592. https://doi.org/10.1111/fwb.13353
Dang CK, Chauvet E, Gessner MO (2005) Magnitude and variability of process rates in fungal diversity-litter decomposition relationships. Ecol Lett 8:1129–1137. https://doi.org/10.1111/j.1461-0248.2005.00815.x
Dang CK, Schindler M, Chauvet E, Gessner MO (2009) Temperature oscillation coupled with fungal community shifts can modulate warming effects on litter decomposition. Ecology 90:122–131
Datry T, Corti R, Claret C, Philippe M (2011) Flow intermittence controls leaf litter breakdown in a French temporary alluvial river: the “drying memory.” Aquat Sci 73:471–483. https://doi.org/10.1007/s00027-011-0193-8
del Campo R, Martí E, Bastias E et al (2021) Floodplain preconditioning of leaf litter modulates the subsidy of terrestrial C and nutrients in fluvial ecosystems. Ecosystems 24:137–152. https://doi.org/10.1007/s10021-020-00508-5
Dowse R, Palmer CG, Hills K et al (2017) The mayfly nymph Austrophlebioides pusillus Harker defies common osmoregulatory assumptions. R Soc Open Sci 4:160520. https://doi.org/10.1098/rsos.160520
Drake TW, Raymond PA, Spencer RGM (2017) Terrestrial carbon inputs to inland waters: a current synthesis of estimates and uncertainty. Limnol Oceanogr Lett 3(3):132–142
Dunlop JE, Horrigan N, McGregor G et al (2008) Effect of spatial variation on salinity tolerance of macroinvertebrates in Eastern Australia and implications for ecosystem protection trigger values. Environ Pollut 151:621–630
Evans-White MA, Halvorson HM (2017) Comparing the ecological stoichiometry in green and brown food webs - a review and meta-analysis of freshwater food webs. Front Microbiol 8:1–14. https://doi.org/10.3389/fmicb.2017.01184
Feld CK, Lorenz AW, Peise M et al (2023) Direct and indirect effects of salinisation on riverine biota: a case study from river Wipper, Germany. Hydrobiologia 850:3043–3059. https://doi.org/10.1007/s10750-023-05229-z
Fenoy E, Casas JJ, Díaz-López M et al (2016) Temperature and substrate chemistry as major drivers of interregional variability of leaf microbial decomposition and cellulolytic activity in headwater streams. FEMS Microbiol Ecol 92:1–13
Fenoy E, Moyano FJ, Casas JJ (2020) Warming and nutrient-depleted food: two difficult challenges faced simultaneously by an aquatic shredder. Freshw Sci 39:393–404
Fenoy E, Pradhan A, Pascoal C et al (2022) Elevated temperature may reduce functional but not taxonomic diversity of fungal assemblages on decomposing leaf litter in streams. Glob Chang Biol 28:115–127. https://doi.org/10.1111/gcb.15931
Fernandes I, Pascoal C, Guimarães H et al (2012) Higher temperature reduces the effects of litter quality on decomposition by aquatic fungi. Freshw Biol 57:2306–2317
Follstad Shah JJ, Kominoski JS, Ardón M et al (2017) Global synthesis of the temperature sensitivity of leaf litter breakdown in streams and rivers. Glob Chang Biol 23:3064–3075. https://doi.org/10.1111/gcb.13609
Gessner MO, Chauvet E (1993) Ergosterol-to-biomass conversion factors for aquatic hyphomycetes. Appl Environ Microbiol 59:502–507
Gessner MO, Chauvet E, Dobson M (1999) A perspective on leaf litter breakdown in streams. Oikos 85:377. https://doi.org/10.2307/3546505
Gómez R, Asencio AD, Picón JM et al (2016) The effect of water salinity on wood breakdown in semiarid Mediterranean streams. Sci Total Environ 541:491–501
Gonçalves AL, Lírio AV, Pratas J, Canhoto C (2011) Uranium contaminated water does not affect microbial activity but decreases feeding by the shredder Sericostoma vittatum. Fundam Appl Limnol/arch Für Hydrobiol 179:17–25
Gonçalves AL, Graça MAS, Canhoto C (2013) The effect of temperature on leaf decomposition and diversity of associated aquatic hyphomycetes depends on the substrate. Fungal Ecol 6:546–553
Gonçalves AL, Chauvet E, Bärlocher F et al (2014) Top-down and bottom-up control of litter decomposers in streams. Freshw Biol 59:2172–2182
Gonçalves AL, Graça MAS, Canhoto C (2015a) Is diversity a buffer against environmental temperature fluctuations? A decomposition experiment with aquatic fungi. Fungal Ecol 17:96–102. https://doi.org/10.1016/j.funeco.2015.05.013
Gonçalves AL, Carvalho A, Bärlocher F, Canhoto C (2019a) Are fungal strains from salinized streams adapted to salt-rich conditions? Philos Trans R Soc B Biol Sci 374:20180018
