Abstract
Upon stress challenges, proteins/RNAs undergo liquid–liquid phase separation (LLPS) to fine-tune cell physiology and metabolism to help cells adapt to adverse environments. The formation of LLPS has been recently linked with intracellular pH, and maintaining proper intracellular pH homeostasis is known to be essential for the survival of organisms. However, organisms are constantly exposed to diverse stresses, which are accompanied by alterations in the intracellular pH. Aging processes and human diseases are also intimately linked with intracellular pH alterations. In this review, we summarize stress-, aging-, and cancer-associated pH changes together with the mechanisms by which cells regulate cytosolic pH homeostasis. How critical cell components undergo LLPS in response to pH alterations is also discussed, along with the functional roles of intracellular pH fluctuation in the regulation of LLPS. Further studies investigating the interplay of pH with other stressors in LLPS regulation and identifying protein responses to different pH levels will provide an in-depth understanding of the mechanisms underlying pH-driven LLPS in cell adaptation. Moreover, deciphering aging and disease-associated pH changes that influence LLPS condensate formation could lead to a deeper understanding of the functional roles of biomolecular condensates in aging and aging-related diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Liquid–liquid phase separation (LLPS) refers to a demixing transition of an initially homogeneous solution that rearranges and separates into two phases that can coexist stably in solution: a dense and a dilute phase. During this physiochemical process, supersaturated macromolecules are separated from the solution to form a dense phase, while these supersaturated components in the dilute phase are depleted. The macromolecular dense phase has liquid-like properties and can exchange molecules rapidly with the dilute phase [1]. Studies in recent years have shown that LLPS is the driving force for the assembly of nonmembrane organelles and other functional biomolecular condensates, which can achieve spatiotemporal control of their internal complex biochemical reactions without physical barriers [2, 3].
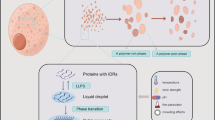
Currently, the formation mechanisms of the resulting condensates formed by LLPS are preliminarily understood and are thought to rely on a network of weak and multivalent protein–protein interactions. Many proteins exhibit LLPS behaviors, and a common feature of such proteins is the presence of multivalent binding domains. Among these, intrinsically disordered regions (IDRs) are the main drivers that provide multivalent interactions [4]. Studies have shown that IDRs are rich in hydrophilic amino acids such as asparagine, glycine, proline, serine, arginine, and aspartate, whereas they are lacking in hydrophobic amino acids such as valine, threonine, leucine, cysteine, isoleucine, histidine, and tryptophan [4, 5]. The enrichment of only a few amino acids in these domains results in low complexity that could mediate weak interactions. Other proteins that contain oligomerization domains and multiple-folded modular domains are also contribute to the multivalent interactions [5]. Furthermore, emerging roles of RNA molecules in the assembly of biomolecular condensates have been revealed. RNAs help establish the promiscuous interaction network through interactions with the RNA-binding domains of proteins, and their intermolecular interactions and self-assembly define the compositions of higher-order condensates [6, 7]. Accumulating evidence shows that protein post-translational modification (PTM) is another important mechanism that achieves cellular control of protein phase separation and condensate formation through processes such as phosphorylation, acetylation, SUMOylation, ubiquitination, methylation, and ADP-ribosylation [8, 9]. These modifications can alter the weak multivalent interactions by changing the charge, structure, hydrophobicity, and other properties of proteins, thus affecting phase separation behavior [8, 9]. In addition, not only protein PTM but also RNA PTM affects condensate dynamics. For instance, N6-methyladenosine (m6A) of RNA can modulate condensate formation and composition by regulating mRNA distribution into distinct condensates or changing the phase separation behaviors of its binding partners [10, 11].
There is mounting evidence that LLPS and condensate formation are widely present in the cells and play important roles in a wide range of physiological processes (Fig. 1), including chromatin organization, cytoskeletal assembly, signal transduction, transcriptional regulation, protein degradation, cell division and differentiation, and environmental response and adaptation [2]. The condensates can transition into different material states such as gel- or solid-like states [1, 12]. Therefore, maintenance of normal condensate material properties can ensure the assembly and disassembly of condensates in a tightly controlled manner to fulfill original functions, while aberrant phase transition is causatively associated with the onset and development of age-related neurodegenerative diseases and cancers (Fig. 1) [13]. In recent research, the development of methods to study LLPS has become an important objective. A series of tools have been developed to predict and analyze the phase separation capabilities of proteins [1]. Fluorescence microscopy observation techniques, including fluorescence recovery after photobleaching (FRAP) and superresolution imaging, can also provide more detailed information on the material properties, composition, and dynamics of biomolecular condensates. In addition, in vitro reconstitution using purified proteins is an accessory method used for studying LLPS [1]. These methods can help researchers to further elucidate the compositions of biological molecules and related biological reactions and explore the factors that drive or influence condensate formation, eventually providing new opportunities for the prevention and treatment of human diseases.
Liquid–liquid phase separation in mammalian cells and its involvement in aging-related neurodegenerative diseases and cancers. Under physiological conditions, scaffold biomacromolecules undergoing liquid–liquid phase separation (LLPS) can interact with and recruit other client molecules to form reversible liquid-like condensates, which participate in a wide range of physiological processes. During aging, multiple factors, including protein mutation and repeated expansions, cellular environmental and metabolic changes, damage to protein quality-control systems, and abnormal protein localization and post-translational modification, can affect the LLPS process and promote aberrant gel-like condensate or pathological protein aggregate formation, ultimately leading to the onset and progression of neurodegenerative diseases. Tumorigenesis is also related to LLPS. a Mutations in the substrate recognition domain of the tumor suppressor SPOP prevent its binding to oncogenic substrates and subsequent condensate formation with ubiquitin ligase complex, causing a failure of oncogenic substrate ubiquitination and proteasomal degradation. b Mutation of p53 can accelerate its solid-phase transition into amyloid aggregates, which is found in more than 50% of human cancers. c Chromosomal translocations lead to aberrant condensate formation of transcriptional regulators (TRs) at enhancers and promoters of oncogenes, driving abnormal oncogenic transcriptional programs. d Mutation or overexpression of signaling receptors alter the formation of signaling clusters and activates aberrant signaling cascades, contributing to cancer development
Phase separation of proteins is a multifactor dynamic process, and it occurs not only spontaneously under normal conditions, but also upon stimulation from an array of environmental factors, including changes in temperature, pH, ATP/energy, macromolecule concentration, and ionic strength [14]. These physiological parameters constitute a continuous phase boundary, and crossing this boundary by changing one or more parameters, such as by raising the temperature, depriving nutrients, lowering the pH, or changing other factors, can cause phase separation and the formation of condensates, which is an adaptive tuned response of cells [15]. Homeostasis of pH is a prerequisite for the normal survival of organisms. Many proteins are very sensitive to pH alterations, and a very small change in pH can induce phase transition of proteins. Phase separation in most proteins is triggered at low pH; while in others, it is induced by alkaline pH [16]. In vivo, the mechanism by which pH regulates protein phase separation is not completely clear. Here, we review the literature on stress-associated pH fluctuation in cells, how cells maintain and regulate cytosolic pH, and the effects of pH changes on protein phase separation. The mechanisms by which pH can mediate phase separation are also discussed. Further research on these topics will not only advance our understanding of compartment formation affected by pH changes but will also provide important insight into the relationship between pH and a diverse set of human diseases.
Diverse stresses induce intracellular pH fluctuation
Cytosolic pH is a tightly controlled physiological parameter in all cellular systems, as almost all cellular processes depend on a constant pH for normal functions [17,18,19,20,21]. For instance, in yeast, pH is involved in replicative senescence of mother cells and rejuvenation of nascent daughter cells [22], and cytoplasmic acidification is critical for yeast cells to enter dormancy under stress conditions [23]. In plants, intracellular pH changes are components of a number of phytohormone signaling pathways, modulating gene expression and defence [21, 24]. In mammals, the maintenance of pH homeostasis is of key importance for the proper execution and regulation of neurotransmission [25]. Small changes in cytosolic pH can lead to major changes in metabolism, signal transduction, protein folding, and protein–lipid interactions [19, 20]. However, organisms are often exposed to diverse adverse conditions throughout their life cycles, and stress-induced cytosolic pH fluctuations are broadly present in the cells; these fluctuations are induced, for example, by osmotic stress, heat shock, and nutrient restriction [26,27,28,29,30]. Aging processes and human diseases, including neurodegenerative diseases and cancers, are also strongly linked with intracellular pH alterations [22, 31, 32]. Table 1 summarizes the stresses that can induce pH fluctuation in the cells of mammals, plants, and microorganisms. Here, we describe the relationships between pH changes and certain stresses, such as temperature perturbation, starvation, and osmotic challenges, as well as aging and aging-related diseases, including neurodegenerative diseases and cancers.
Temperature perturbation
Proper environmental temperature is a critical factor for cell survival. When the temperature becomes harsh, organisms must respond rapidly to adapt and thrive. The best-known stress response, the heat shock response (HSR), is a conserved transcriptional program mediated by heat shock factor 1, which is activated upon heat stress. It upregulates the transcription of a set of molecular chaperones to help the cell to manage the accumulation of heat-induced aberrantly folded proteins and aggregates [70]. On the one hand, upregulated chaperones can efficiently refold nonnative proteins or promote the degradation of protein aggregates through autophagy or the ubiquitin–proteasome system (UPS). On the other hand, they can regulate the deposition of certain misfolded proteins into specialized cellular locations to shield them from degradation and to refold them after stress [71]. In addition to HSR, another adaptive mechanism called the unfolded protein response induced by endoplasmic reticulum stress is also activated upon heat exposure and helps to mitigate the damage caused by heat [72, 73]. Moreover, in different research models, ubiquitination-dependent [74,75,76,77] and autophagy-dependent degradation [78,79,80,81,82] have been observed to be induced after heat shock, and the activation of these degradation systems is essential for cell survival and recovery from thermally induced protein aggregation [74, 82].
In addition to the activation of evolutionarily conserved systems that contribute to thermotolerance, temperature change is often coupled with fluctuations in cytoplasmic pH [28, 83]. Some studies have shown that heat shock acidifies the cytoplasm. For instance, in yeast, an intracellular pH drop can be induced by heat shock [35]. The same heat-associated pH changes have also been observed in Drosophila melanogaster [28] and rat hepatoma cells [41]. Stress-associated acidification is thought to be toxic to cells in some cases [29, 84]; whereas in other cases, it might be a cytoprotective strategy that promotes cellular fitness under stress [23, 33, 85]. For example, cytosolic acidification is required for HSR induction in translationally inhibited cells under heat shock, which allows the cells to adapt to high temperature by increasing the transcription of quality-control components [35, 86]. Furthermore, some stress granule (SG) resident proteins, such as DEAD-box RNA helicase Ded1 [87], poly(A)-binding protein 1 (Pab1) [34], and poly(U)-binding protein 1 (Pub1) [88] in yeast, and Ras-GTPase-activating protein SH3-domain-binding protein (G3BP1) in mammalian cells [89], have been reported to respond to elevated temperatures to undergo LLPS. Likewise, they can also respond to low pH that mimics the pH conditions during heat stress. Therefore, heat-induced acidification may play a key role in protein LLPS following heat exposure and then regulate SG dynamics and cell survival under or after stress.
The mechanism by which heat shock acidifies the cytoplasm is not fully understood. However, evidence has shown that the compositions and structures of the cell membrane are very sensitive to changes in temperature. In yeast, heat shock increases membrane permeability, resulting in proton influx and a rapid decrease in intracellular pH [90]. Studies have shown that intracellular pH disturbance is the triggering mechanism of thermotolerance in yeast [33], and changes in plasma membrane compositions contribute to the thermotolerance of cells, which may also be related to changes in membrane permeability [90]. In turn, heat-induced proton influx and pH decreases can activate plasma membrane ATPase, whose activity is necessary for cell survival under heat shock [33, 91]. The plasma membrane ATPase pumps intracellular protons out of the cell, partially offsetting the internal acidification resulting from the heat-induced increase in membrane permeability [33]. In mammalian cells, heat shock leads to a dramatic loss of plasma membrane Na+–K+ ATPase activity, which then results in loss of the inwardly directed electrochemical Na+ gradient across the membrane [39]. Therefore, it is speculated that Na+ gradient-dependent H+ export from the cytoplasm to the outside by Na+–K+ ATPase is affected and that the cytoplasm is acidified during heat stress.
Starvation
A decrease in cytosolic pH can also be caused by starvation. In yeast cells, numerous studies have shown that the cytoplasmic pH decreases from approximately 7.4 to approximately 6.0 under starvation conditions [43, 45, 47]. Pma1, the plasma membrane-localized P-type H+-ATPase in yeast, is involved in pumping protons out of the cells and is a primary contributor to the maintenance of cytosolic pH stability near neutrality [92, 93]. Importantly, its activation requires glucose-regulated phosphorylation [94]. In addition, other pumps, such as V-type H+-ATPases (V-ATPases), are also responsible for cytosolic pH regulation [95]. They work by pumping excess protons into the vacuole to regulate cytosolic pH homeostasis; they also maintain effective localization of Pma1 at the plasma membrane [95, 96]. Glucose is also required for V-ATPase activation because it mediates reversible associations between the V1 and V0 domains of V-ATPase [43, 97]. Under favorable conditions (with glucose), V-ATPase cooperates with Pma1 to pump protons out of the cytoplasm and help cells stabilize cytoplasmic pH in an ATP-dependent manner. However, upon glucose depletion, a drop in cytoplasmic pH is observed, as starved yeast cells lack efficient H+-ATPase assembly and activation to support the proton gradient across the membrane [46]. Intracellular protons cannot be discharged outside of the cell. Instead, they accumulate inside the cell; thus, cytosolic pH decreases. The increased concentrations of intracellular protons cause the phase transition of the cytoplasm from a fluid-like to a solid-like state, and such a dormant or quiescent state is a protective strategy for cell survival under conditions of starvation [23]. Likewise, evidence suggests that nutrient supply is also closely related to cytoplasmic pH in Physarum plasmodium. The cycle of intracellular pH corresponds to the period of the cell cycle of P. plasmodium. When P. plasmodium is growing in non-nutrient medium, the intracellular pH remains stable and then begins to decline gradually, which serves to block normal mitosis. However, upon refeeding of starved P. plasmodium with the nutrient medium, intracellular pH can recover to normal values and the cell cycle resumes [48].
