Abstract
Signal transducer and activator of transcription (STAT) proteins act downstream of cytokine receptors to facilitate changes in gene expression that impact a range of developmental and homeostatic processes. Patients harbouring loss-of-function (LOF) STAT5B mutations exhibit postnatal growth failure due to lack of responsiveness to growth hormone as well as immune perturbation, a disorder called growth hormone insensitivity syndrome with immune dysregulation 1 (GHISID1). This study aimed to generate a zebrafish model of this disease by targeting the stat5.1 gene using CRISPR/Cas9 and characterising the effects on growth and immunity. The zebrafish Stat5.1 mutants were smaller, but exhibited increased adiposity, with concomitant dysregulation of growth and lipid metabolism genes. The mutants also displayed impaired lymphopoiesis with reduced T cells throughout the lifespan, along with broader disruption of the lymphoid compartment in adulthood, including evidence of T cell activation. Collectively, these findings confirm that zebrafish Stat5.1 mutants mimic the clinical impacts of human STAT5B LOF mutations, establishing them as a model of GHISID1.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The signal transducer and activator of transcription (STAT) proteins are a family of transcription factors that regulate gene transcription in response to cytokines and other extracellular signals [1]. Two closely-related STAT proteins, STAT5A and STAT5B, are stimulated by a wide variety of cytokines including growth hormone (GH), prolactin (PRL) and a range of interleukins (ILs) [2]. GH utilises STAT5B to facilitate much of its effects on somatic growth [3] and sexual dimorphism [4], PRL employs STAT5A to stimulate mammopoiesis and lactogenesis [5], with IL-2 and related cytokines engaging both STAT5B and STAT5A to regulate lymphocyte development and function [6].
Growth hormone insensitivity syndrome (GHIS) represents a group of rare genetic disorders characterised by postnatal growth failure due to functional insulin-like growth factor (IGF)-1 deficiency despite normal or elevated serum GH [7]. GHIS may be acquired through chronic illness or due to genetic defects in components of the GH–IGF-1 axis [8]. The latter include mutations impacting the GH receptor (GHR) [9], IGF-1 [10], the IGF binding protein IGFALS [11], the regulator of IGF-binding proteins PAPPA2 [12] as well as STAT5B [13]. The STAT5B mutations additionally result in immune defects, particularly impacting T lymphocyte development, with this multi-faceted disorder referred to as GHIS with immune dysregulation (GHISID) [7]. Two types have been identified, an autosomal-recessive form due to loss-of-function (LOF) STAT5B mutations, termed GHISID1 [14,15,16], and a less severe autosomal-dominant form due to alternate STAT5B lesions, termed GHISID2 [17].
Zebrafish represents a powerful animal model to mimic human inherited diseases that has been widely exploited to study growth and immunity [18, 19]. Moreover, there is high conservation of cytokine signalling pathway components between zebrafish and mammals, including cytokine receptors [20] and STAT proteins [21]. Two STAT5-related genes have been identified in zebrafish, with stat5.1 most similar to STAT5B [22]. This study used genome editing to target stat5.1 and generate a zebrafish model of GHISID1 that was characterised throughout the life-course.
Materials and methods
Fish husbandry and genetic manipulations
Zebrafish were maintained using standard husbandry practices [23], following national guidelines for their care and use, with all studies approved by the Deakin University Animal Ethics Committee. Synthetic guide RNA (gRNA) targeting exon 5 and 16 of the stat5.1 gene were generated using Zifit to design relevant oligonucleotides (5'-TAGGAGCTGCGAATACTGACTC and 5'-AAACGAGTCAGTATTCGCAGCT, 5'-TAGGAGCGGATAGAGAAGTCTT and 5'-AAACAAGACTTCTCTATCCGCT, respectively) as described [24]. Wild-type (WT) embryos were injected with 12.5 ng/μl gRNA and 100 ng/μl Cas9 mRNA (Sigma), raised to adulthood and out-crossed with WT fish. Identified carriers of stat5.1 mutant alleles were again out-crossed with WT fish to produce heterozygotes that were in-crossed to generate a homozygote mutant line.
Genomic DNA analysis
Genomic DNA from adult fin clips and whole embryos was isolated with QuickExtract following the manufacturer’s instructions. This was subjected to polymerase chain reaction (PCR) with stat5.1 specific primers for exon 5 (5'-GTGGGCGGGTTAATGGACAG, 5'-TACACGCATACCCTGTATTCTGAG) and 16 (5'-GTAACCATTTTAAAGCATCT, 5'-GTAACCATTTTAAAGCATC) for high resolution melt (HRM) analysis [25] to identify potential mutants, which was confirmed with gel electrophoresis using the same primers for exon 5 and an alternate downstream primer for exon 16 (5'-GTGTAGTATTTGGAGAA) and ultimately by Sanger sequencing at the Australian Genome Research Facility.
Body measurements
Embryos and juvenile fish were imaged with a MVX10 monozoom microscope using a 1 × MVXPlan Apochromat lens (NA = 0.25) and DP74 camera using CellSens Dimension 1.6 software (Olympus) and length determined using ImageJ software, with growth rates derived from that data. Adult fish was weighed and imaged using a camera phone (Apple iPhone 6, 8-megapixel, 1/3-inch sensor, 1.5 µm pixel size) next to a ruler, with standard length measured from snout to caudal peduncle [26].
