Abstract
Pluripotent stem cells (PSCs) are a unique type of cells because they exhibit the characteristics of self-renewal and pluripotency. PSCs may be induced to differentiate into any cell type, even male and female germ cells, suggesting their potential as novel cell-based therapeutic treatment for infertility problems. Spermatogenesis is an intricate biological process that starts from self-renewal of spermatogonial stem cells (SSCs) and leads to differentiated haploid spermatozoa. Errors at any stage in spermatogenesis may result in male infertility. During the past decade, much progress has been made in the derivation of male germ cells from various types of progenitor stem cells. Currently, there are two main approaches for the derivation of functional germ cells from PSCs, either the induction of in vitro differentiation to produce haploid cell products, or combination of in vitro differentiation and in vivo transplantation. The production of mature and fertile spermatozoa from stem cells might provide an unlimited source of autologous gametes for treatment of male infertility. Here, we discuss the current state of the art regarding the differentiation potential of SSCs, embryonic stem cells, and induced pluripotent stem cells to produce functional male germ cells. We also discuss the possible use of livestock-derived PSCs as a novel option for animal reproduction and infertility treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Approximately 50–60 % of human infertility is caused by defects in the male germ line [1]. Current infertility treatments include intrauterine insemination, ovulation induction for in vitro fertilization, and intracytoplasmic sperm injection (ICSI), which usually is associated with low efficiency and unwanted side effects in the offspring most likely caused by epigenetic aberrations [2]. However, these treatments are available only to patients who are able to produce functional gametes.
Stem cells are pluripotent cells that have the capacity for indefinite self-renewal and can generate multiple cell types with specific functions in the body [3]. Spermatogenesis is an intricate process that starts with self-renewal of spermatogonial stem cells (SSCs) and leads to fully differentiated functional haploid spermatozoa (Fig. 1). Perturbations at any stage of spermatogenesis may result in infertility; because the process is error prone, and defective sperm production is thought to be responsible for 15–50 % of all infertility cases [2].
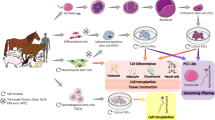
Schematic representation of differentiation of mammalian PSCs into germ cells in vitro. The totipotent zygote is the earliest cell. The ICM in blastocysts contains all cell types forming the entire organism, and ESCs have been established from ICM cells under suitable in vitro culture conditions. Following germ line specification, PGCs appear first in the extraembryonic mesoderm. The germ line potential is preserved during embryo development in OCT4+ cells located in ICM cells of the blastocyst, epiblast stem cells, PGCs, and gonocytes in male gonads. Both ESCs and iPSCs can be differentiated to PGC-like cells under in vitro culture conditions with BMP4 and/or GDNF. The development of germ cells, already during the postnatal period, is sex-specific. Male germ cells enter mitotic arrest and are reactivated to initiate spermatogenesis after birth. Female germ cells enter meiosis and undergo meiotic arrest until after birth [80, 83]. eBlastocyst equine blastocyst, mESCs mouse embryonic stem cells, mEpiblast mouse epiblast, hiPSCs human induced pluripotent stem cells
Oct4 expression is critically involved in the regulation of pluripotency and is found in the inner cell mass (ICM) of blastocysts, the epiblast, and the primordial germ cells (PGCs), but is repressed in somatic cells [4]. PGCs migrate through the hindgut to the genital ridge, where the ovaries and testis are formed. After termination of migration, PGCs start to express a marker gene for post-migratory germ cells, Ddx4 (mouse vasa homologue: Mvh) [5], which initiates sex-specific development. Following migration, male PGCs enter mitotic arrest, and after birth, male germ cells are reactivated to start spermatogenesis. By day E15.5, oogonia are formed in females and gonocytes are formed in males. Gonocytes persist until shortly after birth, and SSCs are formed between postpartum days 0 and 6 in male mice. The transition of gonocytes to SSCs lasts several months in livestock and years in humans and other primates [6].
Male germ cells grown from gonocytes continue to self-renew as SSCs throughout life. SSCs from neonatal and adult mice can develop into pluripotent stem cells (PSCs) when cultured under specific conditions in vitro [7, 8]. The establishment of human adult germ line stem cells from human testicular tissue has been reported [9, 10].