Osmotic stress
In addition to heat shock and starvation, osmotic stress is another important environmental factor affecting cell survival and growth. Organisms including microbes, plants, and mammals, are commonly confronted with hyperosmotic conditions, which trigger a series of actions resulting in downregulation of cellular activity and progression of disease [98,99,100]. When osmolarity changes, cells adjust their volumes accordingly in response to the changing environment. Cells mainly regulate volume changes by controlling substance influx and efflux, which is usually manifests as cell contraction or expansion, so that cells can return to a normal resting state [101,102,103]. A variety of membrane transporters are involved in this complex regulation process. For example, in a hypotonic environment, mammalian cells initially expand via water uptake and subsequently undergo compensatory shrinkage to partially regulate volume reduction, usually through efflux of KCl and organic osmolytes [104, 105]. In contrast, in hypertonic environments, cells undergo transient dehydrating contraction by absorbing Na+, K+ and CI− and then pumping out Na+ to regulate the increase in cell volume [104].
Intracellular osmotic homeostasis is necessary to maintain normal cell function and survival, and osmotic dysregulation is the basis of many diseases and their complications, including cataracts [106], epilepsy [107], inflammation [100, 108], and hypernatremia [109]. For instance, in hyperglycemia or hypergalactosemia, activated aldose reductase converts glucose and lactose to galactose and sorbitol, respectively, which accumulate in the lens and cause osmotic swelling, leading to diabetic cataracts [106]. In addition, cancer and aging processes are also closely related to intracellular osmotic regulation. Many studies have shown that ion channels and ion pumps are beneficial to the development and progression of cancer [110]. Given the importance of ion channels for osmotic homeostasis and the abnormal expression of transporters in many cancers [111], it is likely that the original homeostasis in cells will be disrupted, creating a more favorable internal environment for cancer development. Interestingly, Yes-associated protein (YAP), is a transcriptional coactivator that is widely activated in cancer cells [112], can sense the tumor microenvironment and modify the physicochemical properties of the surrounding environment by activating transcription, thereby promoting tumor development [113]. Moreover, YAP-activated transcription is mediated by the LLPS process, which also occurs under hypertonic conditions [114]. Aging does not directly cause disease, but in this process, the homeostasis of water in the human body is often disturbed [115]. Thus, normal osmotic regulation is impaired, and this impairment is followed by increases in the incidence and severity of diseases, such as hypoosmolality and hyperosmolality [116].
Interestingly, a growing body of evidence implicates hyperosmotic stress as a factor leading to internal pH alteration. In Listeria monocytogenes, a ubiquitous gram-positive food-borne pathogen, the initial response to osmotic stress caused by sorbitol or NaCl is a decrease in intracellular pH [50]. Hyperosmotic stress also leads to cytosolic acidification in Dictyostelium discoideum, which works as a novel signal mediator responsible for hyperosmotic stress responses [54]. Moreover, another study has indicated that hyperosmotic shock elicits a transient increase in Escherichia coli cytoplasmic pH, but the pH returns to normal values after osmotic adaptation [52]. However, whether and how osmotic dysregulation in mammalian cells alters pH and whether it is relevant to human diseases remain unclear; thus, these aspects require further investigation to advance our understanding of pH-related condensate formation and diseases.
Taken together, the evidence indicates that diverse environmental alterations contribute to intracellular pH fluctuation. Manipulating intracellular pH not only serves to maintain the morphology and function of cells to ensure normal growth and metabolic activities, but also is associated with the preservation of cellular equilibrium in response to several environmental factors, which could promote cellular fitness.
Aging and neurodegenerative diseases
Aging is usually an irreversible biological process and is considered to be a predominant risk factor for many neurodegenerative diseases [117]. Nine hallmarks of aging have been tentatively identified in different organisms, including genomic instability, telomere attrition, epigenetic alterations, loss of proteostasis, deregulated nutrient sensing, mitochondrial dysfunction, cellular senescence, stem cell exhaustion, and altered intercellular communication. These hallmarks can be classified into three layers: primary hallmarks, antagonistic hallmarks, and integrative hallmarks, which co-occur during aging and are usually interconnected with each other; defining the exact relationships and causal network of these hallmarks may contribute to future studies on aging and aging-related diseases [118].
In addition to the hallmarks of aging discussed above, growing evidence shows that intracellular pH alterations are also intimately linked to aging processes and aging-related neurodegenerative diseases. In mammals, the intracellular pH of central neurons is tightly regulated, and its fluctuations are important for signaling and synaptic plasticity [119, 120]. Specifically, in cortical neurons, a mild intracellular pH decrease occurs following an excitability increase, and this decrease acts as feedback to reduce local bioelectric activity and excitability. However, when the intracellular pH is outside a certain range and reaches its limits, there may be an increased risk of cell death [25, 119, 121, 122]. Importantly, a decrease in neural pH levels has been observed in a number of neurodegenerative disorders [123, 124] and even in the normal aging process [32, 125, 126]. Moreover, acute neuroinflammation has been observed to provoke intracellular acidification in the mouse hippocampus [127]. For example, in mammalian cortical neurons, intracellular pH is negatively correlated with aging, as evidenced by significantly lower pH in hippocampal slices from aged rats than in slices from young rats [32, 128]. Likewise, in human neurons, the intracellular pH has also been observed to decrease with aging [126, 129]. The mechanisms involved in decreased intracellular pH may be the disruption and overwhelmed of pH regulatory systems through processes including aging-related decreases in buffering capacity and disruption of diverse transmembrane acid/base-transporters [32, 128,129,130]. For instance, considering that Na+–H+ exchange is the dominant regulatory mechanism for proton extrusion in cultured hippocampal neurons, altered H+ homeostasis might be attributable to impaired Na+–H+ exchange (Fig. 2), which utilizes the inwardly directed electrochemical Na+ gradient generated by Na+–K+ ATPase to export H+ [32]. Moreover, limited ATP synthesis during aging might also affect ATP-driven ion pumping, including Na+ gradient generation by Na+–K+ ATPase [131]. The impacts of aging-related alterations on pH regulation are controversial. A slight decrease in intracellular pH may provide neuroprotection [132], while successively greater acidification may increase the vulnerability of brain tissue to stressful conditions [125, 133,134,135].
Aging affects intracellular pH. When cells are young, P-type H+-ATPases distributed on the plasma membrane act in concert with V-type H+-ATPases localized on the lysosomal/vacuolar membrane to regulate intracellular pH. However, during aging, for example, in yeast, P-type H+-ATPase Pma1 accumulates on the plasma membrane, and excessive H+ is pumped out of the cell, resulting in reduced cytosolic H+ availability for V-type H+-ATPase. This leads to a decrease in vacuolar acidity. In other cases, such as in the aged rat hippocampus, the Na+–K+ pump and Na+–H+ exchange may be impaired; as a result, H+ accumulates in the cytoplasm, and cytosolic pH decreases. Moreover, cell buffering capacity is also impaired during aging. V-type H+-ATPase is a target of oxidative stress in aging. Increased oxidative modification of V-type H+-ATPase might inhibit V-type H+-ATPase-mediated vacuolar acidification. Alternatively, aging might alter lysosomal/vacuolar acidification by downregulating V-type H+-ATPase subunit expression, lowering the availability of V-type H+-ATPase. The solid lines represent normal ion transport. The dashed lines represent impaired ion transport. In the young cell cytoplasm, yellow represents cytoplasm with a normal pH. In the aged cell cytoplasm, red represents cytoplasm with a decreased pH
In addition to cytosolic pH dysregulation, lysosomal/vacuolar pH dysregulation has also been implicated in aging and aging-related neurodegenerative diseases. Evidence is now emerging that defective lysosomal function is a major factor in the pathogeneses of different types of neurodegenerative diseases, specifically, a failure of the maintenance of a highly acidic lysosomal/vacuolar pH [136]. There is also increasing evidence for aging-related compromise of lysosomal function [22]. In yeast, vacuolar pH is a critical regulator of mitochondrial function and replicative lifespan. Vacuolar acidity declines with aging, and reduced vacuolar acidity disrupts pH-dependent amino acid homeostasis in the vacuolar lumen, resulting in age-related dysfunction of mitochondria and a shortened lifespan [67]. In addition, lifespan extension via calorie restriction and methionine restriction requires vacuolar acidification [67, 137, 138]. The decrease in vacuolar acidification in yeast is due to excess accumulation of the major regulator of cytosolic pH, Pma1, in mother cells (Fig. 2). Vacuole acidity is thus antagonized by reduced cytosolic proton availability [22]. Importantly, V-ATPase is implicated in lysosomal acidification. Mutations in V-ATPase or proteins that regulate V-ATPase function are observed in aging-related neurodegeneration [136]. It is conceivable that during aging, oxidative stress might impair V-ATPase activity through increased oxidative modification of V-ATPase (Fig. 2), which is inspired by the observation that hydrogen peroxide inhibits bovine brain synaptic vesicle V-ATPase activity [69]. In fact, increased oxidative modification of V-ATPase subunits has been observed in aged rat brain tissue [139], and oxidative modification is known to impair the activity of certain enzymes [140, 141]. Alternatively, aging might alter lysosomal/vacuolar acidification via dynamic transcriptional regulation of V-ATPase subunits (Fig. 2), a mechanism that is supported by the observation of reduced V-ATPase subunit mRNA levels in hippocampal neurons in sporadic Alzheimer’s disease (AD) [142]. In conclusion, intracellular pH alterations, including cytosolic pH changes and lysosomal/vacuolar pH dysregulation, are also striking features that occur during the aging process and aging-related diseases onset.
The processes of aging and aging-related neurodegenerative diseases onset are typically accompanied by the formation of widespread intracellular protein aggregates [143]. Many RNA-binding proteins, such as fused in sarcoma (FUS), tau, alpha synuclein (α-Syn), and TAR DNA-binding protein 43 (TDP-43), are the main components of protein inclusions or aggregates in diverse neurodegenerative diseases, including amyotrophic lateral sclerosis (ALS) [144], frontotemporal dementia (FTD) [144, 145], Parkinson’s disease (PD)[146], and AD [147]. Furthermore, these disease-associated proteins are well known to undergo LLPS, and the failure to maintain their liquid-phase homeostasis may serve as a trigger of solid protein aggregate formation (Fig. 1) [148]. Diverse layers of regulation may affect their transition from a liquid-like state with physiological function to solid pathological aggregates. Therefore, it is reasonable to speculate that alterations in the intracellular microenvironment, such as pH changes during aging, provide these phase-separated neurological disorder-related proteins with the opportunity to change their phase separation behaviors and increase the risk of aggregation. In fact, evidence has already shown that LLPS of α-Syn and its subsequent maturation into protein aggregation are pH-mediated [149]. Therefore, further investigations on aging-induced pH dysregulation will not only advance our understanding of aberrant LLPS and compartment formation but will also provide important insight into the onset of aging-associated pathologies.
Cancers
In recent years, increasing evidence has linked LLPS and condensates to tumorigenesis. A growing number of cancer-associated proteins have been reported to have the ability to undergo LLPS and form biomolecular condensates, such as speckle-type POZ protein, which is involved in oncogenic substrate degradation [150]; p53-binding protein 1 (53BP1) and FET proteins, which are involved in the DNA damage response and genomic stability [151, 152]; and EWS-FLI1, β-catenin, YAP, and PDZ-binding motif (TAZ), which are involved in transcriptional regulation [114, 153,154,155]. In all of the above cases, disrupting functional condensate assembly of tumor suppressors or promoting aberrant condensate assembly of oncoproteins contributes to the oncogenic process (Fig. 1a–d). In addition, aberrant assembly of other membrane-less compartments formed by LLPS, including SGs [156, 157], PML bodies [158], paraspeckles [159], and amyloid bodies [160], is also associated with cancer. The tumor suppressor p53 has been the “star molecule” of molecular biology and oncology since its discovery. It acts as a transcription factor, activating or inhibiting the transcription of various downstream target genes involved in cell cycle regulation, senescence, and apoptosis [161, 162]. p53 prevents tumor development through cell cycle arrest, DNA repair, and antioxidant protein production to maintain genome integrity and limit cell proliferation under adverse conditions such as DNA damage, hypoxia, oncogene expression, nutrient deprivation, and ribosomal dysfunction [162,163,164]. Moreover, its mutation, which tends to result in protein aggregation, is found in more than 50% of human cancers [165, 166]. Recent evidence has revealed that the p53 core domain can undergo LLPS and then undergo a phase transition to the solid-like state. Mutation of p53 can accelerate its solid-phase transition into amyloid aggregates (Fig. 1b) [167]. Therefore, it is a reasonable assumption that differences in the tumor microenvironment compared to the microenvironment of normal differentiated cells may trigger certain proteins to undergo LLPS and phase transition to solid aggregates, leading to further cancer progression.
As cancer cells grow at an uncontrolled high rate, they are usually challenged with an adverse macroenvironment characterized by hypoxia and nutrient starvation [168]. Apart from this, considerable evidence links cancer directly to pH alterations since a higher intracellular pH and a lower extracellular pH than those of normal differentiated cells are observed in most cancers, regardless of tissue origin and cell type [169, 170]. These differences may be attributable to changes in the expression and/or activity of plasma membrane ion pumps and transporters, as well as changes in metabolic activities [169, 170]. In turn, the increased intracellular pH and the decreased extracellular pH also synergistically enhance cancer progression. On the one hand, the increased intracellular pH can increase cell proliferation, facilitate apoptosis evasion, and promote cytoskeletal remodeling for cell migration. On the other hand, the acidified extracellular environment can increase the activities of acid-activated proteases and promote extracellular matrix degradation, thereby accelerating tumor cell invasion and dissemination [31]. However, during these processes, whether and how pH alterations of cancer cells are related to the aberrant phase behavior of cancer-related proteins or aberrant formation of membrane-less compartments such as SGs, PML bodies, paraspeckles, and amyloid bodies, remains unclear and requires further in-depth investigation. Such research will provide more knowledge about the molecular basis of cancer and facilitate the development of new therapies.
Cytosolic pH control by metabolism-based and transporter-based regulation
Since pH control is a critical requirement for growth in all organisms, different organisms have adopted a number of common strategies to address the challenges of pH maintenance in the face of rapid metabolism and extracellular environment changes [171,172,173]. Cells separate metabolites, proteins, and biochemical processes in a manner dependent on compartmentalized membrane-bound organelles, each of which has distinct pH requirements and pH regulation mechanisms [172]. More importantly, cellular compartments have inherent pH buffering capacities. This buffering is achieved by the presence of various intracellular weak acids and bases, as well as the ionizable groups of macromolecules such as side chains of amino acids [174]. Moreover, cytosolic pH regulation also relies on metabolites produced by pH-dependent biological reactions [175]. Organic acids such as malate can produce or consume H+ via carboxylation and decarboxylation reactions. Therefore, correct synthesis, degradation, and transport of organic acids through the cytoplasm to other organelles are thought to be important strategies to regulate intracellular pH [176, 177]. In addition, the alternative pathways to glycolysis, the cyanide-resistant alternative respiration pathway and malate-derived lactic and alcoholic fermentation, which are unique to plants, jointly regulate pH homeostasis in plants [175]. In mammalian cells and fermenting yeast, CO2 produced during metabolism can diffuse freely through biological membranes. It can react with water to form HCO3−, which is an effective proton buffer and consumes protons to produce carbonic acid when the cells are confronted with an acute drop in intracellular pH [178, 179].