Lipid analysis
Euthanized larvae were stained with 10 μg/ml Nile Red (Sigma) solution for 30 min at 28°C in the dark [27], and imaged with a MVX10 fluorescence microscope at an excitation wavelength of 488 nm using CellSens Dimension 1.6 software (Olympus) with the area of staining quantified using ImageJ software. Total lipid was extracted and quantified from adult fish as described [28].
qRT2-PCR
Total RNA was extracted from juvenile zebrafish with TRIsure™ (Bioline) and dissected adult tissues with an RNeasy Mini Kit (Qiagen) according to the manufacturers’ protocols. RNA samples were subjected to quantitative real-time reverse transcription PCR (qRT2-PCR) with primers for the zebrafish genes actb (5'-TGGCATCACACCTTCTAC, 5'-AGACCATCACCAGAGTCC), cd79a (5'-GCGAGGGTGTGAAAAACAGT, 5'-CCCTTTCTGTCTTCCTGTCCA), cd8 (5'-ACTCTTCTTCGGAGAGGTGAC, 5'-ACAGGCTTCAGTGTTGTTTGAA), ctla4 (5'-GGGAACGGCACTGTTGTTTAC, 5'-TGTCTGGCTCTTGCTTTGAC), fasn (5'-CATATTCTGGGTGTGCGTGAC, 5'-GCTTTACAGGAGACTCCTCTTTC), gh1 (5'-TCTTATGCCTGAGGAACGC, 5'-AGGTCTGGCTGGGAAACTC), igf1 (5'-CCGCATCTCATCCTCTTTCTC, 5'-CCTGTCTCCACACACAAACTGC), igf2a (5'-AGTGTCACAGGCTCTTCACAAG, 5'-GATGGGACTCCTCTCCTTAACC), igf2b (5'-TTCTGTTTGCCATACCTGCTC, 5'-ATCCCACGATTTTGAGAACG), igf3 (5'-GCGGACGAGAACTAGTGGAC, 5'-ATGCCTTTCCCACGAGAGC), ighm (5'-CCGAATACAGTGCCACAAGC, 5'-TCTCCCTGCTATCTTTCCGC), ikzf4 (5'-ATTGCAATGGCCGTTCGTAC, 5'-ATGGAGTTAGCACTGAGTGAGC), nklb (5'-TGGGAGGTTCGTGAAGTGG, 5'-CACTTTAGTACAGATGGTGTTTGGTC), nkld (5'-TGGTGAAATCCCAACAGAGCA, 5'-TTTCATCCTGAGTTGCACCA), prl (5'-TGAAGTGCCGGAGGATGA, 5'-ACGGGAGAGTGGACAGGTTGT), srebf1 (5'-GAAGCTAAGCTCAATAAGTCTGC, 5'-TCAGAGACTTGTTCTTCTGGATG), stat5.1 (5’-CATTACGCTTCACAGCTAAAGAGC, 5’-GTTTATCTGGTGGTATTTCTGTGAC) and tcra (5'-ACTGAAGTGAAGCCGAAT, 5'-CGTTAGCTCATCCACGCT) and Sensifast SYBR Lo-Rox qRT2-PCR kit (Meridian Biosciences) using an AriaMx Real-time PCR System. Data were normalised to actb and fold-change was calculated using the ΔΔCt method [29].
Whole-mount in situ hybridisation (WISH)
Anaesthetised embryos were fixed in 4% (w/v) paraformaldehyde at 4°C before WISH with anti-sense digoxigenin-labelled gene-specific probes, as described [30]. These were imaged on a MVX10 monozoom microscope using a 1 × MVXPlan Apochromat lens (NA = 0.25) with a DP74 camera with the area of staining captured using CellSens Dimension 1.6 software (Olympus) and quantified using ImageJ software.
Histological analysis
Adult peripheral blood smears and kidney marrow cytospin preparations were stained with Giemsa (Sigma). These were viewed on a Leica DM E microscope with a 100 × oil objective (NA = 1.25) to perform manual white blood cell differential counts and imaged using a SC50 camera using CellSens Dimension 1.6 software (Olympus).
Statistical analysis
Statistical analysis was performed with GraphPad Prism (Version 8) software using the unpaired independent student’s t-test, with Welch’s correction where appropriate or two-way ANOVA when considering both genotype and sex. The level of statistical significance was denoted as follows: ns: not significant; significant difference between genotypes: *: p < 0.05, **: p < 0.01, ***: p < 0.001; significant difference between sexes: #: p < 0.05, ##: p < 0.01, ###: p < 0.001.
RNAseq analysis
Total RNA was extracted from 5 dpf embryos using an RNeasy Plus Mini kit (Qiagen) following the manufacturer’s instructions. RNA quality was confirmed using an Agilent 2100 bioanalyzer, and an NGS library prepared using a TruSeq RNA library Prep Kit v2 (Illumina). Sequencing RNAseq was performed using a HiSeq 2500 system (Illumina) with 100 bp single end reads, which were trimmed to remove Illumina adaptor sequences and low quality ends using Trimmomatic v0.36 [31]. Trimmed reads were mapped to the coding sequences (cds) of the Danio rerio reference genome (Build 11) using BBmap v38.3. Mapped read counts per sample were tabulated using a custom Python script and statistically analysed for differential expression using EdgeR [32] and ALDEX2 [33]. The PANTHER classification system version 17 (http://www.pantherdb.org/) was used to determine the pathways in which gene expression was altered [34].
Results
Generation of zebrafish LOF Stat5.1 mutant
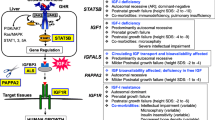
Like other STAT proteins, STAT5B consists of multiple domains, specifically the N-terminal, coiled-coil, DNA binding, linker, SH2 and transactivation domains [1] (Fig. 1A). A variety of autosomal-recessive loss-of-function (LOF) STAT5B mutations have been described [13], including R152X that truncates after the N-terminal domain in a region well-conserved between human STAT5B and zebrafish Stat5.1 (Fig. 1B), which was chosen for targeting by CRISPR/Cas9-mediated genome editing [35].