Here, we review the current status of the differentiation potential of SSCs, embryonic stem cells (ESCs), and induced pluripotent stem cells (iPSCs) towards male germ cells. We discuss their potential for use in reproductive medicine and for gaining a better understanding of stem cell development and spermatogenesis. In addition, we discuss the potential use of large domestic animal-derived PSCs for drug screening, infertility treatment, production of genetically modified (GM) livestock, and human disease models.
Male germ cell generation in vitro
In the past decade, significant progress has been made in the derivation of male germ cells from various types of stem cells. Currently, two approaches are used for generating male germ cells from PSCs: (1) in vitro differentiation to haploid cells, and (2) a combined approach by using in vitro differentiation and in vivo transplantation.
Two main sources of PSCs exist in early mammalian embryos: the ICM of preimplantation blastocysts and the epiblast of pre- and post-implantation embryos, which are termed ESCs and epiblast stem cells (EpiSCs), respectively [11–13]. Mouse embryonic stem cells (mESCs) can be differentiated into all types of cells, including PGCs and undergo further differentiation and meiosis to immature gametes, which in turn form blastocysts after fertilization [14, 15]. Several groups have reported the delivery of live pups from in vivo differentiated sperm cells [16, 17]. A similar developmental capacity was proposed for human and primate ESCs [18–22]. HESCs and hiPSCs are capable of differentiating into the three germ layers and into germ cells. Human iPSCs have been used as a model system to understand the genetic and epigenetic basis of germ cell specifications [23], and germ cell-like cells could be derived by in vitro induction.
It is known that hESCs are more similar to mouse EpiSCs than mESCs [13]. Two different pluripotency states are represented by these cell types: (1) a naïve state, which is characteristic of mESCs, and (2) a primed pluripotent state, which is typical for EpiSCs and hESCs. These cells do not have the capacity to form germ cell line-competent chimeras upon injection into blastocysts [24]. In the laboratory mouse, a properly primed pluripotency state is associated with the induction of an epiblast-like state prior to germ cell derivation, whereas in humans, the correct entry into meiosis led by RNA-binding proteins seems to be the major obstacle (Fig. 2).
Schematic model of germ cell derivation in vitro. a Mouse embryonic stem cells (mESCs) or mouse induced pluripotent stem cells (miPSCs), in general PSCs, can be induced into an epiblastic-like (mEpi-like) cells which are able to respond to the signaling pathway started by BMP4 [17, 120, 121]. A primordial germ cell (PGC)-like cells are induced and these cells, in an appropriate in vivo microenvironment (i.e., transplantation into neonatal mouse testis or ovarian bursa) become functional spermatocytes or oocytes. After intracytoplasmic sperm injection (ICSI) these gametes generate fertile and healthy offspring of both sexes. b Human pluripotent stem cells (hPSCs) either human embryonic stem cells (hESCs) or human induced pluripotent stem cells (hiPSCs) present a primed pluripotency state, more similar to a mEpi-like cells, and they can directly respond to BMP4 signaling to attain a PGC-like status [122–126]. PGC-like cells need the presence of different RNA-binding proteins, to progress meiosis and form haploid cells in vitro after induction by retinoic acid (RA) and to express the correct spermatogonial markers when subjected to in vivo microenvironment control after xenotransplantation in immunosuppressed mouse testes. SSC spermatogonial stem cell, Spg spermatogonia
IPSCs have been generated by over-expression of various combinations of transcription factors (e.g., OCT4, MYC, KLF4, and SOX2) in a broad range of species [25, 26]. Recent reports have shown that hiPSCs can enter meiosis and, in some cases, produce haploid products [27–30]. By contrast, the differentiation potential of ESCs and iPSCs to germ cells has not been reported in livestock animals.