However, when cells are under long-term stress, the major regulatory mechanism to maintain cytosolic pH homeostasis is the membrane transport of H+, which involves a large array of distinct transport pathways. For example, P-type proton pumps are widely distributed on eukaryotic cell membranes, and they are the main determinants of proton efflux and cytoplasmic pH control in plants and yeast [19, 173]. As mentioned above, Pma1 is the most abundant protein in the plasma membrane of yeast and actively coordinates with V-ATPases to regulate cytosolic pH [19, 173]. V-ATPases can also acidify compartments in an ATP-dependent manner and are distributed in acidic organelles such as the Golgi apparatus, vacuole/lysosomes, and endosomes of all eukaryotic cells [180]. In yeast cells, V-ATPase activity is indispensable for vacuolar acidification during glucose metabolism and homeostasis of cytoplasmic pH in the short term. In the long term, V-ATPase is very important for the stability of Pma1 localization [95]. F-type proton pumps are mainly distributed in the bacterial plasma membrane, the mitochondrial membrane, and the plant endomembrane. In enterococci, when the cytoplasm is acidified, the level and activity of F-type H+-ATPase increase synchronously, leading to cytoplasmic alkalization [181]. When the pH value is restored to the initial value, the decrease in the amount and activity of the enzymes terminates proton extrusion. Thus, changes in the amount and activity of enzymes seem to be necessary for pH regulation [182].
Moreover, these proton pumps act in concert with a large array of other transporters. Increasing evidence indicates that a number of ion/H+ exchangers are also important for intracellular pH regulation in different organisms, including yeast, plants, and mammals [19, 172, 173]. These exchangers couple the transfer of H+ across biological membranes to counter-transport of other cations, such as Na+ or K+, to protect against excess acidification. Furthermore, Na+-coupled HCO3− transporters, which are involved in the uptake of extracellular HCO3−, have also been reported to play key roles in the regulation of cytosolic pH. They contribute to the maintenance of CO2–HCO3− equilibrium, the most important pH buffering system [183, 184]. Although the importance of proton extrusion in pH control has been revealed, acid-importing transporters such as Cl−–HCO3− exchangers, which allow HCO3− efflux, can efficiently prevent overalkalization of the cells by working counter to CO2–HCO3− transporters to enable the fine control of cytosolic pH [185].
In summary, cells exhibit a complicated pH regulation network dependent on the interplay among multiple transporters that import or export proton equivalents and metabolism-based regulatory mechanisms, and this network can accurately regulate and maintain cytosolic pH. More details can be found in recent reviews [19, 171,172,173].
pH-dependent phase separation condensate formation induced by stress
As discussed above, many types of stress cause a decrease in cytoplasmic pH, and these stress conditions are known to induce phase separation of proteins to form condensates. Here, we summarize the proteins that are known to form phase separation condensates in response to pH stress together with other stresses in which phase separations are mainly affected by pH alterations, such as heat shock and starvation (Table 2).
Many biomolecules undergo LLPS to form liquid-like condensates that mediate diverse cellular functions [222, 223]. For example, autophagosome formation is a process that is precisely regulated by protein phase separation. Atg1 complex formation is a prerequisite for preautophagosomal structure (PAS) assembly and autophagy initiation [202]. Recent research suggests that the PAS is a liquid-like condensate formed by phase separation of the Atg1 complex, which is critical for further dynamic recruitment of other proteins or factors during autophagosome formation. Notably, this process occurs under low pH and starvation conditions [200,201,202]. TORC1 is a modulator of PAS organization that targets Atg1 complex assembly by regulating the phosphorylation/dephosphorylation of Atg13, a component of the Atg1 complex [202, 224]. Its activity is also modulated by phase-separated compartments such as SGs. Under stressful conditions, including heat, starvation, and osmotic stress, TORC1 is recruited into SGs; as a result, TORC1 signaling is inhibited [225,226,227]. For example, in yeast, TORC1 is partitioned into heat shock-induced SGs, which then prevents an increase in the frequency of heat-induced DNA mutations [225]. Under osmotic stress, TORC1 in mammalian cells is similarly sequestered into SGs, thereby blocking its signal transduction to downstream effectors [227].
SGs are also dynamic membrane-less organelles, the formation of which is driven by LLPS [228, 229]. It has been reported that many proteins in SGs exhibit phase separation behavior under stress-associated pH changes. Pab1, is an RNA-binding protein consisting of a short N-terminal sequence, four RNA recognition motifs (RRMs), a proline-rich low-complexity region (LCR) and a C-terminal peptide-binding domain. It plays a key role in controlling the polyadenylation, stability, and translation of mRNA in yeast cells [34, 190]. Pub1 is similar to Pab1 in that it is an RNA-binding protein with three RRMs and one LCR [191]. Both Pub1 and Pab1 are core components of SGs and are prone to phase separation when temperature increases, pH decreases, or nutrients are lacking to help cells survive during stress [34, 88, 189]. In addition, G3BP1 is a central node and molecular switch in SG assembly. Its phase separation also occurs in an RNA-dependent manner under low pH and heat shock [89, 203]. Moreover, members of the Asp–Glu–Ala–Asp (DEAD)-box ATPase (DDX)3 family are widely present in both eukaryotes and prokaryotes [192, 194], and studies have suggested that many proteins in the DDX family undergo LLPS in vivo or in vitro, including Ded1, Dbp1, and Dbp2 in yeast; DDX3X, DDX4, and DDX6 in humans; and DeaD, SrmB, and RhlE in E. coli [192, 193]. Ded1p, an ATP-dependent DEAD-box RNA helicase in yeast, is an indispensable translation initiation factor and a component of SGs [188]. It can parse the secondary structure of mRNA 5′ untranslated regions for ribosomal scanning and recognition of the initiation codon [186, 187]. Studies have shown that Ded1p undergoes phase separation and forms condensates at elevated temperatures, or at lower temperatures when the pH is adjusted to that of the heat-stressed cytosol (heat-shocked cells experience a decrease in cytosolic pH). When in condensate form, Ded1p is translationally inactivated, which leads to a switch in translation from housekeeping transcripts to stress-responsive transcripts [87]. Therefore, heat shock-induced and temperature-associated pH change-induced Ded1p condensation in SGs is an adaptive response to survive heat shock. It promotes an evolutionarily conserved heat shock response that selectively translates housekeeping or heat shock transcripts [87]. Similarly, another DDX family member in yeast, Dhh1, is responsible for the assembly and disassembly of RNA-containing membrane-less organelles. Dhh1 also exhibits enhanced phase separation at low pH, which mimics the pH conditions during glucose starvation [192].
Moreover, evidence indicates that in changed growth conditions, enzyme activities can be acutely regulated through the formation of phase separation-induced enzyme condensates, which restrict or promote specific biochemical reactions in membrane-less organelles, suggesting the importance of phase separation in regulating the metabolism of cells [47, 195]. For instance, glutamine synthetase (Gln1) is an indispensable metabolic enzyme that catalyzes the synthesis of glutamate and ammonium into glutamine, a process that requires ATP. Gln1 forms filaments during a state of advanced cellular starvation, and filament formation leads to enzymatic inactivation [197]. Further evidence demonstrates that starvation-induced cytosolic acidification is the trigger for Gln1 condensate formation, and many metabolic enzymes follow this principle to help cells endure and recover from severe starvation conditions [47].
In addition to the above-mentioned findings, there are other proteins for which LLPS is directly or indirectly affected by pH changes, increasing cell fitness or inducing diseases. For instance, Sup35 is a translation termination factor in budding yeast [198]. It can form condensates upon energy depletion or at a low pH. This pH-dependent phase separation of Sup35 can serve as a means for Sup35 to rescue itself from stress-induced damage and promote recovery of the yeast cell from stress [44]. The nucleocapsid protein (N) of the severe acute respiratory syndrome coronavirus (SARS-CoV-2) is a multivalent RNA-binding protein that is essential for viral RNA replication and virion packaging [206]. The N protein can partition into SGs and interact with G3BP1/2 to block the assembly of SGs through RNA-dependent liquid phase separation and thus disrupt the immune response of host cells [204]. Notably, phase separation of the N protein occurs under physiological conditions and is enhanced at low pH [205]. α-Syn is an IDP for which aggregation into amyloid-like fibrils is associated with PD pathology [207, 208]. One study found that α-Syn initially undergoes phase separation and becomes rigid over time and eventually transforms into solid-like aggregates. Low pH can promote α-Syn LLPS and further increase the maturation and nucleation of α-Syn aggregates, which is relevant to PD pathogenesis [149]. Additionally, pathological inclusions of the microtubule-associated protein Tau have been reported to accumulate in patients with several neurodegenerative diseases [210,211,212]. Evidence indicates that the microtubule-binding repeats of the Tau protein have a strong propensity for liquid demixing, which occurs over a wide range of pH values. The phase separation of these four repeats at different pH values wound concentrate the most aggregation-prone Tau residues and further promote amyloid formation [16]. Interestingly, in addition to natural proteins, artificially constructed polypeptides can also undergo phase separation. Elastin-like polypeptides (ELPs) are recombinant protein polymers composed of pentapeptide (Val–Pro–Gly–Xaa–Gly)L repeat units, which are recurring motifs in tropoelastin in a wide range of species. ELPs are often used as new biomaterials for drug delivery and tissue engineering [218,219,220,221]. One study found that ELPs can exhibit reversible phase separation triggered by a wide range of pH values, and this pH responsiveness is controlled by the type and number of ionizable residues and the molecular weight of the ELPs. This property of specific pH-controlled ELP phase separation can be applied in drug delivery systems for local cancer therapy, as various tumors types usually have different pH values than healthy tissues [217].
Finally, as we discussed above, many cancer-associated proteins have been reported to undergo LLPS and to be involved in biomolecular condensate formation. 53BP1 is a binding partner of p53 [230] that can directly regulate p53 and affect p53 target gene expression [231]. It is also one of the main regulators of the DNA damage response, loss of which has been associated with apoptosis and cancer cell proliferation [232]. Studies have found that 53BP1 undergoes LLPS at DNA damage sites, forming DNA repair condensates that recruit and stabilize p53 [151]. If the expression of 53BP1 is changed or LLPS behavior is affected, the disruption of condensate formation leads to destabilization of p53 and reduced induction of its target genes as well as cell cycle arrest [151]. Interestingly, it has been reported that 53BP1 can respond to low pH to form 53BP1 droplets [151]; thus, further studies on the relationships of pH regulation and 53BP1 LLPS will help enhance our understanding of tumorigenesis. However, besides 53BP1, research on the relationships between cancer-associated proteins and pH dysregulation are limited. Considering that the physiochemical properties and microenvironment of cancer cells are different from those of normal cells [168], two important research topics in the future are whether these proteins undergo pH-regulated LLPS and how pH-regulated LLPS is relevant to tumorigenesis. In addition, research on how microenvironmental changes in cancer cells affect the dynamics of intracellular membrane-less organelles such as SGs, PML bodies, paraspeckles, and amyloid bodies, whose aberrant assembly is associated with cancer, is also needed. Such research will provide further evidence regarding the links among pH, LLPS, and cancer.
Mechanisms underlying pH-mediated phase separation
pH changes mediate protein–protein/RNA interactions
Some proteins or their specific domains possess the ability to sense stresses directly and thus undergo phase separation in response to these stresses [34, 88]. It is known that LLPS is driven by multivalent weak macromolecular interactions (protein–protein, protein–RNA, and RNA–RNA interactions), disruption or alteration of which would affect protein phase separation behaviors [5, 233]. Therefore, pH changes can influence intramolecular or intermolecular protein–protein/RNA interactions by changing the net charges of components, thereby driving LLPS (Fig. 3). For example, G3BP1 is a multidomain protein composed of two folded domains and two IDRs. Under nonstress conditions, its central negatively charged, glutamate-rich IDR can interact with the C-terminal positively charged RG-rich region to allow G3BP1 to fold into a compact state. This compact state is an autoinhibitory conformation that disrupts G3BP1 phase separation. However, at a low pH, protonation of the clustered glutamates changes the net charge of the acidic IDR and disrupts its stable electrostatic interactions with the RG-rich region, allowing G3BP1 to expand from its original tightly self-inhibited state and release the RG-rich region. G3BP1 can then further facilitate intermolecular protein–RNA/protein interactions to drive LLPS, which is consistent with the observation that heterotypic interactions among G3BP1 and RNA molecules drive SG assembly [89, 203]. In addition, a low pH can directly trigger Pub1 assembly, and this pH-dependent assembly formation is sensitive to salt concentrations, suggesting that electrostatic interactions promote Pub1 assembly. Self-interactions among the RRM domains are the main drivers for Pub1 phase separation, and acidic pH may change the charge distribution in the RRM domains, thereby mediating the electrostatic interactions [88]. Moreover, the pH range that can induce artificially recombinant ELP phase separation is related to the pKa. This suggests to a certain extent that pH can affect protein–solution or protein–protein interactions by changing the number of ionizable residues of proteins, thus triggering phase separation [217]. Likewise, phase separation of Pab1 at low pH is also an electrostatically mediated process [34]; thus, the principle of pH-dependent protein condensate formation mediated by electrostatic interactions may be generalizable to many proteins.