Generation of a zebrafish Stat5.1 mutation mimicking those in human STAT5B associated with GHISID1. A Schematic diagram of the STAT5B protein domain architecture consisting of N-terminal (yellow), coiled-coil (blue), DNA-binding (green), linker (orange), SH2 (pink), and transactivation (TA) (dark green) domains. B Alignment of human STAT5B with zebrafish stat5.1 sequences around the site of the R152X mutation (green) seen in human GHISID1, showing identical (*), highly similar (:) and similar (.) amino acids. C Nucleotide sequence of zebrafish stat5.1 genomic DNA targeted using CRISPR/Cas9 with the gRNA (orange) shown and the coding phase indicated. D HRM analysis of a sample of embryos injected with stat5.1 gRNA and Cas9, with wild-type embryos in green and other colours representing potential mutants. E–F Genotyping by PCR and gel electrophoresis of representative F1 wild-type (WT) and heterozygous (HET) mutant carriers (E) and F3 WT, HET and homozygous (HOM) fish (F), with the wild-type allele indicated with the green arrow and the mutant allele with red. G Sequence of homozygous wild-type (WT, wt/wt) and loss-of-function (LOF, mdu022/mdu022) mutant stat5.1 showing the chromatogram along with corresponding nucleotides, including a 47 bp insertion (pink text) in the LOF mutant. The coding phases and protein translations are shown, including the alternate phase and resultant five de novo amino acid residues and stop codon (orange text) following T155 in the LOF mutant. H Expression analysis of stat5.1 using qRT.2-PCR on the indicated tissues from WT and LOF adult female (F) zebrafish. Data was normalised to actb and represented as relative fold-change compared to WT, with mean ± SD shown and statistical significance indicated (n = 6)
Embryos were injected with gRNA targeting the corresponding region of the stat5.1 gene (Fig. 1C) as well as Cas9 mRNA. High resolution melt (HRM) analysis identified multiple mutagenic events in a sample of injected embryos (Fig. 1D) and so the remainder were raised to adulthood and out-crossed with wild-type (WT) fish and progeny carrying a potential stat5.1 mutation identified by genotyping (Fig. 1E). These carriers were again out-crossed with WT fish, and heterozygous progeny in-crossed with homozygous mutants identified (Fig. 1F) that were characterised by sequence analysis. This identified the mutant allele mdu022, which represents a 47 bp insertion that results in the addition of five de novo amino acid residues after T155 followed by a stop codon (Fig. 1G), resulting in a large truncation of Stat5.1 similar to that observed with the human STAT5B R152X LOF mutation [16]. Expression analysis confirmed a large reduction in stat5.1 expression in key tissues, including the liver and kidney, the latter being the major site of adult zebrafish haematopoiesis [36], but not muscle (Fig. 1H).
LOF Stat5.1 mutation impacts growth and adiposity
Both female and male adult stat5.1mdu022/mdu022 LOF mutant zebrafish were clearly smaller than their respective WT counterparts (Fig. 2A), with significantly reduced standard body length in both sexes (Fig. 2B) and wet weight in females (Fig. 2C). In contrast, female but not male LOF fish displayed increased adiposity compared to respective WT fish as judged visually (Fig. 2A) and by analysis of lipid content (Fig. 2D). Female LOF fish also showed significantly increased lipid content compared to male LOF fish, a difference not evident between female and male WT fish (Fig. 2D), whereas the increased length of female compared to male WT fish was not evident in LOF fish (Fig. 2B).
Effect of LOF Stat5.1 mutation on growth and adiposity. A–D Assessment of adult size and body composition in female (F) and male (M) homozygous stat5.1wt/wt wild-type (WT) and stat5.1mdu022/mdu022 loss-of-function (LOF) mutants at 5 months post-fertilisation (mpf), presenting representative images (A) along with quantification of standard length (B), wet weight (C) and lipid content (D), showing values for individual fish as well as mean ± standard deviation (SD) with statistical significance indicated (n = 6). E–H Growth trajectory during early life, presenting light microscopic images of representative WT and LOF individuals at the 1 cell stage (E) and at the indicated times post-fertilisation (F), along with quantification of length showing mean ± SD and statistical significance (n = 10–33) (G) and relative growth rate in the indicated age ranges calculated from these data with p-values shown (H). Scale bar = 1 mm. I–J Lipid analysis in juveniles, presenting fluorescent images of representative 14 dpf WT and LOF stained with Nile Red (I) and quantification of the area of Nile Red staining showing individual values as well as mean ± SD, with statistical significance indicated (n = 24) (J). Scale bar = 1 mm. K Expression analysis of growth and metabolic genes in 28 dpf juveniles (K) using qRT2-PCR on WT and LOF samples. Data for the indicated genes were normalised to actb and represented as relative fold-change compared to WT, with mean ± SD shown and statistical significance indicated (n = 6)
To further investigate these differences, individuals were closely monitored throughout the life-course. Eggs from LOF parents were found to be smaller in size than those from WT parents (Fig. 2E), which reached statistical significance (WT: 0.67 ± 0.029 mm, LOF: 0.64 ± 0.027 mm, p = 0.028). The reduction in size continued from 3 to 28 days post-fertilisation (dpf) (Fig. 2F–G), with close analysis revealing a significantly decreased growth rate in LOF individuals between 5–7, 7–10 and 14–28 dpf (Fig. 2H). Relative adiposity was also assessed by visualisation of lipid droplets using Nile Red, which identified a significant increase in lipid accumulation in 14 dpf LOF larvae (Fig. 2I–J).
To consider potential molecular pathways involved in the observed growth deficiency and increased adiposity, 28 dpf juveniles were analysed for the expression of genes specific for growth and lipid metabolism (Fig. 2K). A significant increase in expression of growth hormone (gh1) [37] was observed, while that of prolactin (prl) [38] was unaltered in LOF compared to WT fish. Amongst the four zebrafish igf genes [39], there was a significantly decreased expression of igf2a but not the other igf genes. Moreover, expression of the lipid-metabolism related genes, fasn, encoding fatty acid synthase [40], and srebf1, involved in hepatic lipogenesis [41], was significantly increased in LOF fish.
LOF Stat5.1 mutation impacts lymphopoiesis
Early T cell lymphopoiesis was assessed with the lymphocyte precursor marker, ikzf1 [42], and the mature T cell markers, rag1 [43] and tcra [44], using WISH on 5 dpf embryos. A reduced area of expression was observed for each of these markers in LOF compared to WT embryos (Fig. 3A–F).