Recently, endocrine disruptors have been suggested to have profound trans-generational effects on male germ cell function and have been associated with infertility and tumor formation [31–35]. Exploitation of in vitro culture systems to support mammalian germ cells might improve the development of novel methods for monitoring putative detrimental effects of reproductive toxicants. We have demonstrated that bovine testicular iPSCs are useful for screening the toxicity of environmental disruptors, such as phthalate esters by examining their effects on the maintenance of stemness and pluripotency, and for identifying signaling pathways that might be affected by disruptors [36, 37]. Modeling spermatogenesis in vitro has been employed to examine the effects of environmental toxicants on the differentiation process to spermatozoa [38]. This represents a unique platform for assessing the toxicity of various environmental disruptors on human reproductive functions in a rather straightforward manner.
Restoring fertility following SSC transplantation into the testis
The most direct assay to confirm the biological capacity of SSCs is functional transplantation. Re-transplantation of SSCs obtained from testicular biopsies restored fertility in infertile recipient mice [39–45]. For SSC transplantation, a donor testis-derived cell suspension is injected into the seminiferous tubules of a recipient male, in which the endogenous germ cells have been depleted by treatment with chemotoxic drugs (e.g., busulfan), or it is injected into an animal that is naturally devoid of germ cells (e.g., W/Wv mutant males). Successful transplantation of SSCs with the production of viable spermatozoa has also been reported in livestock animals, including pigs, cattle, sheep, and goats [46–49]. Functional sperm derived from sheep and goat SSCs in the host testis produced donor-derived progeny [48, 54]. SSC transplantation is the only method for identifying fully functional SSCs and confirming their biological activity.
The testicles are an immune-privileged site that is crucial for successful allogenic SSC transplantation between unrelated, immunocompetent individuals [46, 48, 50]. In nonhuman primates, treatment with a humanized monoclonal antibody against CD154 prevented acute renal allograft rejection [51]. SSC transplantation leads to restoration of fertility in males after successful tumor treatment, suggesting SSC transplantation as an emerging clinical application [43, 52–56]. Recently, SSCs were successfully transplanted into the testes of recipient macaques that had been treated with busulfan to destroy the endogenous sperm cell population [57]. The donor genotypes were found in ejaculated sperm of the recipients and mature ejaculated sperm led to blastocyst development after ICSI in Rhesus oocytes, clearly indicating functional spermatogenesis in the foster testes that had been rendered sterile by prior chemotherapy. Thus, in cases of a deficient testicular environment or in the absence of differentiated haploid germ cells or spermatozoa, SSC transplantation may be a valuable therapeutic option to restore fertility. The findings in large animals and nonhuman primates are promising for the application of transplantation of human SSCs; for example, tissue biopsies obtained from adolescent male patients prior to chemotherapy may be stored to produce functional germ cells for later use after successful cancer treatment [57, 58].
Enhancement of SSC self-renewal and stemness in vitro
The core ESC regulatory transcription factors that regulate self-renewal and pluripotency include OCT4, SOX2, and NANOG [59–64]. Expression of Oct4, Sox2, Klf4, and c-Myc, rather than Nanog, was observed in mouse SSC in vitro, but tumor formation after transplantation was not observed [65]. NANOG expression was shown to be essential for PGC maturation in the genital ridge during fetal development [66]. In our studies, bovine testicular cells did not express endogenous OCT4, NANOG, or SOX2; instead, they expressed KLF4 and c-MYC [67]. By contrast, bovine iPSCs expressing pluripotency markers, including OCT4, NANOG, SOX2, STAT3, c-MYC, KLF4, TERT, and DNA methyltransferase 3 (DNMT3) have been reported; benign cystic teratomas containing derivatives of the three germ layers were observed after subcutaneous transplantation into nude mice [36, 37]. These data suggest that NANOG plays a critical role in the ability to contribute to teratoma formation as an ultimate proof of pluripotency. Speculatively, silencing of NANOG expression may be essential for maturation of SSCs from PGCs or gonocytes.