Roles of pH in biomolecular condensate formation. Under nonstress conditions, proteins and RNAs are dispersed in the cytoplasm. When cells are exposed to stresses such as starvation, heat shock, or acid stress, the intracellular pH changes, and this change is accompanied by the formation of protein- and RNA-containing biomolecular liquid-like condensates. During this process, pH plays multiple functional roles in triggering liquid–liquid phase separation (LLPS)-driven condensate formation; for example, it affects protein–protein/RNA interactions, alters protein solubility, or acts as a messenger to transmit stress signals. pH changes can also enhance phase separation, which may gradually mature and result in transformation into an irreversible gel-/solid-like state
Notably, pH changes not only initiate protein LLPS by facilitating intramolecular or intermolecular interactions but also enhance phase separation and its further maturation into a gel state or a pathological solid state (Fig. 3). Phase separation of α-Syn is mediated by an interplay of electrostatic interactions in the unstructured N-terminal domain and hydrophobic interactions in the central NAC domain, while the charge distribution in these domains is strongly dependent on the pH value. A lower pH serves to change the net charges and hydrophobicity of different domains as well as the interactions between these domains, leading to significant structural reorganization. Thus, pH-mediated diverse changes in α-Syn accelerate the maturation of phase separation and subsequent protein aggregation [149, 209]. Similarly, a reduction in pH can enhance the intramolecular interactions of the phase-separated SARS-CoV-2 N protein and lead to irregularly shaped assemblies with less liquidity, in vitro [205]. Indeed, phase separation proteins that contain flexible LCDs are highly prone to forming pathogenic aggregates. This could explain, to some extent, why hundreds of proteins are highly prone to forming aggregates during aging. During aging or chronic pH stress, these phase separation proteins can transition into irreversible aggregates, which could then lead to persistent condensate formation, such as persistent SG formation, even after the stress subsides. Persistent condensates typically exhibit solid-like properties; and as a consequence, other pathological changes and neurodegenerative disorders occur [233].
pH changes affect protein solubility
In addition to engaging in the promiscuous interactions that function in phase separation, macromolecules must reach a critical concentration threshold to start LLPS. Evidence indicates that not all LCRs and IDRs function as autonomous modules that drive phase separation; instead, they function as modifier sequences, regulating the solubility of phase-separating proteins and the material properties of condensates [234]. Long-term evolutionary pressure has tuned the solubility of Pub1 to be very close to the critical threshold for phase separation. This not only endows Pub1 with a solubility that is conducive to growth but also enables Pub1 to quickly sense and respond to stress. In fact, changes in pH can affect the solubility of Pub1 and lead to the formation of stress-responsive Pub1 condensates [88]. Changes in pH appear to be able to decrease the solubilities of many proteins. In yeast, a decrease in pH results in a phase transition of cytoplasm from a fluid-like to a solid-like state, which might be caused by decreased solubilities of a series of proteins and subsequent formation of intracellular solid-like assemblies, such as SGs [23, 235]. Moreover, the relationship between pH and protein isoelectric point is closely related to solubility. The closer the pH value is to the isoelectric point of the protein, the lower its solubility, and the more likely it is that phase separation occurs [236, 237]. This could explain to a certain degree why the microtubule-binding repeats of Tau are most prone to phase separation when the pH is close to the isoelectric point but less prone to demixing when the protein solubility is increased in response to pH that is substantially higher or lower than the isoelectric point [16]. Therefore, it is believed that one of the mechanisms by which pH triggers protein phase separation is the alteration of protein solubility (Fig. 3).
pH changes act as messengers to transmit stress signals
In the face of adverse conditions, intracellular pH might act as a messenger to signal changes in the environment, triggering phase separation of proteins to promote cell fitness. Upon heat shock, cells can integrate signals of different temperatures and temperature-induced pH changes into a unified response to provide a trigger for phase separation. For example, Pab1 undergoes LLPS autonomously through temperature-dependent structural changes under conditions of stressful temperatures [34].
However, how does a cell sense other stresses, such as starvation, to trigger LLPS to help cells survive diverse adverse conditions? Previous studies have indicated that proteins and protein-associated condensates that undergo LLPS under starvation conditions, such as Pub1, Gln1, Dhh1, and PAS, can also respond to low pH [47, 88, 192, 200, 201]. Considering that cytosolic pH is rapidly and reversibly regulated by glucose metabolism, the stress information perceived by these proteins is most likely transmitted through pH. Evidence has shown that cytosolic pH is a second messenger for glucose to mediate activation of the PKA pathway through V-ATPase [43]. Therefore, a change in pH might be an extremely sensitive readout of other changes in the environment, especially starvation, to induce protein LLPS and cellular adaptive responses (Fig. 3).
In this way, pH is capable of playing diverse functional roles in the regulation of LLPS, including by affecting protein–protein/RNA interactions, altering protein solubility, and acting as a messenger to transmit stress signals.
Conclusion and perspective
From viruses to prokaryotes and eukaryotes, the formation of macromolecular condensates by phase separation is emerging as a principle means for cells to regulate cellular functions and adapt to environmental changes. Cells encounter a variety of stresses, some of which can cause cytoplasmic pH fluctuations. In this review, we have summarized the relationships between pH changes and certain stresses, such as heat shock, nutrient stress, and osmotic stress, and described which proteins or physiological processes can respond to stress-associated pH changes through phase separation. We have also highlighted the diverse ways by which pH fluctuation can influence protein phase separation. For example, pH can act as a signal to transmit stress information, mediate protein–protein/RNA interactions, and affect protein solubility, thereby regulating protein/RNA phase separation.
Despite the research progress concerning the relationships between stress-associated pH changes and phase separation discussed in this review, further in-depth investigations are still needed. It is worth noting that pH might not be the sole determinant of stress-induced phase separation and condensate formation. For stresses such as heat shock, changes in both intracellular temperature and pH are involved, which can lead them to differences in protein phase separation behavior and condensate material properties [88]. The interplay of pH, temperature, ion strength, RNA concentration, protein concentration and other factors forms a sophisticated network that dynamically affects the phase behavior of proteins. However, some questions remain. How does pH interact with other factors in this process? What are the differences and similarities in the roles of pH among the different stress-induced phase separation processes? Preliminary evidence suggests that the properties of different condensate materials formed by different groups of proteins can be used by cells to build a hierarchical stress-adaptive system that is fine-tuned to different conditions [88]. In other words, when encountering different types of stresses or the same stress with different intensity or duration, a cell can regulate the activities of multiple proteins to achieve specific biological functions by concentrating specific cellular components in the condensates (or excluding them from the condensates) for a favorable period of time. The cells can then determine when to restart growth. In this way, control of condensates can be used by the cells as a method to promote adaptation to stress. Therefore, revealing the differences and similarities among the various roles of pH in addressing different types of stress will provide insights into the mechanisms underlying the protein separation involved in cellular adaptation. Moreover, pH values might be changed considerably by different stresses, and a given protein might display different phase separation behaviors under different pH values. Therefore, the identification of proteins that respond to different pH values or have behavior changes that accompany pH changes may provide vital clues for investigation of the machineries involved in the influences of pH on cellular functions.
Finally, intracellular pH changes and phase separation condensate formation are linked to aging, aging-related neurodegenerative diseases, and cancers. It would be interesting to further investigate how aging-induced pH changes affect protein phase separation. Importantly, innovative drug delivery strategies could be developed for specific local cancer therapy by exploiting the altered intracellular and extracellular pH in tumors. Attempts to modulate pH and SG formation could also spur the development of innovative approaches for cancer therapy.
References
Alberti S, Gladfelter A, Mittag T (2019) Considerations and challenges in studying liquid-liquid phase separation and biomolecular condensates. Cell 176(3):419–434. https://doi.org/10.1016/j.cell.2018.12.035
Hyman AA, Weber CA, Julicher F (2014) Liquid-liquid phase separation in biology. Annu Rev Cell Dev Biol 30:39–58. https://doi.org/10.1146/annurev-cellbio-100913-013325
Boeynaems S, Alberti S, Fawzi NL, Mittag T, Polymenidou M, Rousseau F, Schymkowitz J, Shorter J, Wolozin B, Van Den Bosch L, Tompa P, Fuxreiter M (2018) Protein phase separation: a new phase in cell biology. Trends Cell Biol 28(6):420–435. https://doi.org/10.1016/j.tcb.2018.02.004
Oldfield CJ, Dunker AK (2014) Intrinsically disordered proteins and intrinsically disordered protein regions. Annu Rev Biochem 83:553–584. https://doi.org/10.1146/annurev-biochem-072711-164947
Gomes E, Shorter J (2019) The molecular language of membraneless organelles. J Biol Chem 294(18):7115–7127. https://doi.org/10.1074/jbc.TM118.001192
Van Treeck B, Parker R (2018) Emerging roles for intermolecular RNA-RNA interactions in RNP assemblies. Cell 174(4):791–802. https://doi.org/10.1016/j.cell.2018.07.023
Van Treeck B, Protter DSW, Matheny T, Khong A, Link CD, Parker R (2018) RNA self-assembly contributes to stress granule formation and defining the stress granule transcriptome. Proc Natl Acad Sci USA 115(11):2734–2739. https://doi.org/10.1073/pnas.1800038115
Hofweber M, Dormann D (2019) Friend or foe-post-translational modifications as regulators of phase separation and RNP granule dynamics. J Biol Chem 294(18):7137–7150. https://doi.org/10.1074/jbc.TM118.001189
Luo YY, Wu JJ, Li YM (2021) Regulation of liquid-liquid phase separation with focus on post-translational modifications. Chem Commun (Camb) 57(98):13275–13287. https://doi.org/10.1039/d1cc05266g
Gao Y, Pei G, Li D, Li R, Shao Y, Zhang QC, Li P (2019) Multivalent m6A motifs promote phase separation of YTHDF proteins. Cell Res 29(9):767–769. https://doi.org/10.1038/s41422-019-0210-3
Ries RJ, Zaccara S, Klein P, Olarerin-George A, Namkoong S, Pickering BF, Patil DP, Kwak H, Lee JH, Jaffrey SR (2019) m6A enhances the phase separation potential of mRNA. Nature 571(7765):424–428. https://doi.org/10.1038/s41586-019-1374-1
Alberti S (2017) The wisdom of crowds: regulating cell function through condensed states of living matter. J Cell Sci 130(17):2789–2796. https://doi.org/10.1242/jcs.200295
Wang B, Zhang L, Dai T, Qin Z, Lu H, Zhang L, Zhou F (2021) Liquid-liquid phase separation in human health and diseases. Signal Transduct Target Ther 6(1):290. https://doi.org/10.1038/s41392-021-00678-1
Alberti S, Hyman AA (2016) Are aberrant phase transitions a driver of cellular aging? BioEssays 38(10):959–968. https://doi.org/10.1002/bies.201600042
Ruff KM, Roberts S, Chilkoti A, Pappu RV (2018) Advances in understanding stimulus-responsive phase behavior of intrinsically disordered protein polymers. J Mol Biol 430(23):4619–4635. https://doi.org/10.1016/j.jmb.2018.06.031