Gonçalves AL, Simões S, Bärlocher F, Canhoto C (2019b) Leaf litter microbial decomposition in salinized streams under intermittency. Sci Total Environ 653:1204–1212
Jackson JK, Funk DH (2019) Temperature affects acute mayfly responses to elevated salinity: Implications for toxicity of road de-icing salts. Philos Trans R Soc B Biol Sci. https://doi.org/10.1098/rstb.2018.0081
Jeppesen E, Beklioğlu M, Özkan K, Akyürek Z (2020) Salinization increase due to climate change will have substantial negative effects on inland waters: a call for multifaceted research at the local and global scale. Innov 1:100030. https://doi.org/10.1016/j.xinn.2020.100030
Kaspari M, Yanoviak SP, Dudley R et al (2009) Sodium shortage as a constraint on the carbon cycle in an inland tropical rainforest. Proc Natl Acad Sci 106:19405–19409. https://doi.org/10.1073/pnas.0906448106
Kaushal SS, Likens GE, Pace ML et al (2021) Freshwater salinization syndrome: from emerging global problem to managing risks. Biogeochemistry 154:255–292. https://doi.org/10.1007/s10533-021-00784-w
Kefford BJ, Marchant R, Schäfer RB et al (2011) The definition of species richness used by species sensitivity distributions approximates observed effects of salinity on stream macroinvertebrates. Environ Pollut 159:302–310. https://doi.org/10.1016/j.envpol.2010.08.025
Landeira-Dabarca A, Pérez J, Graça MAS, Boyero L (2019) Joint effects of temperature and litter quality on detritivore-mediated breakdown in streams. Aquat Sci 81:1. https://doi.org/10.1007/s00027-018-0598-8
Liu Y, Zhang B, Zhang Y et al (2022) Organic matter decomposition in river ecosystems: microbial interactions influenced by total nitrogen and temperature in river water. Microb Ecol. https://doi.org/10.1007/s00248-022-02013-9
Marks JC (2019) Revisiting the fates of dead leaves that fall into streams. Annu Rev Ecol Evol Syst 50:547–568
Martínez A, Barros J, Gonçalves AL, Canhoto C (2020) Salinisation effects on leaf litter decomposition in fresh waters: does the ionic composition of salt matter? Freshw Biol 65:1475–1483. https://doi.org/10.1111/fwb.13514
Monroy S, Larrañaga A, Martínez A et al (2022) Temperature sensitivity of microbial litter decomposition in freshwaters: role of leaf litter quality and environmental characteristics. Microb Ecol. https://doi.org/10.1007/s00248-022-02041-5
Niedrist GH (2023) Substantial warming of Central European mountain rivers under climate change. Reg Environ Chang 23:1–11. https://doi.org/10.1007/s10113-023-02037-y
Oliveira R, Martínez A, Gonçalves AL et al (2021) Salt pulses effects on in-stream litter processing and recovery capacity depend on substrata quality. Sci Total Environ 783:147013. https://doi.org/10.1016/j.scitotenv.2021.147013
Orr SE, Buchwalter DB (2020) It’s all about the fluxes: temperature influences ion transport and toxicity in aquatic insects. Aquat Toxicol. https://doi.org/10.1016/j.aquatox.2020.105405
Pérez J, Correa-Araneda F, López-Rojo N et al (2021) Extreme temperature events alter stream ecosystem functioning. Ecol Indic 121:106984
Perkins DM, Reiss J, Yvon-Durocher G, Woodward G (2010) Global change and food webs in running waters. Hydrobiologia 657:181–198. https://doi.org/10.1007/s10750-009-0080-7
Piscart C, Usseglio-Polatera P, Moreteau J-CB (2006) The role of salinity in the selection of biological traits of freshwater invertebrates. Arch Für Hydrobiol 166:185–198. https://doi.org/10.1127/0003-9136/2006/0166-0185
Reid AJ, Carlson AK, Creed IF et al (2019) Emerging threats and persistent conservation challenges for freshwater biodiversity. Biol Rev 94:849–873
Reis F, Nascimento E, Castro H et al (2018) Land management impacts on the feeding preferences of the woodlouse Porcellio dilatatus (Isopoda: Oniscidea) via changes in plant litter quality. Appl Soil Ecol 132:45–52. https://doi.org/10.1016/j.apsoil.2018.08.018
Robinson CH (2001) Cold adaptation in Arctic and Antarctic fungi. New Phytol 151:341–353. https://doi.org/10.1046/j.1469-8137.2001.00177.x
Sauer FG, Bundschuh M, Zubrod JP et al (2016) Effects of salinity on leaf breakdown: dryland salinity versus salinity from a coalmine. Aquat Toxicol 177:425–432. https://doi.org/10.1016/j.aquatox.2016.06.014