Sato et al. [65] demonstrated the derivation of functional sperms from mouse SSCs using an in vitro organ culture system. The cells were cultured in explanted neonatal testis tissues, and sperm cells could be differentiated from SSCs; ultimately viable sperm gave rise to offspring after micro insemination. These results seem to be applicable to other species, including humans and large domestic species. The technology requires explant culture with testicular tissue to serve as host incubator [66], which, however, may pose additional challenges related to hygiene and variability. In contrast, human SSCs that were cultured in medium supplemented with retinoic acid and stem cell factor can differentiate into haploid spermatids that were microinjected into mouse oocytes and showed evidence of fertilization potential [67].
Progress in stem cell technologies might lead to new cell-based infertility treatments if immunologically compatible patient-specific cells can be derived. Using SSCs for autologous cell-based therapy would be superior to ESC-based treatments, because it avoids the ethical problems associated with the use of human ESCs. Moreover, studies on SSCs may offer unique insight into one of the earliest fate decisions of ESCs or EpiSCs and into the biology of SSCs, which are of fundamental importance for the continuity of species [6].
PSCs to screen for environmental toxicant-associated male infertility
Numerous studies have confirmed that environmental endocrine disruptors have adverse effects on male fertility; phthalate derivatives lead to testicular atrophy, decreased testicular weight and lower testosterone level [68–71]. The detachment of germ cells from the seminiferous epithelium and the increased incidence of germ cell apoptosis have been observed in young peripubertal rodents after exposure to mono-(2-ethylhexyl) phthalate (MEHP) [71]. The number of germ cells was significantly reduced in cultured human fetal testes after exposure to 10−4 M MEHP for 3 days, mainly associated with a dramatic increase in apoptosis [72]. The toxicity of environmental disruptors such as cadmium [73], MEHP [74], and uranium [75], was investigated using organ culture systems with human fetal testes. Thus, the use of hESCs and iPSCs is promising for monitoring potentially detrimental effects of environmental disruptors.
Bovine iPSCs and testicular cells have been successfully used as in vitro models to study the toxicity of phthalate esters. We found that bovine iPSCs were more resistant to androgen receptor (AR)-dependent apoptosis than testicular cells, most likely attributed to regulation of the AR-p21Cip1 cascade via p53, which showed significantly enhanced expression. Phthalate esters significantly reduced AR expression in bovine iPSCs. Collectively, these studies indicate that iPSCs may be useful for screening for adverse effects from endocrine disruptor [36, 37]. This screening system has also promised as a useful model for studying the effects of environmental factors on human germ cell development.
Derivation of gametes from mammalian adult tissues, and germ line cell differentiation from ESCs and iPSCs
Functional adult germ line stem cells can be derived from human testes and adult mouse and human ovaries [9, 10, 76–78]. However, stem cells from human testicular tissue did not form teratomas after transplantation into immune-deficient mice, suggesting limited pluripotency [9, 10]. Mitotically active oogonial stem cells could be isolated from the surface of mouse adult ovaries and human ovarian tissues by sorting for DDX-expressing cells [78]. However, other investigators did not find mitotically active female germ line progenitors in mouse ovaries. Moreover, Ddx4-expressing cells from postnatal mouse ovaries did not enter meiosis and did not develop to oocytes during de novo folliculogenesis under their experimental conditions [79]. Gamete derivation in vitro from PSCs is challenging because many PGC markers are identical to PSC markers [80], which makes it extremely difficult to discriminate early embryonic germ line cells from PGCs.
Hübner et al. [81] were the first to report the in vitro gamete production from mouse ESCs carrying the Oct4 reporter gene. Ovarian follicle-like structures were observed under culture conditions without feeder layer or growth factors. Toyooka et al. [14] described for the first time the derivation of male germ cells from mouse ESCs carrying a Ddx4 (Mvh) reporter construct. These authors used embryoid bodies (EBs) as the starting material and induced EBs to differentiate in suspension culture in the absence of leukemia inhibitory factor (LIF). Ddx4+ cells gradually appeared in the EBs, suggesting the presence of cells with the characteristics of post-migratory PGCs in EBs. Subsequently, purified Ddx4+ cells were transplanted together with male genital ridge cells into adult mouse testes. The cell aggregates formed seminiferous tubules that supported complete spermatogenesis derived from purified Ddx4+ cells. This study clearly demonstrates that germ line specification and the emergence of post-migratory PGCs occur spontaneously or are induced in EBs. However, spermatozoa derived from PGCs could not activate oocytes. Male PGCs could be derived from mouse ESCs in vitro with the aid of EBs [15]. The cells spontaneously became post-meiotic and were capable of activating oocytes after injection of PGC-derived male haploid cells into EBs, using an antibody that specifically reacted with specific stages of postnatal male germ cells up to spermatozoa [15].