Ambadipudi S, Biernat J, Riedel D, Mandelkow E, Zweckstetter M (2017) Liquid-liquid phase separation of the microtubule-binding repeats of the Alzheimer-related protein Tau. Nat Commun 8(1):275. https://doi.org/10.1038/s41467-017-00480-0
Khan YM, East JM, Lee AG (1997) Effects of pH on phosphorylation of the Ca2+-ATPase of sarcoplasmic reticulum by inorganic phosphate. Biochem J 321(Pt 3):671–676. https://doi.org/10.1042/bj3210671
Mellman I (1992) The importance of being acid: the role of acidification in intracellular membrane traffic. J Exp Biol 172:39–45. https://doi.org/10.1242/jeb.172.1.39
Orij R, Brul S, Smits GJ (2011) Intracellular pH is a tightly controlled signal in yeast. Biochim Biophys Acta 1810(10):933–944. https://doi.org/10.1016/j.bbagen.2011.03.011
Isom DG, Sridharan V, Baker R, Clement ST, Smalley DM, Dohlman HG (2013) Protons as second messenger regulators of G protein signaling. Mol Cell 51(4):531–538. https://doi.org/10.1016/j.molcel.2013.07.012
Felle HH (2001) pH: signal and messenger in plant cells. Plant Biol 3(6):577–591. https://doi.org/10.1055/s-2001-19372
Henderson KA, Hughes AL, Gottschling DE (2014) Mother-daughter asymmetry of pH underlies aging and rejuvenation in yeast. Elife 3:e03504. https://doi.org/10.7554/eLife.03504
Munder MC, Midtvedt D, Franzmann T, Nüske E, Otto O, Herbig M, Ulbricht E, Müller P, Taubenberger A, Maharana S, Malinovska L, Richter D, Guck J, Zaburdaev V, Alberti S (2016) A pH-driven transition of the cytoplasm from a fluid- to a solid-like state promotes entry into dormancy. Elife 5:e09347. https://doi.org/10.7554/eLife.09347
Ismail A, Takeda S, Nick P (2014) Life and death under salt stress: same players, different timing? J Exp Bot 65(12):2963–2979. https://doi.org/10.1093/jxb/eru159
Obara M, Szeliga M, Albrecht J (2008) Regulation of pH in the mammalian central nervous system under normal and pathological conditions: facts and hypotheses. Neurochem Int 52(6):905–919. https://doi.org/10.1016/j.neuint.2007.10.015
Bright CM, Ellis D (1992) Intracellular pH changes induced by hypoxia and anoxia in isolated sheep heart Purkinje fibres. Exp Physiol 77(1):165–175. https://doi.org/10.1113/expphysiol.1992.sp003570
Diaz FE, Dantas E, Cabrera M, Benitez CA, Delpino MV, Duette G, Rubione J, Sanjuan N, Trevani AS, Geffner J (2016) Fever-range hyperthermia improves the anti-apoptotic effect induced by low pH on human neutrophils promoting a proangiogenic profile. Cell Death Dis 7(10):e2437. https://doi.org/10.1038/cddis.2016.337
Drummond IA, McClure SA, Poenie M, Tsien RY, Steinhardt RA (1986) Large changes in intracellular pH and calcium observed during heat shock are not responsible for the induction of heat shock proteins in Drosophila melanogaster. Mol Cell Biol 6(5):1767–1775. https://doi.org/10.1128/mcb.6.5.1767-1775.1986
Ishizawa K (2014) Intracellular pH regulation of plant cells under anaerobic conditions. Plant Cell Monographs 21:59–74. https://doi.org/10.1007/978-3-7091-1254-0_4
Weitzel G, Pilatus U, Rensing L (1987) The cytoplasmic pH, ATP content and total protein synthesis rate during heat-shock protein inducing treatments in yeast. Exp Cell Res 170(1):64–79. https://doi.org/10.1016/0014-4827(87)90117-0
Webb BA, Chimenti M, Jacobson MP, Barber DL (2011) Dysregulated pH: a perfect storm for cancer progression. Nat Rev Cancer 11(9):671–677. https://doi.org/10.1038/nrc3110
Roberts EL Jr, Sick TJ (1996) Aging impairs regulation of intracellular pH in rat hippocampal slices. Brain Res 735(2):339–342. https://doi.org/10.1016/0006-8993(96)00925-0
Coote PJ, Cole MB, Jones MV (1991) Induction of increased thermotolerance in Saccharomyces cerevisiae may be triggered by a mechanism involving intracellular pH. J Gen Microbiol 137(7):1701–1708. https://doi.org/10.1099/00221287-137-7-1701
Riback JA, Katanski CD, Kear-Scott JL, Pilipenko EV, Rojek AE, Sosnick TR, Drummond DA (2017) Stress-triggered phase separation is an adaptive, evolutionarily tuned response. Cell 168(6):1028-1040.e19. https://doi.org/10.1016/j.cell.2017.02.027
Triandafillou CG, Katanski CD, Dinner AR, Allan Drummond D (2020) Transient intracellular acidification regulates the core transcriptional heat shock response. Elife 9:e54880. https://doi.org/10.7554/eLife.54880
Pauli D, Arrigo A, Tissires A (1992) Heat shock response in Drosophila. Experientia 48:623–629. https://doi.org/10.1007/BF02118306
Zhong M, Kim SJ, Wu C (1999) Sensitivity of Drosophila heat shock transcription factor to low pH. J Biol Chem 274(5):3135–3140. https://doi.org/10.1074/jbc.274.5.3135
Aickin CC, Thomas RC (1977) An investigation of the ionic mechanism of intracellular pH regulation in mouse soleus muscle fibres. J Physiol 273:295–316. https://doi.org/10.1113/jphysiol.1977.sp012095
Burdon RH, Cutmore CMM (1982) Human heat shock gene expression and the modulation of plasma membrane Na+, K+-ATPase activity. FEBS Lett 140(1):45–48. https://doi.org/10.1016/0014-5793(82)80517-6
Kiang JG, McKinney LC, Gallin EK (1990) Heat induces intracellular acidification in human A-431 cells: role of Na+-H+ exchange and metabolism. Am J Physiol 259(5 Pt 1):C727–C737. https://doi.org/10.1152/ajpcell.1990.259.5.c727
Lamarche S, Chretien P, Landry J (1985) Inhibition of the heat shock response and synthesis of glucose-regulated proteins in Ca2+-deprived rat hepatoma cells. Biochem Biophys Res Commun 131(2):868–876. https://doi.org/10.1016/0006-291x(85)91320-8
Lepock JR, Cheng KH, Al-Qysi H, Kruuv J (1983) Thermotropic lipid and protein transitions in chinese hamster lung cell membranes: relationship to hyperthermic cell killing. Can J Biochem Cell Biol 61(6):421–427. https://doi.org/10.1139/o83-057
Dechant R, Binda M, Lee SS, Pelet S, Winderickx J, Peter M (2010) Cytosolic pH is a second messenger for glucose and regulates the PKA pathway through V-ATPase. EMBO J 29(15):2515–2526. https://doi.org/10.1038/emboj.2010.138
Franzmann TM, Jahnel M, Pozniakovsky A, Mahamid J, Holehouse AS, Nüske E, Richter D, Baumeister W, Grill SW, Pappu RV, Hyman AA, Alberti S (2018) Phase separation of a yeast prion protein promotes cellular fitness. Science 359(6371):eaao5654. https://doi.org/10.1126/science.aao5654
Orij R, Urbanus ML, Vizeacoumar FJ, Giaever G, Boone C, Nislow C, Brul S, Smits GJ (2012) Genome-wide analysis of intracellular pH reveals quantitative control of cell division rate by pHc in Saccharomyces cerevisiae. Genome Biol 13(9):R80. https://doi.org/10.1186/gb-2012-13-9-r80
Orij R, Postmus J, Beek AT, Brul S, Smits GJ (2009) In vivo measurement of cytosolic and mitochondrial pH using a pH-sensitive GFP derivative in Saccharomyces cerevisiae reveals a relation between intracellular pH and growth. Microbiology (Reading) 155(1):268–278. https://doi.org/10.1099/mic.0.022038-0
Petrovska I, Nüske E, Munder MC, Kulasegaran G, Malinovska L, Kroschwald S, Richter D, Fahmy K, Gibson K, Verbavatz JM, Alberti S (2014) Filament formation by metabolic enzymes is a specific adaptation to an advanced state of cellular starvation. Elife 2014(3):e02409. https://doi.org/10.7554/eLife.02409
Morisawa M, Steinhardt RA (1982) Changes in intracellular pH of Physarum plasmodium during the cell cycle and in response to starvation. Exp Cell Res 140(2):341–351. https://doi.org/10.1016/0014-4827(82)90123-9
Chakraborty S, Winardhi RS, Morgan LK, Yan J, Kenney LJ (2017) Non-canonical activation of OmpR drives acid and osmotic stress responses in single bacterial cells. Nat Commun 8(1):1587. https://doi.org/10.1038/s41467-017-02030-0
Fang W, Siegumfeldt H, Budde BB, Jakobsen M (2004) Osmotic stress leads to decreased intracellular pH of Listeria monocytogenes as determined by fluorescence ratio-imaging microscopy. Appl Environ Microbiol 70(5):3176–3179. https://doi.org/10.1128/AEM.70.5.3176-3179.2004
Csonka LN (1989) Physiological and genetic responses of bacteria to osmotic stress. Microbiol Rev 53(1):121–147. https://doi.org/10.1128/mmbr.53.1.121-147.1989
Dinnbier U, Limpinsel E, Schmid R, Bakker EP (1988) Transient accumulation of potassium glutamate and its replacement by trehalose during adaptation of growing cells of Escherichia coli K-12 to elevated sodium chloride concentrations. Arch Microbiol 150(4):348–357. https://doi.org/10.1007/BF00408306
Castle AM, Macnab RM, Shulman RG (1986) Coupling between the sodium and proton gradients in respiring Escherichia coli cells measured by 23Na and 31P nuclear magnetic resonance. J Biol Chem 261(17):7797–7806. https://doi.org/10.1016/s0021-9258(19)57471-3
Pintsch T, Satre M, Klein G, Martin JB, Schuster SC (2001) Cytosolic acidification as a signal mediating hyperosmotic stress responses in Dictyostelium discoideum. BMC Cell Biol 2(1):1–15. https://doi.org/10.1186/1471-2121-2-9
Zischka H, Oehme F, Pintsch T, Ott A, Keller H, Kellermann J, Schuster SC (1999) Rearrangement of cortex proteins constitutes an osmoprotective mechanism in Dictyostelium. EMBO J 18(15):4241–4249. https://doi.org/10.1093/emboj/18.15.4241
Oyama M, Kubota K (1997) H+ secretion induced by hypertonic stress in the cellular slime mold Dictyostelium discoideum. J Biochem 122(1):64–70. https://doi.org/10.1093/oxfordjournals.jbchem.a021741
Bracey D, Holyoak CD, Nebe-Von Caron G, Coote PJ (1998) Determination of the intracellular pH (pHi) of growing cells of Saccharomyces cerevisiae: the effect of reduced-expression of the membrane H+-ATPase. J Microbiol Methods 31(3):113–125. https://doi.org/10.1016/S0167-7012(97)00095-X
Cole MB, Keenan MHJ (1987) Effects of weak acids and external pH on the intracellular pH of Zygosaccharomyces bailii, and its implications in weak-acid resistance. Yeast 3(1):23–32. https://doi.org/10.1002/yea.320030105
Salmond CV, Kroll RG, Booth IR (1984) The effect of food preservatives on pH homeostasis in Escherichia coli. J Gen Microbiol 130(11):2845–2850. https://doi.org/10.1099/00221287-130-11-2845
Yao H, Haddad GG (2004) Calcium and pH homeostasis in neurons during hypoxia and ischemia. Cell Calcium 36(3–4):247–255. https://doi.org/10.1016/j.ceca.2004.02.013
Roberts JK, Callis J, Wemmer D, Walbot V, Jardetzky O (1984) Mechanisms of cytoplasmic pH regulation in hypoxic maize root tips and its role in survival under hypoxia. Proc Natl Acad Sci USA 81(11):3379–3383. https://doi.org/10.1073/pnas.81.11.3379
Davies DD (1980) Anaerobic metabolism and the production of organic acids. Metab Resp 2:581–611. https://doi.org/10.1016/b978-0-12-675402-5.50020-9
Leão C, Van Uden N (1984) Effects of ethanol and other alkanols on passive proton influx in the yeast Saccharomyces cerevisiae. Biochim Biophys Acta 774(1):43–48. https://doi.org/10.1016/0005-2736(84)90272-4
Li GC, Shiu EC, Hahn GM (1980) Similarities in cellular inactivation by hyperthermia or by ethanol. Radiat Res 82(2):257–268. https://doi.org/10.2307/3575377
He DY, Yazaki Y, Nishizawa Y, Takai R, Yamada K, Sakano K, Shibuya N, Minami E (1998) Gene activation by cytoplasmic acidification in suspension-cultured rice cells in response to the potent elicitor, N-acetylchitoheptaose. Mol Plant Microbe Interact 11(12):1167–1174. https://doi.org/10.1094/MPMI.1998.11.12.1167
Hansen UP, Moldaenke C, Tabrizi H, Ramm D (1993) The effect of transthylakoid proton uptake on cytosolic pH and the imbalance of ATP and NAPDH/H+ production as measured by CO2- and light-induced depolarisation of the plasmalemma. Plant Cell Physiol 34(5):681–695. https://doi.org/10.1093/oxfordjournals.pcp.a078471
Hughes AL, Gottschling DE (2012) An early age increase in vacuolar pH limits mitochondrial function and lifespan in yeast. Nature 492(7428):261–265. https://doi.org/10.1038/nature11654
van Schalkwyk DA, Saliba KJ, Biagini GA, Bray PG, Kirk K (2013) Loss of pH control in Plasmodium falciparum parasites subjected to oxidative stress. PLoS ONE 8(3):e58933. https://doi.org/10.1371/journal.pone.0058933
Wang Y, Floor E (1998) Hydrogen peroxide inhibits the vacuolar H+-ATPase in brain synaptic vesicles at micromolar concentrations. J Neurochem 70(2):646–652. https://doi.org/10.1046/j.1471-4159.1998.70020646.x
Vihervaara A, Sistonen L (2014) HSF1 at a glance. J Cell Sci 127(Pt 2):261–266. https://doi.org/10.1242/jcs.132605
Cabrera M, Boronat S, Marte L, Vega M, Perez P, Ayte J, Hidalgo E (2020) Chaperone-facilitated aggregation of thermo-sensitive proteins shields them from degradation during heat stress. Cell Rep 30(7):2430-2443.e4. https://doi.org/10.1016/j.celrep.2020.01.077
Kim JH, Park SJ, Kim TS, Park HJ, Park J, Kim BK, Kim GR, Kim JM, Huang SM, Chae JI, Park CK, Lee DS (2013) Testicular hyperthermia induces unfolded protein response signaling activation in spermatocyte. Biochem Biophys Res Commun 434(4):861–866. https://doi.org/10.1016/j.bbrc.2013.04.032
Mizusawa M, Sharmin MM, Yonekura S (2019) Mild heat stress induces transcription of the beta-casein gene via unfolded protein response-activated XBP1 signaling in undifferentiated mammary epithelial cells. Anim Sci J 90(8):1026–1032. https://doi.org/10.1111/asj.13246