Schäfer RB, Bundschuh M, Rouch DA et al (2012) Effects of pesticide toxicity, salinity and other environmental variables on selected ecosystem functions in streams and the relevance for ecosystem services. Sci Total Environ 415:69–78
Senik SV, Psurtseva NV, Shavarda AL, Kotlova ER (2019) Role of lipids in the thermal plasticity of basidial fungus Favolaschia manipularis. Can J Microbiol 65:870–879. https://doi.org/10.1139/cjm-2019-0284
Simões S, Gonçalves AL, Jones TH et al (2022) Air temperature more than drought duration affects litter decomposition under flow intermittency. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2022.154666
Stoler AB, Hintz WD, Jones DK et al (2017) Leaf litter mediates the negative effect of road salt on forested wetland communities. Freshw Sci 36:415–426
Swan CM, Palmer MA (2006) Preferential feeding by an aquatic consumer mediates non-additive decomposition of speciose leaf litter. Oecologia 149:107–114
Swan CM, Boyero L, Canhoto C (2021) The ecology of plant litter decomposition in stream ecosystems. Springer, Cham
Thorslund J, Bierkens MFP, Oude Essink GHP et al (2021) Common irrigation drivers of freshwater salinisation in river basins worldwide. Nat Commun. https://doi.org/10.1038/s41467-021-24281-8
Tickner D, Opperman JJ, Abell R et al (2020) Bending the curve of global freshwater biodiversity loss: an emergency recovery plan. Bioscience 70(4):330–342
Tiegs SD, Costello DM, Isken MW et al (2019) Global patterns and drivers of ecosystem functioning in rivers and riparian zones. Sci Adv 5:eaav0486
Tyree M, Clay N, Polaskey S, Entrekin S (2016) Salt in our streams: even small sodium additions can have negative effects on detritivores. Hydrobiologia 775:109–122. https://doi.org/10.1007/s10750-016-2718-6
Vander Vorste R, Timpano AJ, Cappellin C et al (2019) Microbial and macroinvertebrate communities, but not leaf decomposition, change along a mining-induced salinity gradient. Freshw Biol 64:671–684. https://doi.org/10.1111/fwb.13253
Velasco J, Gutiérrez-Cánovas C, Botella-Cruz M et al (2019) Effects of salinity changes on aquatic organisms in a multiple stressor context. Philos Trans R Soc B Biol Sci 374:20180011. https://doi.org/10.1098/rstb.2018.0011
Verberk WC, Buchwalter DB, Kefford BJ (2020) Energetics as a lens to understanding aquatic insect’s responses to changing temperature, dissolved oxygen and salinity regimes. Curr Opin Insect Sci 41:46–53. https://doi.org/10.1016/j.cois.2020.06.001
Wilmot OJ, Hood JM, Huryn AD, Benstead JP (2021) Decomposing decomposition: isolating direct effects of temperature from other drivers of detrital processing. Ecology 102:1–12. https://doi.org/10.1002/ecy.3467
Zhao Q, Zhang Y, Guo F et al (2021) Increasing anthropogenic salinisation leads to declines in community diversity, functional diversity and trophic links in mountain streams. Chemosphere 263:127994. https://doi.org/10.1016/j.chemosphere.2020.127994
Acknowledgements
This study was supported by (1) F4F—Forest for Future (CENTRO-08-5864-FSE-000031), pilot project—MyFORESt, co-financed by the Regional Operational Programme Centro 2020, Portugal 2020, and the European Union, through the European Social Fund (ESF), to SS and (2) Project UIDP/04004/2020, co-funded by FCT/MEC through national funds, FEDER within the PT2020 Partnership Agreement, and COMPETE 2020.
Funding
Open access funding provided by FCT|FCCN (b-on). Regional Operational Programme Centro 2020, Portugal 2020, and the European Union, through the European Social Fund (ESF), F4F—Forest for Future (CENTRO-08-5864-FSE-000031), FCT/MEC through national funds, FEDER within the PT2020 Partnership Agreement, and COMPETE 2020, Project UIDP/04004/2020.
Author information
Authors and Affiliations
Contributions
SS, ALG, and CC contributed to the study conception and design. Material preparation, data collection, and analysis were performed by SS, TA, and RO. The first draft of the manuscript was written by SS and TA. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Simões, S., Augusto, T., Oliveira, R. et al. Combined effects of salinization and temperature on microbial-mediated oak decomposition and invertebrate consumption. Aquat Sci 85, 116 (2023). https://doi.org/10.1007/s00027-023-01014-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00027-023-01014-1