Effect of LOF Stat5.1 mutation on lymphoid cells. A–F Assessment of early lymphopoiesis in 5 dpf homozygous stat5.1wt/wt wild-type (WT) and stat5.1mdu022/mdu022 loss-of-function (LOF) mutant embryos using WISH analysis, presenting representative individuals for ikzf1 (A), rag1 (C) and tcra (E), along with quantification of the relative area of expression for ikzf1 (B), rag1 (D) and tcra (F), showing results for individual embryos along with mean ± SD and statistical significance (n = 25–31). Scale bar = 200 μm. G–J. Analysis of blood and immune cells in adults, presenting images of representative Giemsa-stained blood (G) and kidney marrow (I) smears from 5 month post-fertilisation WT and LOF female fish and quantification of indicated cell populations in blood (H) and kidney marrow (J) showing mean and SD with statistical significance indicated (n = 6). Scale bar = 20 μm. K–L Expression analysis of immune genes in adults using qRT2-PCR on kidney marrow (K) and spleen (L) samples from 5 month post-fertilisation WT and LOF female fish. Data for the indicated genes were normalised to actb and represented as relative fold-change compared to WT fish, with mean ± SD shown and statistical significance indicated (n = 6)
Adult lymphopoiesis was examined by analysis of peripheral blood, kidney marrow, which plays a similar role as a mammalian bone marrow [36], and spleen. No significant differences in peripheral immune cell populations were observed in female (Fig. 3G–H) or male (Supp. Figure 1A–B) fish. However, a decreased proportion of lymphocytes was observed in female kidney marrow (Fig. 3I–J), although not in male kidney marrow (Supp. Figure 1C–D). Further analysis of female kidney marrow showed significantly decreased expression of T cell markers (cd8, tcra) but no change in markers for NK (nklb, nkld) and B cells (ighm) [45]. In contrast two T cell genes that mark both activated and Treg cells, ikzf4 [46] and ctla4 [47], were significantly increased (Fig. 3K). Similar results were observed in male kidney (Supp. Figure 1E). In the spleen, expression of cd8, tcra and nklb were significantly downregulated in both female and male LOF fish with nkld decreased just in female LOF compared to WT fish. Expression of ighm was increased in the spleens of both female and male LOF fish while expression of ctla4 was significantly increased just in male LOF fish (Fig. 3L and Supp. Figure 1F).
Discussion
STAT5B is an important transcription factor that plays a crucial role in cytokine signalling mediating somatic growth [3] and immune homeostasis [6]. STAT5B LOF mutations underpin an autosomal-recessive disorder characterised by severe growth failure and significant perturbation of lymphoid lineages, termed GHISID1 [7]. CRISPR/Cas9-based genome editing was used to successfully generate a zebrafish Stat5.1 mutant similar to known human STAT5B LOF mutations [16, 48]. Homozygous Stat5.1 mutant zebrafish showed defective growth and immune dysregulation, characterised by reduced size of both males and females, with the latter also displaying increased adiposity, along with reduced numbers of T lymphocytes throughout the life-course and alterations in other lymphocyte subsets in both sexes. No difference in fertilisation, survival or behavioural responses were noted (Supp. Figure 2 and data not shown). Moreover, heterozygous Stat5.1 mutant fish displayed no difference in lymphocyte numbers (Supp. Figure 3A-B) or growth (Supp. Figure 3C), confirming the autosomal-recessive nature of this mutation. Analysis of an alternative Stat5.1 C-terminal deletion mutant, in which the entire transactivation domain and tyrosine motif were absent (Supp. Figure 4A–D), confirmed the reduction in size in adults of both sexes and enhanced adiposity in females (Supp. Figure 4E–H) as well the reduction in embryonic T cells (Supp. Figure 4I–L). Morpholino-mediated knockdown further confirmed the reduced size and decreased T cells during embryogenesis (data not shown). These data collectively confirmed the creation of a zebrafish Stat5.1 LOF model that successfully mimicked human GHISID1.
The zebrafish Stat5.1 mutants showed a reduced growth trajectory during embryogenesis that continued into adulthood with both male and female mutant fish smaller than their wild-type counterparts despite increased gh1 expression. This is consistent with patients harbouring LOF STAT5B mutations, with both males and females experiencing postnatal growth failure despite unaffected or elevated GH levels [16] and GH signalling shown to be directly impacted [14]. Stat5b knockout mice also showed reduced postnatal growth with elevated plasma GH, although this was limited to males where sexual dimorphic growth is strongly mediated by GH [4, 49]. Currently the number of patients with LOF STAT5B mutations is low, but as more are identified it will be of interest to uncover whether any phenotypic dimorphism exists between sexes. Either way these data confirm a conserved key role of STAT5B/Stat5.1 downstream of GHR, consistent with STAT5 binding sites being present on the duplicated zebrafish GHR proteins (Supp. Figure 5A). In humans the growth deficits that result from STAT5B LOF mutations are equivalent to those caused by GHR LOF mutations [7]. In contrast, a more severe growth defect was seen with a GH-deficient zebrafish than observed with the Stat5.1 LOF mutant [50]. This is similar to mice, where both Gh─/─ [51] and Ghr ─ / ─ [52] mutants were smaller than Stat5b─/─ mutants [49], although disruption of both Stat5a and Stat5b genes imparted a more pronounced effect [49]. This suggests that Stat5a can partially compensate for Stat5b in mice, with Stat5.2 potentially able to do the same in zebrafish. STAT5B is expressed more highly than STAT5A [53], explaining why such compensation may not occur in humans.
In STAT5B LOF patients [13] and male Stat5b knockout mice [4, 49] the observed growth defects correlated with reduced IGF-I levels. In contrast, the zebrafish Stat5.1 LOF mutants showed a significant reduction in igf2a, but not igf1, igf2b or igf3. In mammals, IGF-2 is particularly involved in the regulation of foetal growth, being regulated by a complex imprinting mechanism [54]. In fish, however, this mechanism does not exist, conceivably allowing it to play a broader role. In fact it is the sole igf gene expressed in the embryonic zebrafish liver [39], while muscle igf2a has been shown to mediate the hypertrophy associated with GH transgenesis in zebrafish [55], tissues where stat5.1 is strongly expressed (data not shown). In contrast, igf1 appears to have a diminished role, with igf1-deficient zebrafish exhibiting only a mild size reduction [56]. RNAseq analysis of zebrafish Stat5.1 LOF mutant embryos (Table 1) identified down-regulation of two relevant pathways, ‘Insulin/IGF-MAPKK/MAPK’ and ‘Insulin/IGF-PKB’. Collectively, this suggests a core GH/GHR/STAT5/IGF pathway utilised in growth regulation across higher vertebrates, with species-specific diversification at the level of the individual proteins involved.