Maxwell BA, Gwon Y, Mishra A, Peng J, Nakamura H, Zhang K, Kim HJ, Taylor JP (2021) Ubiquitination is essential for recovery of cellular activities after heat shock. Science 372(6549):eabc3593. https://doi.org/10.1126/science.abc3593
Carlson N, Rogers S, Rechsteiner M (1987) Microinjection of ubiquitin: changes in protein degradation in HeLa cells subjected to heat-shock. J Cell Biol 104(3):547–555. https://doi.org/10.1083/jcb.104.3.547
Medicherla B, Goldberg AL (2008) Heat shock and oxygen radicals stimulate ubiquitin-dependent degradation mainly of newly synthesized proteins. J Cell Biol 182(4):663–673. https://doi.org/10.1083/jcb.200803022
Parag HA, Raboy B, Kulka RG (1987) Effect of heat shock on protein degradation in mammalian cells: involvement of the ubiquitin system. EMBO J 6(1):55–61. https://doi.org/10.1002/j.1460-2075.1987.tb04718.x
Dokladny K, Zuhl MN, Mandell M, Bhattacharya D, Schneider S, Deretic V, Moseley PL (2013) Regulatory coordination between two major intracellular homeostatic systems: heat shock response and autophagy. J Biol Chem 288(21):14959–14972. https://doi.org/10.1074/jbc.M113.462408
Zhao Y, Gong S, Shunmei E, Zou J (2009) Induction of macroautophagy by heat. Mol Biol Rep 36(8):2323–2327. https://doi.org/10.1007/s11033-009-9451-4
Hsu SF, Chao CM, Huang WT, Lin MT, Cheng BC (2013) Attenuating heat-induced cellular autophagy, apoptosis and damage in H9c2 cardiomyocytes by pre-inducing HSP70 with heat shock preconditioning. Int J Hyperthermia 29(3):239–247. https://doi.org/10.3109/02656736.2013.777853
Zhang M, Jiang M, Bi Y, Zhu H, Zhou Z, Sha J (2012) Autophagy and apoptosis act as partners to induce germ cell death after heat stress in mice. PLoS ONE 7(7):e41412. https://doi.org/10.1371/journal.pone.0041412
Nivon M, Richet E, Codogno P, Arrigo AP, Kretz-Remy C (2009) Autophagy activation by NFkappaB is essential for cell survival after heat shock. Autophagy 5(6):766–783. https://doi.org/10.4161/auto.8788
Weitzel G, Pilatus U, Rensing L (1985) Similar dose response of heat shock protein synthesis and intracellular pH change in yeast. Exp Cell Res 159(1):252–256. https://doi.org/10.1016/s0014-4827(85)80054-9
Tombaugh GC, Sapolsky RM (1993) Evolving concepts about the role of acidosis in ischemic neuropathology. J Neurochem 61(3):793–803. https://doi.org/10.1111/j.1471-4159.1993.tb03589.x
Joyner RP, Tang JH, Helenius J, Dultz E, Brune C, Holt LJ, Huet S, Muller DJ, Weis K (2016) A glucose-starvation response regulates the diffusion of macromolecules. Elife 5:e09376. https://doi.org/10.7554/eLife.09376
Jayaraj GG, Hipp MS, Hartl FU (2020) Functional modules of the proteostasis network. Cold Spring Harb Perspect Biol 12(1):a033951. https://doi.org/10.1101/cshperspect.a033951
Iserman C, Desroches Altamirano C, Jegers C, Friedrich U, Zarin T, Fritsch AW, Mittasch M, Domingues A, Hersemann L, Jahnel M, Richter D, Guenther UP, Hentze MW, Moses AM, Hyman AA, Kramer G, Kreysing M, Franzmann TM, Alberti S (2020) Condensation of Ded1p promotes a translational switch from housekeeping to stress protein production. Cell 181(4):818-831.e19. https://doi.org/10.1016/j.cell.2020.04.009
Kroschwald S, Munder MC, Maharana S, Franzmann TM, Richter D, Ruer M, Hyman AA, Alberti S (2018) Different material states of Pub1 condensates define distinct modes of stress adaptation and recovery. Cell Rep 23(11):3327–3339. https://doi.org/10.1016/j.celrep.2018.05.041
Yang P, Mathieu C, Kolaitis RM, Zhang P, Messing J, Yurtsever U, Yang Z, Wu J, Li Y, Pan Q, Yu J, Martin EW, Mittag T, Kim HJ, Taylor JP (2020) G3BP1 is a tunable switch that triggers phase separation to assemble stress granules. Cell 181(2):325-345.e28. https://doi.org/10.1016/j.cell.2020.03.046
Piper PW (1993) Molecular events associated with acquisition of heat tolerance by the yeast Saccharomyces cerevisiae. FEMS Microbiol Rev 11(4):339–355. https://doi.org/10.1111/j.1574-6976.1993.tb00005.x
Panaretou B, Piper PW (1990) Plasma-membrane ATPase action affects several stress tolerances of Saccharomyces cerevisiae and Schizosaccharomyces pombe as well as the extent and duration of the heat shock response. J Gen Microbiol 136:1763–1770. https://doi.org/10.1099/00221287-136-9-1763
Morsomme P, Slayman CW, Goffeau A (2000) Mutagenic study of the structure, function and biogenesis of the yeast plasma membrane H+-ATPase. Biochim Biophys Acta 1469(3):133–157. https://doi.org/10.1016/s0304-4157(00)00015-0
Perlin DS, San Francisco MJ, Slayman CW, Rosen BP (1986) H+/ATP stoichiometry of proton pumps from Neurospora crassa and Escherichia coli. Arch Biochem Biophys 248(1):53–61. https://doi.org/10.1016/0003-9861(86)90400-5
Lecchi S, Nelson CJ, Allen KE, Swaney DL, Thompson KL, Coon JJ, Sussman MR, Slayman CW (2007) Tandem phosphorylation of Ser-911 and Thr-912 at the C terminus of yeast plasma membrane H+-ATPase leads to glucose-dependent activation. J Biol Chem 282(49):35471–35481. https://doi.org/10.1074/jbc.M706094200
Martinez-Munoz GA, Kane P (2008) Vacuolar and plasma membrane proton pumps collaborate to achieve cytosolic pH homeostasis in yeast. J Biol Chem 283(29):20309–20319. https://doi.org/10.1074/jbc.M710470200
Perzov N, Nelson H, Nelson N (2000) Altered distribution of the yeast plasma membrane H+-ATPase as a feature of vacuolar H+-ATPase null mutants. J Biol Chem 275(51):40088–40095. https://doi.org/10.1074/jbc.M007011200
Parra KJ, Kane PM (1998) Reversible association between the V1 and V0 domains of yeast vacuolar H+-ATPase is an unconventional glucose-induced effect. Mol Cell Biol 18(12):7064–7074. https://doi.org/10.1128/MCB.18.12.7064
Alexandrov AI, Grosfeld EV, Dergalev AA, Kushnirov VV, Chuprov-Netochin RN, Tyurin-Kuzmin PA, Kireev II, Ter-Avanesyan MD, Leonov SV, Agaphonov MO (2019) Analysis of novel hyperosmotic shock response suggests “beads in liquid” cytosol structure. Biol Open 8(7):bio044529. https://doi.org/10.1242/bio.044529
Miermont A, Waharte F, Hu S, McClean MN, Bottani S, Leon S, Hersen P (2013) Severe osmotic compression triggers a slowdown of intracellular signaling, which can be explained by molecular crowding. Proc Natl Acad Sci USA 110(14):5725–5730. https://doi.org/10.1073/pnas.1215367110
Brocker C, Thompson DC, Vasiliou V (2012) The role of hyperosmotic stress in inflammation and disease. Biomol Concepts 3(4):345–364. https://doi.org/10.1515/bmc-2012-0001
Hohmann S (2002) Osmotic stress signaling and osmoadaptation in yeasts. Microbiol Mol Biol Rev 66(2):300–372. https://doi.org/10.1128/MMBR.66.2.300-372.2002
Shank BB, Rosenberg HM, Horowitz C (1973) Ionic basis of volume regulation in mammalian cells following osmotic shock. J Cell Physiol 82(2):257–265. https://doi.org/10.1002/jcp.1040820214
Wood JM (2015) Bacterial responses to osmotic challenges. J Gen Physiol 145(5):381–388. https://doi.org/10.1085/jgp.201411296
Eveloff JL, Warnock DG (1987) Activation of ion transport systems during cell volume regulation. Am J Physiol 252(1 Pt 2):F1-10. https://doi.org/10.1152/ajprenal.1987.252.1.F1
Christensen O, Hoffmann EK (1992) Cell swelling activates K+ and Cl- channels as well as nonselective, stretch-activated cation channels in Ehrlich ascites tumor cells. J Membr Biol 129(1):13–36. https://doi.org/10.1007/BF00232052
Dvornik E, Simard-Duquesne N, Krami M, Sestanj K, Gabbay KH, Kinoshita JH, Varma SD, Merola LO (1973) Polyol accumulation in galactosemic and diabetic rats: control by an aldose reductase inhibitor. Science 182(4117):1146–1148. https://doi.org/10.1126/science.182.4117.1146
Andrew RD (1991) Seizure and acute osmotic change: clinical and neurophysiological aspects. J Neurol Sci 101(1):7–18. https://doi.org/10.1016/0022-510x(91)90013-w
Neuhofer W (2010) Role of NFAT5 in inflammatory disorders associated with osmotic stress. Curr Genom 11(8):584–590. https://doi.org/10.2174/138920210793360961
Palevsky PM, Bhagrath R, Greenberg A (1996) Hypernatremia in hospitalized patients. Ann Intern Med 124(2):197–203. https://doi.org/10.7326/0003-4819-124-2-199601150-00002
Prevarskaya N, Skryma R, Shuba Y (2010) Ion channels and the hallmarks of cancer. Trends Mol Med 16(3):107–121. https://doi.org/10.1016/j.molmed.2010.01.005
Fiske JL, Fomin VP, Brown ML, Duncan RL, Sikes RA (2006) Voltage-sensitive ion channels and cancer. Cancer Metastasis Rev 25(3):493–500. https://doi.org/10.1007/s10555-006-9017-z
Lian I, Kim J, Okazawa H, Zhao J, Zhao B, Yu J, Chinnaiyan A, Israel MA, Goldstein LS, Abujarour R, Ding S, Guan KL (2010) The role of YAP transcription coactivator in regulating stem cell self-renewal and differentiation. Genes Dev 24(11):1106–1118. https://doi.org/10.1101/gad.1903310
Zanconato F, Cordenonsi M, Piccolo S (2019) YAP and TAZ: a signalling hub of the tumour microenvironment. Nat Rev Cancer 19(8):454–464. https://doi.org/10.1038/s41568-019-0168-y
Cai D, Feliciano D, Dong P, Flores E, Gruebele M, Porat-Shliom N, Sukenik S, Liu Z, Lippincott-Schwartz J (2019) Phase separation of YAP reorganizes genome topology for long-term YAP target gene expression. Nat Cell Biol 21(12):1578–1589. https://doi.org/10.1038/s41556-019-0433-z
Cowen LE, Hodak SP, Verbalis JG (2013) Age-associated abnormalities of water homeostasis. Endocrinol Metab Clin N Am 42(2):349–370. https://doi.org/10.1016/j.ecl.2013.02.005
Terzian C, Frye EB, Piotrowski ZH (1994) Admission hyponatremia in the elderly: factors influencing prognosis. J Gen Intern Med 9(2):89–91. https://doi.org/10.1007/BF02600208
Hou Y, Dan X, Babbar M, Wei Y, Hasselbalch SG, Croteau DL, Bohr VA (2019) Ageing as a risk factor for neurodegenerative disease. Nat Rev Neurol 15(10):565–581. https://doi.org/10.1038/s41582-019-0244-7
Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G (2013) The hallmarks of aging. Cell 153(6):1194–1217. https://doi.org/10.1016/j.cell.2013.05.039
Chesler M (2003) Regulation and modulation of pH in the brain. Physiol Rev 83(4):1183–1221. https://doi.org/10.1152/physrev.00010.2003
Sinning A, Hubner CA (2013) Minireview: pH and synaptic transmission. FEBS Lett 587(13):1923–1928. https://doi.org/10.1016/j.febslet.2013.04.045
Xiang Z, Bergold PJ (2000) Synaptic depression and neuronal loss in transiently acidic hippocampal slice cultures. Brain Res 881(1):77–87. https://doi.org/10.1016/s0006-8993(00)02795-5
Lee BK, Jung YS (2017) Sustained intracellular acidosis triggers the Na(+)/H(+) exchager-1 activation in glutamate excitotoxicity. Biomol Ther (Seoul) 25(6):593–598. https://doi.org/10.4062/biomolther.2017.018
Hamakawa H, Murashita J, Yamada N, Inubushi T, Kato N, Kato T (2004) Reduced intracellular pH in the basal ganglia and whole brain measured by 31P-MRS in bipolar disorder. Psychiatry Clin Neurosci 58(1):82–88. https://doi.org/10.1111/j.1440-1819.2004.01197.x
Mandal PK, Akolkar H, Tripathi M (2012) Mapping of hippocampal pH and neurochemicals from in vivo multi-voxel 31P study in healthy normal young male/female, mild cognitive impairment, and Alzheimer’s disease. J Alzheimers Dis 31(Suppl 3):S75-86. https://doi.org/10.3233/JAD-2012-120166
Roberts EL Jr, Chih CP (1997) The influence of age of pH regulation in hippocampal slices before, during, and after anoxia. J Cereb Blood Flow Metab 17(5):560–566. https://doi.org/10.1097/00004647-199705000-00010
Forester BP, Berlow YA, Harper DG, Jensen JE, Lange N, Froimowitz MP, Ravichandran C, Iosifescu DV, Lukas SE, Renshaw PF, Cohen BM (2010) Age-related changes in brain energetics and phospholipid metabolism. NMR Biomed 23(3):242–250. https://doi.org/10.1002/nbm.1444
Tyrtyshnaia AA, Lysenko LV, Madamba F, Manzhulo IV, Khotimchenko MY, Kleschevnikov AM (2016) Acute neuroinflammation provokes intracellular acidification in mouse hippocampus. J Neuroinflammation 13(1):283. https://doi.org/10.1186/s12974-016-0747-8
Roberts EL Jr, Chih CP (1998) The pH buffering capacity of hippocampal slices from young adult and aged rats. Brain Res 779(1–2):271–275. https://doi.org/10.1016/s0006-8993(97)01120-7
Bonnet U, Bingmann D, Speckmann EJ, Wiemann M (2018) Aging is associated with a mild acidification in neocortical human neurons in vitro. J Neural Transm (Vienna) 125(10):1495–1501. https://doi.org/10.1007/s00702-018-1904-2
Ruffin VA, Salameh AI, Boron WF, Parker MD (2014) Intracellular pH regulation by acid-base transporters in mammalian neurons. Front Physiol 5:43. https://doi.org/10.3389/fphys.2014.00043
Raffin CN, Sick TJ, Rosenthal M (1988) Inhibition of glycolysis alters potassium ion transport and mitochondrial redox activity in rat brain. J Cereb Blood Flow Metab 8(6):857–865. https://doi.org/10.1038/jcbfm.1988.143
Bonnet U, Bingmann D, Wiltfang J, Scherbaum N, Wiemann M (2010) Modulatory effects of neuropsychopharmaca on intracellular pH of hippocampal neurones in vitro. Br J Pharmacol 159(2):474–483. https://doi.org/10.1111/j.1476-5381.2009.00540.x
Baram TZ, Eghbal-Ahmadi M, Bender RA (2002) Is neuronal death required for seizure-induced epileptogenesis in the immature brain? Prog Brain Res 135:365–375. https://doi.org/10.1016/S0079-6123(02)35033-7
Roberts EL Jr, Rosenthal M, Sick TJ (1990) Age-related modifications of potassium homeostasis and synaptic transmission during and after anoxia in rat hippocampal slices. Brain Res 514(1):111–118. https://doi.org/10.1016/0006-8993(90)90441-d
Yao H, Sadoshima S, Ooboshi H, Sato Y, Uchimura H, Fujishima M (1991) Age-related vulnerability to cerebral ischemia in spontaneously hypertensive rats. Stroke 22(11):1414–1418. https://doi.org/10.1161/01.str.22.11.1414
Colacurcio DJ, Nixon RA (2016) Disorders of lysosomal acidification-the emerging role of v-ATPase in aging and neurodegenerative disease. Ageing Res Rev 32:75–88. https://doi.org/10.1016/j.arr.2016.05.004
Ruckenstuhl C, Netzberger C, Entfellner I, Carmona-Gutierrez D, Kickenweiz T, Stekovic S, Gleixner C, Schmid C, Klug L, Sorgo AG, Eisenberg T, Buttner S, Marino G, Koziel R, Jansen-Durr P, Frohlich KU, Kroemer G, Madeo F (2014) Lifespan extension by methionine restriction requires autophagy-dependent vacuolar acidification. PLoS Genet 10(5):e1004347. https://doi.org/10.1371/journal.pgen.1004347
Molin M, Demir AB (2014) Linking peroxiredoxin and vacuolar-ATPase functions in calorie restriction-mediated life span extension. Int J Cell Biol 2014:913071. https://doi.org/10.1155/2014/913071
Di Domenico F, Perluigi M, Butterfield DA, Cornelius C, Calabrese V (2010) Oxidative damage in rat brain during aging: interplay between energy and metabolic key target proteins. Neurochem Res 35(12):2184–2192. https://doi.org/10.1007/s11064-010-0295-z
Fujisawa Y, Kato K, Giulivi C (2009) Nitration of tyrosine residues 368 and 345 in the β-subunit elicits FoF1-ATPase activity loss. Biochem J 423(2):219–231. https://doi.org/10.1042/BJ20090594
Haynes V, Traaseth NJ, Elfering S, Fujisawa Y, Giulivi C (2010) Nitration of specific tyrosines in FoF1 ATP synthase and activity loss in aging. Am J Physiol Endocrinol Metab 298(5):E978–E987. https://doi.org/10.1152/ajpendo.00739.2009
Ginsberg SD, Alldred MJ, Counts SE, Cataldo AM, Neve RL, Jiang Y, Wuu J, Chao MV, Mufson EJ, Nixon RA, Che S (2010) Microarray analysis of hippocampal CA1 neurons implicates early endosomal dysfunction during Alzheimer’s disease progression. Biol Psychiatry 68(10):885–893. https://doi.org/10.1016/j.biopsych.2010.05.030
Taylor RC, Dillin A (2011) Aging as an event of proteostasis collapse. Cold Spring Harb Perspect Biol 3(5):a004440. https://doi.org/10.1101/cshperspect.a004440
Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, Bruce J, Schuck T, Grossman M, Clark CM, McCluskey LF, Miller BL, Masliah E, Mackenzie IR, Feldman H, Feiden W, Kretzschmar HA, Trojanowski JQ, Lee VM (2006) Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 314(5796):130–133. https://doi.org/10.1126/science.1134108
Neumann M, Bentmann E, Dormann D, Jawaid A, DeJesus-Hernandez M, Ansorge O, Roeber S, Kretzschmar HA, Munoz DG, Kusaka H, Yokota O, Ang LC, Bilbao J, Rademakers R, Haass C, Mackenzie IR (2011) FET proteins TAF15 and EWS are selective markers that distinguish FTLD with FUS pathology from amyotrophic lateral sclerosis with FUS mutations. Brain 134(Pt 9):2595–2609. https://doi.org/10.1093/brain/awr201
Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M (1997) Alpha-synuclein in Lewy bodies. Nature 388(6645):839–840. https://doi.org/10.1038/42166
Grundke-Iqbal I, Iqbal K, Quinlan M, Tung YC, Zaidi MS, Wisniewski HM (1986) Microtubule-associated protein tau. A component of Alzheimer paired helical filaments. J Biol Chem 261(13):6084–6089. https://doi.org/10.1016/S0021-9258(17)38495-8
Zbinden A, Perez-Berlanga M, De Rossi P, Polymenidou M (2020) Phase separation and neurodegenerative diseases: a disturbance in the force. Dev Cell 55(1):45–68. https://doi.org/10.1016/j.devcel.2020.09.014
Ray S, Singh N, Kumar R, Patel K, Pandey S, Datta D, Mahato J, Panigrahi R, Navalkar A, Mehra S, Gadhe L, Chatterjee D, Sawner AS, Maiti S, Bhatia S, Gerez JA, Chowdhury A, Kumar A, Padinhateeri R, Riek R, Krishnamoorthy G, Maji SK (2020) α-Synuclein aggregation nucleates through liquid–liquid phase separation. Nat Chem 12(8):705–716. https://doi.org/10.1038/s41557-020-0465-9
Bouchard JJ, Otero JH, Scott DC, Szulc E, Martin EW, Sabri N, Granata D, Marzahn MR, Lindorff-Larsen K, Salvatella X, Schulman BA, Mittag T (2018) Cancer mutations of the tumor suppressor SPOP disrupt the formation of active, phase-separated compartments. Mol Cell 72(1):19-36.e8. https://doi.org/10.1016/j.molcel.2018.08.027
Kilic S, Lezaja A, Gatti M, Bianco E, Michelena J, Imhof R, Altmeyer M (2019) Phase separation of 53BP1 determines liquid-like behavior of DNA repair compartments. EMBO J 38(16):e101379. https://doi.org/10.15252/embj.2018101379
Altmeyer M, Neelsen KJ, Teloni F, Pozdnyakova I, Pellegrino S, Grofte M, Rask MD, Streicher W, Jungmichel S, Nielsen ML, Lukas J (2015) Liquid demixing of intrinsically disordered proteins is seeded by poly(ADP-ribose). Nat Commun 6:8088. https://doi.org/10.1038/ncomms9088
Boulay G, Sandoval GJ, Riggi N, Iyer S, Buisson R, Naigles B, Awad ME, Rengarajan S, Volorio A, McBride MJ, Broye LC, Zou L, Stamenkovic I, Kadoch C, Rivera MN (2017) Cancer-specific retargeting of BAF complexes by a prion-like domain. Cell 171(1):163-178.e19. https://doi.org/10.1016/j.cell.2017.07.036
Zamudio AV, Dall’Agnese A, Henninger JE, Manteiga JC, Afeyan LK, Hannett NM, Coffey EL, Li CH, Oksuz O, Sabari BR, Boija A, Klein IA, Hawken SW, Spille JH, Decker TM, Cisse II, Abraham BJ, Lee TI, Taatjes DJ, Schuijers J, Young RA (2019) Mediator condensates localize signaling factors to key cell identity genes. Mol Cell 76(5):753-766.e6. https://doi.org/10.1016/j.molcel.2019.08.016
Lu Y, Wu T, Gutman O, Lu H, Zhou Q, Henis YI, Luo K (2020) Phase separation of TAZ compartmentalizes the transcription machinery to promote gene expression. Nat Cell Biol 22(4):453–464. https://doi.org/10.1038/s41556-020-0485-0
Arimoto K, Fukuda H, Imajoh-Ohmi S, Saito H, Takekawa M (2008) Formation of stress granules inhibits apoptosis by suppressing stress-responsive MAPK pathways. Nat Cell Biol 10(11):1324–1332. https://doi.org/10.1038/ncb1791
Thedieck K, Holzwarth B, Prentzell MT, Boehlke C, Klasener K, Ruf S, Sonntag AG, Maerz L, Grellscheid SN, Kremmer E, Nitschke R, Kuehn EW, Jonker JW, Groen AK, Reth M, Hall MN, Baumeister R (2013) Inhibition of mTORC1 by astrin and stress granules prevents apoptosis in cancer cells. Cell 154(4):859–874. https://doi.org/10.1016/j.cell.2013.07.031
Hsu KS, Kao HY (2018) PML: Regulation and multifaceted function beyond tumor suppression. Cell Biosci 8:5. https://doi.org/10.1186/s13578-018-0204-8
Adriaens C, Standaert L, Barra J, Latil M, Verfaillie A, Kalev P, Boeckx B, Wijnhoven PW, Radaelli E, Vermi W, Leucci E, Lapouge G, Beck B, van den Oord J, Nakagawa S, Hirose T, Sablina AA, Lambrechts D, Aerts S, Blanpain C, Marine JC (2016) p53 induces formation of NEAT1 lncRNA-containing paraspeckles that modulate replication stress response and chemosensitivity. Nat Med 22(8):861–868. https://doi.org/10.1038/nm.4135
Audas TE, Audas DE, Jacob MD, Ho JJ, Khacho M, Wang M, Perera JK, Gardiner C, Bennett CA, Head T, Kryvenko ON, Jorda M, Daunert S, Malhotra A, Trinkle-Mulcahy L, Gonzalgo ML, Lee S (2016) Adaptation to stressors by systemic protein amyloidogenesis. Dev Cell 39(2):155–168. https://doi.org/10.1016/j.devcel.2016.09.002
Lane DP (1992) Cancer. p53, guardian of the genome. Nature 358(6381):15–16. https://doi.org/10.1038/358015a0
Levine AJ (1997) p53, the cellular gatekeeper for growth and division. Cell 88(3):323–331. https://doi.org/10.1016/s0092-8674(00)81871-1
Levine AJ, Momand J, Finlay CA (1991) The p53 tumour suppressor gene. Nature 351(6326):453–456. https://doi.org/10.1038/351453a0
Vousden KH, Lu X (2002) Live or let die: the cell’s response to p53. Nat Rev Cancer 2(8):594–604. https://doi.org/10.1038/nrc864
Freed-Pastor WA, Prives C (2012) Mutant p53: one name, many proteins. Genes Dev 26(12):1268–1286. https://doi.org/10.1101/gad.190678.112
Silva JL, De Moura Gallo CV, Costa DC, Rangel LP (2014) Prion-like aggregation of mutant p53 in cancer. Trends Biochem Sci 39(6):260–267. https://doi.org/10.1016/j.tibs.2014.04.001
Petronilho EC, Pedrote MM, Marques MA, Passos YM, Mota MF, Jakobus B, de Sousa GDS, Pereira da Costa F, Felix AL, Ferretti GDS, Almeida FP, Cordeiro Y, Vieira T, de Oliveira GAP, Silva JL (2021) Phase separation of p53 precedes aggregation and is affected by oncogenic mutations and ligands. Chem Sci 12(21):7334–7349. https://doi.org/10.1039/d1sc01739j
Ackerman D, Simon MC (2014) Hypoxia, lipids, and cancer: surviving the harsh tumor microenvironment. Trends Cell Biol 24(8):472–478. https://doi.org/10.1016/j.tcb.2014.06.001
Gillies RJ, Raghunand N, Karczmar GS, Bhujwalla ZM (2002) MRI of the tumor microenvironment. J Magn Reson Imaging 16(4):430–450. https://doi.org/10.1002/jmri.10181
Stuwe L, Muller M, Fabian A, Waning J, Mally S, Noel J, Schwab A, Stock C (2007) pH dependence of melanoma cell migration: protons extruded by NHE1 dominate protons of the bulk solution. J Physiol 585(Pt 2):351–360. https://doi.org/10.1113/jphysiol.2007.145185
Kane PM (2016) Proton transport and pH control in fungi. Adv Exp Med Biol 892:33–68. https://doi.org/10.1007/978-3-319-25304-6_3
Casey JR, Grinstein S, Orlowski J (2010) Sensors and regulators of intracellular pH. Nat Rev Mol Cell Biol 11(1):50–61. https://doi.org/10.1038/nrm2820
Pittman JK (2012) Multiple transport pathways for mediating intracellular pH homeostasis: the contribution of H+/ion exchangers. Front Plant Sci 3:11. https://doi.org/10.3389/fpls.2012.00011
Roos A, Boron WF (1981) Intracellular pH. Physiol Rev 61(2):296–434. https://doi.org/10.1152/physrev.1981.61.2.296
Sakano K (2001) Metabolic regulation of pH in plant cells: role of cytoplasmic pH in defense reaction and secondary metabolism. Int Rev Cytol 206:1–44. https://doi.org/10.1016/s0074-7696(01)06018-1
Davies DD (1986) The fine control of cytosolic pH. Physiol Plant 67(4):702–706. https://doi.org/10.1111/j.1399-3054.1986.tb05081.x
Hurth MA, Suh SJ, Kretzschmar T, Geis T, Bregante M, Gambale F, Martinoia E, Neuhaus HE (2005) Impaired pH homeostasis in Arabidopsis lacking the vacuolar dicarboxylate transporter and analysis of carboxylic acid transport across the tonoplast. Plant Physiol 137(3):901–910. https://doi.org/10.1104/pp.104.058453
Sigler K, Kotyk A, Knotkova A, Opekarova M (1981) Processes involved in the creation of buffering capacity and in substrate-induced proton extrusion in the yeast Saccharomyces cerevisiae. Biochim Biophys Acta 643(3):583–592. https://doi.org/10.1016/0005-2736(81)90354-0
Michenkova M, Taki S, Blosser MC, Hwang HJ, Kowatz T, Moss FJ, Occhipinti R, Qin X, Sen S, Shinn E, Wang D, Zeise BS, Zhao P, Malmstadt N, Vahedi-Faridi A, Tajkhorshid E, Boron WF (2021) Carbon dioxide transport across membranes. Interface Focus 11(2):20200090. https://doi.org/10.1098/rsfs.2020.0090
Kane PM (2006) The where, when, and how of organelle acidification by the yeast vacuolar H+-ATPase. Microbiol Mol Biol Rev 70(1):177–191. https://doi.org/10.1128/MMBR.70.1.177-191.2006
Kobayashi H, Suzuki T, Unemoto T (1986) Streptococcal cytoplasmic pH is regulated by changes in amount and activity of a proton-translocating ATPase. J Biol Chem 261(2):627–630. https://doi.org/10.1016/s0021-9258(17)36138-0
Kobayashi H (2003) Computer simulation of cytoplasmic pH regulation mediated by the F-type H+-ATPase. Biochim Biophys Acta 1607(2–3):211–216. https://doi.org/10.1016/j.bbabio.2003.10.001
Cordat E, Casey JR (2009) Bicarbonate transport in cell physiology and disease. Biochem J 417(2):423–439. https://doi.org/10.1042/BJ20081634
Gotz R, Gnann A, Zimmermann FK (1999) Deletion of the carbonic anhydrase-like gene NCE103 of the yeast Saccharomyces cerevisiae causes an oxygen-sensitive growth defect. Yeast 15(10A):855–864. https://doi.org/10.1002/(SICI)1097-0061(199907)15:10A%3c855::AID-YEA425%3e3.0.CO;2-C
Gawenis LR, Ledoussal C, Judd LM, Prasad V, Alper SL, Stuart-Tilley A, Woo AL, Grisham C, Sanford LP, Doetschman T, Miller ML, Shull GE (2004) Mice with a targeted disruption of the AE2 Cl-/HCO3- exchanger are achlorhydric. J Biol Chem 279(29):30531–30539. https://doi.org/10.1074/jbc.M403779200
Guenther UP, Weinberg DE, Zubradt MM, Tedeschi FA, Stawicki BN, Zagore LL, Brar GA, Licatalosi DD, Bartel DP, Weissman JS, Jankowsky E (2018) The helicase Ded1p controls use of near-cognate translation initiation codons in 5′ UTRs. Nature 559(7712):130–134. https://doi.org/10.1038/s41586-018-0258-0