Eggs from Stat5.1 LOF mutants were smaller than those from WT fish. As expected, this correlated with having a Stat5.1 LOF mother regardless of the genotype of the father (Supp. Figure 6). This is consistent with previous studies that showed fish egg size was positively related to the size of the mother [57]. To date, no data are available on the birth weight of progeny from homozygous STAT5B LOF women. However, the mean birth weight of Ghr ─/─ piglets was 40% lower when derived from Ghr ─/─ rather than Ghr +/─ mothers, attributed to reduced foetal growth capacity within the smaller Ghr ─/─ mothers [58]. Therefore, impacts on foetal/egg size constitute an important alternate mechanism by which mutations in the GH/GHR/STAT5/IGF pathway can impact organism size.
Zebrafish Stat5.1 LOF mutants demonstrated greater adiposity. This was evident as increased lipid deposits in juveniles and elevated relative lipid content in adults, although the latter was restricted to females. The heightened adiposity correlated with a significant increase in expression of genes involved in lipid metabolism, including srebf1, and was consistent with a similarly enhanced adiposity observed in GH-deficient zebrafish [50]. Several patients harbouring STAT5B LOF mutations also displayed central obesity [15, 48], while those with acquired GH deficiency possess increased visceral and abdominal fat mass in particular [59, 60]. Stat5b knockout mice [4], as well as mice expressing a truncated GHR unable to activate STAT5 [61], exhibited a similar increase in adiposity, with Stat5b-deficient mice also shown to have elevated Srebf1 expression [62]. In combination, these observations indicate a conserved role for STAT5B/Stat5.1 downstream of GHR in regulating key genes involved in lipid metabolism, with enhanced adiposity resulting from disruption of this pathway.
Zebrafish Stat5.1 LOF mutants showed decreased numbers of lymphocyte precursors and T lymphocytes during embryogenesis and reduced T lymphocytes in adulthood, with splenic NK cells also reduced. Patients carrying STAT5B LOF mutations, including those harbouring a R152X mutation, exhibited lymphoid defects [16, 63], with reduced numbers of T cells (CD4+, CD8+), NK cells and Treg cells [48, 63,64,65], with signalling by IL-2 cytokines shown to be affected [66]. Stat5b knockout mice also possessed a subtle reduction in the number and proliferation of T lymphocytes [67] as well as reduced NK cell number and function [68, 69]. In each case the impact on lymphoid cells was similar but less severe than observed with mutation of the common IL-2R gamma chain, which mediates signalling for the IL-2 cytokine family in humans [70], mice [71] and zebrafish [72]. This might be due to redundancy with STAT5A/Stat5.2, since Stat5a─/─/Stat5b─/─ double mutant mice exhibited a more severe immune phenotype [49], but also likely the result of alternate STAT proteins and indeed other pathways activated in parallel [73]. We have previously demonstrated Stat5.1 acting downstream of zebrafish II-2rγc [74] and Jak3 [74, 75], with conserved STAT5 docking sites on the II-2rβ receptor chain that utilises II-2rγc (Supp. Figure 5B). Moreover, RNAseq analysis confirmed down-regulation of the ‘Interleukin signalling’ as well as the ‘Inflammation mediated by chemokine and cytokine signalling’ pathways (Table 1), while stat5.1 was expressed in the major zebrafish haematopoietic organ, the kidney. Together this provides further evidence of a conserved IL-2Rγc/JAK3/STAT5 pathway in lymphopoiesis. Zebrafish Stat5.1 LOF mutants additionally showed increased expression of the gene encoding IgM as well as markers of activated T cells [46, 47]. This is in line with some STAT5B LOF human patients that show high levels of IgG and activated T cells along with autoimmune disease [63, 64]. Further analysis of this aspects of the clinical phenotype appears worthwhile.
An alternate zebrafish Stat5.1 LOF mutant has been described that also showed a similar reduction in growth to that reported here, although this was associated with reduced GH expression and loss of female-biased size dimorphism but not increased female adiposity [76]. It remains unclear why such different phenotypes were observed. Further analysis of this mutant revealed loss of sexually dimorphic gene expression, with multiple pathways related to metabolism affected [77]. The immune system was not investigated in this alternate mutant. However, a strong interplay between metabolism and immunity has been identified [78] providing a potential additional mechanism to explain the impacts of STAT5B/Stat5.1 mutations. A range of other clinically important mutations impacting cytokine receptor signalling via the JAK-STAT pathway have been identified [79]. Our success of modelling STAT5B-mediated GHISID1 in this study, as well as IL-2Rγc-mediated SCID [72] and gain-of-function (GOF) mutations of JAK2 [80], JAK3 [75] and STAT5B [81] associated with haematological malignancy, along with the work of others investigating the impact of LOF STAT3 mutations [82], highlight the strong potential for using zebrafish to explore the role of JAK-STAT perturbation in disease. We hope to extend this research to autosomal-dominant STAT5B mutations associated with GHISID2 [17], as well as GOF STAT3 mutations implicated in immune dysregulation [83], and to utilise these platforms for therapeutic testing.
Availability of data and materials
All data generated or analysed during this study are included in this article (and its Supplementary Material files).