Sen ND, Zhou F, Ingolia NT, Hinnebusch AG (2015) Genome-wide analysis of translational efficiency reveals distinct but overlapping functions of yeast DEAD-box RNA helicases Ded1 and eIF4A. Genome Res 25(8):1196–1205. https://doi.org/10.1101/gr.191601.115
Hilliker A, Gao Z, Jankowsky E, Parker R (2011) The DEAD-box protein Ded1 modulates translation by the formation and resolution of an eIF4F-mRNA complex. Mol Cell 43(6):962–972. https://doi.org/10.1016/j.molcel.2011.08.008
Kroschwald S, Alberti S (2017) Gel or die: phase separation as a survival strategy. Cell 168(6):947–948. https://doi.org/10.1016/j.cell.2017.02.029
Yao G, Chiang Y-C, Zhang C, Lee DJ, Laue TM, Denis CL (2007) PAB1 self-association precludes its binding to poly(A), thereby accelerating CCR4 deadenylation in vivo. Mol Cell Biol 27(17):6243–6253. https://doi.org/10.1128/mcb.00734-07
Alberti S, Halfmann R, King O, Kapila A, Lindquist S (2009) A systematic survey identifies prions and illuminates sequence features of prionogenic proteins. Cell 137(1):146–158. https://doi.org/10.1016/j.cell.2009.02.044
Hondele M, Sachdev R, Heinrich S, Wang J, Vallotton P, Fontoura BMA, Weis K (2019) DEAD-box ATPases are global regulators of phase-separated organelles. Nature 573(7772):144–148. https://doi.org/10.1038/s41586-019-1502-y
Nott TJ, Petsalaki E, Farber P, Jervis D, Fussner E, Plochowietz A, Craggs TD, Bazett-Jones DP, Pawson T, Forman-Kay JD, Baldwin AJ (2015) Phase transition of a disordered nuage protein generates environmentally responsive membraneless organelles. Mol Cell 57(5):936–947. https://doi.org/10.1016/j.molcel.2015.01.013
Linder P, Jankowsky E (2011) From unwinding to clamping—the DEAD box RNA helicase family. Nat Rev Mol Cell Biol 12(8):505–516. https://doi.org/10.1038/nrm3154
Prouteau M, Loewith R (2018) Regulation of cellular metabolism through phase separation of enzymes. Biomolecules 8(4):160. https://doi.org/10.3390/biom8040160
Shen QJ, Kassim H, Huang Y, Li H, Zhang J, Li G, Wang PY, Yan J, Ye F, Liu JL (2016) Filamentation of metabolic enzymes in Saccharomyces cerevisiae. J Genet Genomics 43(6):393–404. https://doi.org/10.1016/j.jgg.2016.03.008
Narayanaswamy R, Levy M, Tsechansky M, Stovall GM, O’Connell JD, Mirrielees J, Ellington AD, Marcotte EM (2009) Widespread reorganization of metabolic enzymes into reversible assemblies upon nutrient starvation. Proc Natl Acad Sci USA 106(25):10147–10152. https://doi.org/10.1073/pnas.0812771106
Stansfield I, Jones KM, Kushnirov VV, Dagkesamanskaya AR, Poznyakovski AI, Paushkin SV, Nierras CR, Cox BS, Ter-Avanesyan MD, Tuite MF (1995) The products of the SUP45 (eRF1) and SUP35 genes interact to mediate translation termination in Saccharomyces cerevisiae. EMBO J 14(17):4365–4373. https://doi.org/10.1002/j.1460-2075.1995.tb00111.x
Zhao YG, Zhang H (2020) Phase separation in membrane biology: the interplay between membrane-bound organelles and membraneless condensates. Dev Cell 55(1):30–44. https://doi.org/10.1016/j.devcel.2020.06.033
Fujioka Y, Alam JM, Noshiro D, Mouri K, Ando T, Okada Y, May AI, Knorr RL, Suzuki K, Ohsumi Y, Noda NN (2020) Phase separation organizes the site of autophagosome formation. Nature 578(7794):301–305. https://doi.org/10.1038/s41586-020-1977-6
Yamamoto H, Fujioka Y, Suzuki SW, Noshiro D, Suzuki H, Kondo-Kakuta C, Kimura Y, Hirano H, Ando T, Noda NN, Ohsumi Y (2016) The intrinsically disordered protein Atg13 mediates supramolecular assembly of autophagy initiation complexes. Dev Cell 38(1):86–99. https://doi.org/10.1016/j.devcel.2016.06.015
Fujioka Y, Suzuki SW, Yamamoto H, Kondo-Kakuta C, Kimura Y, Hirano H, Akada R, Inagaki F, Ohsumi Y, Noda NN (2014) Structural basis of starvation-induced assembly of the autophagy initiation complex. Nat Struct Mol Biol 21(6):513–521. https://doi.org/10.1038/nsmb.2822
Guillén-Boixet J, Kopach A, Holehouse AS, Wittmann S, Jahnel M, Schlüßler R, Kim K, Trussina IREA, Wang J, Mateju D, Poser I, Maharana S, Ruer-Gruß M, Richter D, Zhang X, Chang YT, Guck J, Honigmann A, Mahamid J, Hyman AA, Pappu RV, Alberti S, Franzmann TM (2020) RNA-induced conformational switching and clustering of G3BP drive stress granule assembly by condensation. Cell 181(2):346-361.e17. https://doi.org/10.1016/j.cell.2020.03.049
Luo L, Li Z, Zhao T, Ju X, Ma P, Jin B, Zhou Y, He S, Huang J, Xu X, Zou Y, Li P, Liang A, Liu J, Chi T, Huang X, Ding Q, Jin Z, Huang C, Zhang Y (2021) SARS-CoV-2 nucleocapsid protein phase separates with G3BPs to disassemble stress granules and facilitate viral production. Sci Bull (Beijing) 66(12):1194–1204. https://doi.org/10.1016/j.scib.2021.01.013
Perdikari TM, Murthy AC, Ryan VH, Watters S, Naik MT, Fawzi NL (2020) SARS-CoV-2 nucleocapsid protein phase-separates with RNA and with human hnRNPs. EMBO J 39(24):e106478. https://doi.org/10.15252/embj.2020106478
You J, Dove BK, Enjuanes L, DeDiego ML, Alvarez E, Howell G, Heinen P, Zambon M, Hiscox JA (2005) Subcellular localization of the severe acute respiratory syndrome coronavirus nucleocapsid protein. J Gen Virol 86(Pt 12):3303–3310. https://doi.org/10.1099/vir.0.81076-0
Mehra S, Sahay S, Maji SK (2019) α-Synuclein misfolding and aggregation: implications in Parkinson’s disease pathogenesis. Biochim Biophys Acta Proteins Proteom 1867(10):890–908. https://doi.org/10.1016/j.bbapap.2019.03.001
Lashuel HA, Overk CR, Oueslati A, Masliah E (2013) The many faces of α-synuclein: from structure and toxicity to therapeutic target. Nat Rev Neurosci 14(1):38–48. https://doi.org/10.1038/nrn3406
Wu KP, Weinstock DS, Narayanan C, Levy RM, Baum J (2009) Structural reorganization of α-Synuclein at low pH observed by NMR and REMD simulations. J Mol Biol 391(4):784–796. https://doi.org/10.1016/j.jmb.2009.06.063
Ballatore C, Lee VMY, Trojanowski JQ (2007) Tau-mediated neurodegeneration in Alzheimer’s disease and related disorders. Nat Rev Neurosci 8(9):663–672. https://doi.org/10.1038/nrn2194
Williams DR (2006) Tauopathies: classification and clinical update on neurodegenerative diseases associated with microtubule-associated protein tau. Intern Med J 36(10):652–660. https://doi.org/10.1111/j.1445-5994.2006.01153.x
Drubin DG, Kirschner MW (1986) Tau protein function in living cells. J Cell Biol 103(6):2739–2746. https://doi.org/10.1083/jcb.103.6.2739
Patel A, Lee HO, Jawerth L, Maharana S, Jahnel M, Hein MY, Stoynov S, Mahamid J, Saha S, Franzmann TM, Pozniakovski A, Poser I, Maghelli N, Royer LA, Weigert M, Myers EW, Grill S, Drechsel D, Hyman AA, Alberti S (2015) A liquid-to-solid phase transition of the ALS protein FUS accelerated by disease mutation. Cell 162(5):1066–1077. https://doi.org/10.1016/j.cell.2015.07.047
Burke KA, Janke AM, Rhine CL, Fawzi NL (2015) Residue-by-residue view of in vitro FUS granules that bind the C-terminal domain of RNA polymerase II. Mol Cell 60(2):231–241. https://doi.org/10.1016/j.molcel.2015.09.006
Iwabuchi K, Li B, Massa HF, Trask BJ, Date T, Fields S (1998) Stimulation of p53-mediated transcriptional activation by the p53-binding proteins, 53BP1 and 53BP2. J Biol Chem 273(40):26061–26068. https://doi.org/10.1074/jbc.273.40.26061
Panier S, Boulton SJ (2014) Double-strand break repair: 53BP1 comes into focus. Nat Rev Mol Cell Biol 15(1):7–18. https://doi.org/10.1038/nrm3719
Mackay JA, Callahan DJ, Fitzgerald KN, Chilkoti A (2010) Quantitative model of the phase behavior of recombinant pH-responsive elastin-like polypeptides. Biomacromol 11(11):2873–2879. https://doi.org/10.1021/bm100571j
Liu W, MacKay JA, Dreher MR, Chen M, McDaniel JR, Simnick AJ, Callahan DJ, Zalutsky MR, Chilkoti A (2010) Injectable intratumoral depot of thermally responsive polypeptide-radionuclide conjugates delays tumor progression in a mouse model. J Control Release 144(1):2–9. https://doi.org/10.1016/j.jconrel.2010.01.032
Lim DW, Nettles DL, Setton LA, Chilkoti A (2008) In situ cross-linking of elastin-like polypeptide block copolymers for tissue repair. Biomacromol 9(1):222–230. https://doi.org/10.1021/bm7007982
Betre H, Ong SR, Guilak F, Chilkoti A, Fermor B, Setton LA (2006) Chondrocytic differentiation of human adipose-derived adult stem cells in elastin-like polypeptide. Biomaterials 27(1):91–99. https://doi.org/10.1016/j.biomaterials.2005.05.071
McHale MK, Setton LA, Chilkoti A (2005) Synthesis and in vitro evaluation of enzymatically cross-linked elastin-like polypeptide gels for cartilaginous tissue repair. Tissue Eng 11(11–12):1768–1779. https://doi.org/10.1089/ten.2005.11.1768
Banani SF, Lee HO, Hyman AA, Rosen MK (2017) Biomolecular condensates: organizers of cellular biochemistry. Nat Rev Mol Cell Biol 18(5):285–298. https://doi.org/10.1038/nrm.2017.7
Shin Y, Brangwynne CP (2017) Liquid phase condensation in cell physiology and disease. Science 357(6357):eaaf4382. https://doi.org/10.1126/science.aaf4382
Memisoglu G, Eapen VV, Yang Y, Klionsky DJ, Haber JE (2019) PP2C phosphatases promote autophagy by dephosphorylation of the Atg1 complex. Proc Natl Acad Sci U S A 116(5):1613–1620. https://doi.org/10.1073/pnas.1817078116
Takahara T, Maeda T (2012) Transient sequestration of TORC1 into stress granules during heat stress. Mol Cell 47(2):242–252. https://doi.org/10.1016/j.molcel.2012.05.019
Yang YS, Kato M, Wu X, Litsios A, Sutter BM, Wang Y, Hsu CH, Wood NE, Lemoff A, Mirzaei H, Heinemann M, Tu BP (2019) Yeast Ataxin-2 Forms an Intracellular Condensate Required for the Inhibition of TORC1 Signaling during Respiratory Growth. Cell 177(3):697-710.e17. https://doi.org/10.1016/j.cell.2019.02.043
Wippich F, Bodenmiller B, Trajkovska MG, Wanka S, Aebersold R, Pelkmans L (2013) Dual specificity kinase DYRK3 couples stress granule condensation/dissolution to mTORC1 signaling. Cell 152(4):791–805. https://doi.org/10.1016/j.cell.2013.01.033
Wheeler JR, Matheny T, Jain S, Abrisch R, Parker R (2016) Distinct stages in stress granule assembly and disassembly. Elife 5:e18413. https://doi.org/10.7554/eLife.18413
Molliex A, Temirov J, Lee J, Coughlin M, Kanagaraj AP, Kim HJ, Mittag T, Taylor JP (2015) Phase separation by low complexity domains promotes stress granule assembly and drives pathological fibrillization. Cell 163(1):123–133. https://doi.org/10.1016/j.cell.2015.09.015
Iwabuchi K, Bartel PL, Li B, Marraccino R, Fields S (1994) Two cellular proteins that bind to wild-type but not mutant p53. Proc Natl Acad Sci USA 91(13):6098–6102. https://doi.org/10.1073/pnas.91.13.6098
Cuella-Martin R, Oliveira C, Lockstone HE, Snellenberg S, Grolmusova N, Chapman JR (2016) 53BP1 integrates DNA repair and p53-dependent cell fate decisions via distinct mechanisms. Mol Cell 64(1):51–64. https://doi.org/10.1016/j.molcel.2016.08.002
Bi J, Huang A, Liu T, Zhang T, Ma H (2015) Expression of DNA damage checkpoint 53BP1 is correlated with prognosis, cell proliferation and apoptosis in colorectal cancer. Int J Clin Exp Pathol 8(6):6070–6082
Cao X, Jin X, Liu B (2020) The involvement of stress granules in aging and aging-associated diseases. Aging Cell 19(4):e13136. https://doi.org/10.1111/acel.13136
Franzmann TM, Alberti S (2019) Prion-like low-complexity sequences: key regulators of protein solubility and phase behavior. J Biol Chem 294(18):7128–7136. https://doi.org/10.1074/jbc.TM118.001190
Kroschwald S, Maharana S, Mateju D, Malinovska L, Nuske E, Poser I, Richter D, Alberti S (2015) Promiscuous interactions and protein disaggregases determine the material state of stress-inducible RNP granules. Elife 4:e06807. https://doi.org/10.7554/eLife.06807
Lobaskin V, Qamhieh K (2003) Effective macroion charge and stability of highly asymmetric electrolytes at various salt conditions. J Phys Chem B 107(32):8022–8029. https://doi.org/10.1021/jp027608+
Zhang FJ, Roosen-Runge F, Sauter A, Wolf M, Jacobs RMJ, Schreiber F (2014) Reentrant condensation, liquid-liquid phase separation and crystallization in protein solutions induced by multivalent metal ions. Pure Appl Chem 86(2):191–202. https://doi.org/10.1515/pac-2014-5002
Funding
Open access funding provided by University of Gothenburg. This work was supported by grants from the National Natural Science Foundation of China (32000387) to XC, Scientific Research Foundation of Zhejiang A&F University (2021LFR053) to XJ, and the Swedish Cancer Fund (Cancerfonden) [CAN 2017/643 and 19 0069] and the Swedish Natural Research Council (Vetenskapsrådet) [VR 2015-04984 and VR 2019-03604] to BL.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Author contributions
XJ and MZ wrote the manuscript. XJ, MZ, SC, and DL compiled the tables and created the figures. Both XC and BL designed and edited the manuscript.
Data availability
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jin, X., Zhou, M., Chen, S. et al. Effects of pH alterations on stress- and aging-induced protein phase separation. Cell. Mol. Life Sci. 79, 380 (2022). https://doi.org/10.1007/s00018-022-04393-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00018-022-04393-0