Abbreviations
- dpf:
-
Days post-fertilisation
- GH:
-
Growth hormone
- GHISID:
-
Growth hormone insensitivity syndrome with immune dysregulation
- GOF:
-
Gain-of-function
- gRNA:
-
Guide ribonucleic acid
- HRM:
-
High resolution melt
- IGF:
-
Insulin-like growth factor
- IL:
-
Interleukin
- IL-2Rγc:
-
IL-2 receptor gamma common
- LOF:
-
Loss-of-function
- mpf:
-
Months post-fertilisation
- PCR:
-
Polymerase chain reaction
- PRL:
-
Prolactin
- qRT2-PCR:
-
Quantitative real-time reverse-transcription-PCR
- R:
-
Receptor
- SD:
-
Standard deviation
- STAT:
-
Signal transducer and activator of transcription
- WISH:
-
Whole-mount in situ hybridisation
- WT:
-
Wild-type
References
Awasthi N, Liongue C, Ward AC (2021) STAT proteins: a kaleidoscope of canonical and non-canonical functions in immunity and cancer. J Hematol Oncol 14(1):1–17
Buitenhuis M, Coffer PJ, Koenderman L (2004) Signal transducer and activator of transcription 5 (STAT5). Int J Biochem Cell Biol 36(11):2120–2124
Baik M, Yu JH, Hennighausen L (2011) Growth hormone-STAT5 regulation of growth, hepatocellular carcinoma, and liver metabolism. Annals NY Acad Sci 1229:29–37
Udy GB, Towers RP, Snell RG, Wilkins RJ, Park SH, Ram PA et al (1997) Requirement of STAT5b for sexual dimorphism of body growth rates and liver gene expression. Proc Natl Acad Sci USA 94(14):7239–7244
Liu X, Robinson GW, Wagner K-U, Garrett L, Wynshaw-Boris A, Hennighausen L (1997) Stat5a is mandatory for adult mammary gland development and lactogenesis. Genes Dev 11:179–186
Maurer B, Kollmann S, Pickem J, Hoelbl-Kovacic A, Sexl V (2019) STAT5A and STAT5B-twins with different personalities in hematopoiesis and leukemia. Cancers 11(11):1726
Hwa V (2021) Human growth disorders associated with impaired GH action: defects in STAT5B and JAK2. Mol Cell Endocrinol 519:111063
David A, Hwa V, Metherell LA, Netchine I, Camacho-Hübner C, Clark AJ et al (2011) Evidence for a continuum of genetic, phenotypic, and biochemical abnormalities in children with growth hormone insensitivity. Endocrine Rev 32(4):472–497
Lin S, Li C, Li C, Zhang X (2018) Growth hormone receptor mutations related to individual dwarfism. Int J Mol Sci 19(5):1433
Woods KA, Camacho-Hübner C, Savage MO, Clark AJ (1996) Intrauterine growth retardation and postnatal growth failure associated with deletion of the insulin-like growth factor I gene. New Engl J Med 335(18):1363–1367
Domené HM, Bengolea SV, Martínez AS, Ropelato MG, Pennisi P, Scaglia P et al (2004) Deficiency of the circulating insulin-like growth factor system associated with inactivation of the acid-labile subunit gene. New Engl J Med 350(6):570–577
Dauber A, Muñoz-Calvo MT, Barrios V, Domené HM, Kloverpris S, Serra-Juhé C et al (2016) Mutations in pregnancy-associated plasma protein A2 cause short stature due to low IGF-I availability. EMBO Mol Med 8(4):363–374
Hwa V (2016) STAT5B deficiency: impacts on human growth and immunity. Growth Hormone IGF Res 28:16–20
Kofoed EM, Hwa V, Little B, Woods KA, Buckway CK, Tsubaki J et al (2003) Growth hormone insensitivity associated with a STAT5b mutation. N Engl J Med 349:1139–1147
Vidarsdottir S, Walenkamp MJ, Pereira AM, Karperien M, van Doorn J, van Duyvenvoorde HA et al (2006) Clinical and biochemical characteristics of a male patient with a novel homozygous STAT5b mutation. J Clin Endocrinol Metab 91:3482–3485
Bernasconi A, Marino R, Ribas A, Rossi J, Ciaccio M, Oleastro M et al (2006) Characterization of immunodeficiency in a patient with growth hormone insensitivity secondary to a novel STAT5b gene mutation. Pediatrics 118(5):1584–1592
Klammt J, Neumann D, Gevers EF, Andrew SF, Schwartz ID, Rockstroh D et al (2018) Dominant-negative STAT5B mutations cause growth hormone insensitivity with short stature and mild immune dysregulation. Nat Commun 9(1):1–10
Rasighaemi P, Basheer F, Liongue C, Ward AC (2015) Zebrafish as a model for leukemia and other hematopoietic disorders. J Hematol Oncol 8:29
Zang L, Maddison LA, Chen W (2018) Zebrafish as a model for obesity and diabetes. Front Cell Dev Biol 6:91
Liongue C, Ward AC (2007) Evolution of class I cytokine receptors. BMC Evol Biol 7:120
Liongue C, Ward AC (2013) Evolution of the Jak-Stat pathway. JAK-STAT 2:e22756
Lewis RS, Ward AC (2004) Conservation, duplication and divergence of the zebrafish stat5 genes. Gene 338(1):65–74
Lawrence C (2011) Advances in zebrafish husbandry and management. Meth Cell Biol 104:429–451
Hwang WY, Fu Y, Reyon D, Maeder ML, Tsai SQ, Sander JD et al (2013) Efficient genome editing in zebrafish using a CRISPR-Cas system. Nat Biotech 31(3):227–229
Krypuy M, Ahmed AA, Etemadmoghadam D, Hyland SJ, DeFazio A, Australian Ovarian Cancer Study G et al (2007) High resolution melting for mutation scanning of TP53 exons 5–8. BMC Cancer 7:168
Parichy DM, Elizondo MR, Mills MG, Gordon TN, Engeszer RE (2009) Normal table of postembryonic zebrafish development: staging by externally visible anatomy of the living fish. Dev Dyn 238(12):2975–3015
Tingaud-Sequeira A, Ouadah N, Babin PJ (2011) Zebrafish obesogenic test: a tool for screening molecules that target adiposity. J Lipid Res 52(9):1765–1772
Folch J, Lees M, Sloane Stanley GH (1957) A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226(1):497–509
Giulietti A, Overbergh L, Valckx D, Decallonne B, Bouillon R, Mathieu C (2001) An overview of real-time quantitative PCR: applications to quantify cytokine gene expression. Methods 25(4):386–401
Thisse C, Thisse B (2008) High-resolution in situ hybridization to whole-mount zebrafish embryos. Nat Protoc 3(1):59–69
Bolger AM, Lohse M, Usadel B (2014) Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30(15):2114–2120
Robinson MD, McCarthy DJ, Smyth GK (2010) edgeR: a bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26(1):139–140
Fernandes AD, Macklaim JM, Linn TG, Reid G, Gloor GB (2013) ANOVA-like differential expression (ALDEx) analysis for mixed population RNA-Seq. PLoS ONE 8(7):e67019
Mi H, Muruganujan A, Ebert D, Huang X, Thomas PD (2019) PANTHER version 14: more genomes, a new PANTHER GO-slim and improvements in enrichment analysis tools. Nucleic Acids Res 47(D1):D419–D426
Sertori R, Trengove M, Basheer F, Ward AC, Liongue C (2016) Genome editing in zebrafish: a practical overview. Brief Funct Genomics 15(4):322–330
Chen AT, Zon LI (2009) Zebrafish blood stem cells. J Cell Biochem 108(1):35–42
Herzog W, Zeng X, Lele Z, Sonntag C, Ting J-W, Chang C-Y et al (2003) Adenohypophysis formation in the zebrafish and its dependence on sonic hedgehog. Dev Biol 254(1):36–49
Liu NA, Liu Q, Wawrowsky K, Yang Z, Lin S, Melmed S (2006) Prolactin receptor signaling mediates the osmotic response of embryonic zebrafish lactotrophs. Mol Endocrinol 20(4):871–880
Li J, Wu P, Liu Y, Wang D, Cheng CH (2014) Temporal and spatial expression of the four Igf ligands and two Igf type 1 receptors in zebrafish during early embryonic development. Gene Expr Patterns 15(2):104–111
Yu Q, Huo J, Zhang Y, Liu K, Cai Y, Xiang T et al (2020) Tamoxifen-induced hepatotoxicity via lipid accumulation and inflammation in zebrafish. Chemosphere 239:124705
Shochat C, Wang Z, Mo C, Nelson S, Donaka R, Huang J et al (2021) Deletion of SREBF1, a functional bone-muscle pleiotropic gene, alters bone density and lipid signaling in zebrafish. Endocrinology 162(1):bqaa189
Willett CE, Kawasaki H, Amemiya CT, Lin S, Steiner LA (2001) Ikaros expression as a marker for lymphoid progenitors during zebrafish development. Dev Dyn 222(4):694–698
Willett CE, Cherry JJ, Steiner L (1997) Characterization and expression of the recombination activating genes (rag1 and rag2) of zebrafish. Immunogenetics 45(6):394–404
Danilova N, Hohman VS, Sacher F, Ota T, Willett CE, Steiner LA (2004) T cells and the thymus in developing zebrafish. Dev Comp Immunol 28(7–8):755–767
Moore FE, Garcia EG, Lobbardi R, Jain E, Tang Q, Moore JC et al (2016) Single-cell transcriptional analysis of normal, aberrant, and malignant hematopoiesis in zebrafish. J Exp Med 213(6):979–992
Rieder SA, Metidji A, Glass DD, Thornton AM, Ikeda T, Morgan BA et al (2015) Eos is redundant for regulatory T cell function but plays an important role in IL-2 and Th17 production by CD4+ conventional T cells. J Immunol 195(2):553–563
Kasheta M, Painter CA, Moore FE, Lobbardi R, Bryll A, Freiman E et al (2017) Identification and characterization of T reg–like cells in zebrafish. J Exp Med 214(12):3519–3530
Pugliese-Pires PN, Tonelli CA, Dora JM, Silva PC, Czepielewski M, Simoni G et al (2010) A novel STAT5B mutation causing GH insensitivity syndrome associated with hyperprolactinemia and immune dysfunction in two male siblings. Eur J Endocrinol 163(2):349
Teglund S, McKay C, Schuetz E, van Deursen JM, Stravopodis D, Wang D et al (1998) Stat5a and Stat5b proteins have essential and nonessential, or redundant, roles in cytokine responses. Cell 93:841–850
McMenamin SK, Minchin JE, Gordon TN, Rawls JF, Parichy DM (2013) Dwarfism and increased adiposity in the gh1 mutant zebrafish vizzini. Endocrinology 154(4):1476–1487
List EO, Basu R, Duran-Ortiz S, Krejsa J, Jensen EA (2021) Mouse models of growth hormone deficiency. Rev Endocrine Metab Disord 22(1):3–16
List EO, Sackmann-Sala L, Berryman DE, Funk K, Kelder B, Gosney ES et al (2011) Endocrine parameters and phenotypes of the growth hormone receptor gene disrupted (GHR-/-) mouse. Endocrinol Rev 32(3):356–386
Su AI, Cooke MP, Ching KA, Hakak Y, Walker JR, Wiltshire T et al (2002) Large-scale analysis of the human and mouse transcriptomes. Proc Natl Acad Sci USA 99(7):4465–4470
Livingstone C, Borai A (2014) Insulin-like growth factor-II: its role in metabolic and endocrine disease. Clin Endocrinol 80(6):773–781
Nornberg BF, Figueiredo MA, Marins LF (2016) Expression profile of IGF paralog genes in liver and muscle of a GH-transgenic zebrafish. Gen Compar Endocrinol 226:36–41
Zeng N, Bao J, Shu T, Shi C, Zhai G, Xia J et al (2022) Sexual dimorphic effects of igf1 deficiency on metabolism in zebrafish. Front Endocrinol 13:1645
Hagmayer A, Furness AI, Reznick DN, Pollux BJ (2018) Maternal size and body condition predict the amount of post-fertilization maternal provisioning in matrotrophic fish. Ecol Evol 8(24):12386–12396
Hinrichs A, Kessler B, Kurome M, Blutke A, Kemter E, Bernau M et al (2018) Growth hormone receptor-deficient pigs resemble the pathophysiology of human Laron syndrome and reveal altered activation of signaling cascades in the liver. Mol Metab 11:113–128
Lanes R, Soros A, Gunczler P, Paoli M, Carrillo E, Villaroel O et al (2006) Growth hormone deficiency, low levels of adiponectin, and unfavorable plasma lipid and lipoproteins. J Pediatrics 149(3):324–329
Misra M, Bredella MA, Tsai P, Mendes N, Miller KK, Klibanski A (2008) Lower growth hormone and higher cortisol are associated with greater visceral adiposity, intramyocellular lipids, and insulin resistance in overweight girls. Am J Physiol - Endocrinol Metab 295(2):E385–E392
Rowland JE, Lichanska AM, Kerr LM, White M, d’Aniello EM, Maher SL et al (2005) In vivo analysis of growth hormone receptor signaling domains and their associated transcripts. Mol Cell Biol 25(1):66–77
Mueller KM, Kornfeld JW, Friedbichler K, Blaas L, Egger G, Esterbauer H et al (2011) Impairment of hepatic growth hormone and glucocorticoid receptor signaling causes steatosis and hepatocellular carcinoma in mice. Hepatology 54(4):1398–1409
Nadeau K, Hwa V, Rosenfeld RG (2011) STAT5b deficiency: an unsuspected cause of growth failure, immunodeficiency, and severe pulmonary disease. J Pediatrics 158(5):701–708
Cohen AC, Nadeau KC, Tu W, Hwa V, Dionis K, Bezrodnik L et al (2006) Decreased accumulation and regulatory function of CD4+ CD25high T cells in human STAT5b deficiency. J Immunol 177(5):2770–2774
Scaglia PA, Martínez AS, Feigerlová E, Bezrodnik L, Gaillard MI, Di Giovanni D et al (2012) A novel missense mutation in the SH2 domain of the STAT5B gene results in a transcriptionally inactive STAT5b associated with severe IGF-I deficiency, immune dysfunction, and lack of pulmonary disease. J Clin Endocrinol 97(5):E830–E839
Jenks JA, Seki S, Kanai T, Huang J, Morgan AA, Scalco RC et al (2013) Differentiating the roles of STAT5B and STAT5A in human CD4+ T cells. Clin Immunol 148(2):227–236
Snow JW, Abraham N, Ma MC, Herndier BG, Pastuszak AW, Goldsmith MA (2003) Loss of tolerance and autoimmunity affecting multiple organs in STAT5A/5B-deficient mice. J Immunol 171(10):5042–5050
Moriggl R, Topham DJ, Teglund S, Sexl V, McKay C, Wang D et al (1999) Stat5 is required for IL-2-induced cell cycle progression of peripheral T cells. Immunity 10(2):249–259
Wiedemann GM, Grassmann S, Lau CM, Rapp M, Villarino AV, Friedrich C et al (2020) Divergent role for STAT5 in the adaptive responses of natural killer cells. Cell Rep 33(11):108498
Noguchi M, Yi H, Rosenblatt HM, Filipovich AH, Adelstein S, Modi WS et al (1993) Interleukin-2 receptor g chain mutation results in X-linked severe combined immunodeficiency in humans. Cell 73:147–157
Bosma GC, Custer RP, Bosma MJ (1983) A severe combined immunodeficiency mutation in the mouse. Nature 301(5900):527–530
Sertori R, Jones R, Basheer F, Rivera L, Dawson S, Loke S et al (2022) Generation and characterization of a zebrafish IL-2Rgc SCID model. Int J Mol Sci 23(4):2385
Yao Z, Cui Y, Watford WT, Bream JH, Yamaoka K, Hissong BD et al (2006) Stat5a/b are essential for normal lymphoid development and differentiation. Proc Natl Acad Sci USA 104:1000–1004
Sertori R, Liongue C, Basheer F, Lewis KL, Rasighaemi P, de Coninck D et al (2016) Conserved IL-2Rgc signaling mediates lymphopoiesis in zebrafish. J Immunol 196(1):135–143
Basheer F, Bulleeraz V, Ngo VQT, Liongue C, Ward AC (2022) In vivo impact of JAK3 A573V mutation revealed using zebrafish. Cell Mol Life Sci 79(6):322
Xiong S, Mei J, Huang P, Jing J, Li Z, Kang J et al (2017) Essential roles of stat5.1/stat5b in controlling fish somatic growth. J Genet Genom 44(12):577–585
Huang P, Xiong S, Kang J, Mei J, Gui JF (2018) Stat5b regulates sexually dimorphic gene expression in zebrafish liver. Front Physiol 9:676
Braun TP, Okhovat M, Coblentz C, Carratt SA, Foley A, Schonrock Z et al (2022) Author correction: myeloid lineage enhancers drive oncogene synergy in CEBPA/CSF3R mutant acute myeloid leukemia. Nat Commun 13(1):3471
Luo Y, Alexander M, Gadina M, O’Shea JJ, Meylan F, Schwartz DM (2021) JAK-STAT signaling in human disease: from genetic syndromes to clinical inhibition. J Allergy Clin Immunol 148(4):911–925
Ma AC, Fan A, Ward AC, Liongue C, Lewis RS, Cheng SH et al (2009) A novel zebrafish jak2a(V581F) model shared features of human JAK2(V617F) polycythemia vera. Exp Hematol 37(12):1379–1386
Lewis RS, Stephenson SEM, Ward AC (2006) Constitutive activation of zebrafish stat5 expands hematopoietic cell populations in vivo. Exp Hematol 34(2):179–187
Xiong ST, Wu JJ, Jing J, Huang PP, Li Z, Mei J et al (2017) Loss of stat3 function leads to spine malformation and immune disorder in zebrafish. Sci Bull 62(3):185–196
Vogel TP, Milner JD, Cooper MA (2015) The ying and yang of STAT3 in human disease. J Clin Immunol 35(7):615–623
Acknowledgements
The authors would like to thank the Deakin University Animal House staff for superb aquarium management and Rachel Mahony for technical assistance.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. The authors recognize the support of postgraduate research scholarships (SH, NA, RSL) and project costs (ACW) from Deakin University. The funder had no role in study design, data collection and interpretation or manuscript preparation.
Author information
Authors and Affiliations
Contributions
ACW: conceived the project based on preliminary work performed by RSL. CL and NA: generated the zebrafish Stat5.1 mutants, which was characterized by SH, NA, NP and TA. ACW: supervised RSL and with CL co-supervised SH, NA and NP. SH and ACW: drafted the paper. All authors contributed to data interpretation, and all approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
This study was approved by the Deakin University Animal Ethics Committee.
Consent to participate and publish
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Heidary, S., Awasthi, N., Page, N. et al. A zebrafish model of growth hormone insensitivity syndrome with immune dysregulation 1 (GHISID1). Cell. Mol. Life Sci. 80, 109 (2023). https://doi.org/10.1007/s00018-023-04759-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00018-023-04759-y