Nayernia et al. [16] reported the induction of male gametes from ESCs and the successful production of offspring derived thereof. However, the low viability and growth abnormalities in the progeny derived from in vitro-derived germ cells indicated imprinting errors, suggesting erroneous epigenetic reprogramming associated with the development of male-specific germ cells under in vitro conditions. Moreover, the remaining undifferentiated stem cells in culture might cause teratomas after transplantation. Further investigations into the epigenetic reprogramming status in induced germ cells might provide valuable information regarding sex-specific germ cell differentiation in vitro. In vitro germ cell induction mechanisms have not yet been sufficiently defined to allow for examining the normal development of germ cells ex vivo. Further in vivo studies are needed to establish the effectiveness of in vitro systems as a reliable assay of germ cell development [80].
The expression profiles of marker genes in germ cells and PSCs may provide important information for deriving germ cells from those cells. Marker molecules for specific types of stem cells are shown in Table 1. Basic fibroblast growth factor (bFGF) and feeder cells increased the expression of PGC marker genes such as VASA (DDX4), DAZL, and OCT4 in human germ-like cells differentiated from hESCs [85]. Tilgner et al. [20] reported the enrichment of putative PGCs from hESCs that had been sorted using an antibody specific for stage specific embryonic antigen-1 (SSEA-1). Gelatin-bound monolayers are obviously a robust system for generating large number of differentiated cells. However, these cells do not enter meiosis.
Transplantation of ESC-derived somatic cells or tissues is promising for curing many human diseases. However, derivation of gametes from unrelated ESCs is associated with incompatibilities of the immune systems. Well-characterized iPSCs may be a good option for obtaining sufficient numbers of autologous cells. HiPSCs could be successfully differentiated to post-meiotic cells without over-expression of germ line-related transcription factors [26]. Cells were cultured without bFGF as monolayers for 3 weeks and the pluripotency markers SSEA-4 and OCT4 were down-regulated at the end of this period. Under these conditions, male germ-like haploid cells were obtained from hiPSCs. Tilgner et al. [20] demonstrated for the first time the meiotic competence of hiPSC-derived cells, which suggests the possibility of producing human gametes in vitro. The ability of hiPSCs and hESCs to differentiate into presumptive SSC-like cells in vitro, and to contribute to advanced spermatogenesis, including round spermatids, was reported recently [30]. However, round spermatids could not fertilize human oocytes. The feasibility and safety of the culture systems will need to be established in animal models.
Mouse ESCs and iPSCs can be induced to form epiblast-like cells that, in turn, develop into PGC-like cells when the culture medium is supplemented with BMP4 [17] (Table 2). The resulting PGC-like cells were then transferred to the testes of infertile mice and produced sperm that were used for ICSI; transfer of the resulting embryos into recipient females gave rise to viable offspring. This is the most advanced protocol for the deviation of functional gametes from PSCs until now. Further experiments are required before this system could be used for therapeutic treatments in human patients because some of the offspring showed malignant tumors in the neck area [17]. Human iPSC-derived cells should be monitored carefully to eliminate mutations, specifically in tumor suppressor genes [83, 84].
Epigenetic control of germ cell development
A bimodal pattern of DNA methylation has been detected during the specification and maturation of mouse male germ cells (Fig. 3). PGCs derived from the epiblast at E6.5–E7.5 are stimulated by BMP4, then migrate from the epiblast to the hindgut at E7.5–E9, and finally to the genital ridge at E9.5–E11.5. In E6.5 mouse embryos, PGCs show DNA hypermethylation with repression of certain genes [85]. The epigenetic marks are erased during migration of PGCs [86], particularly in imprinted genes and transposons of PGCs. The re-establishment of DNA methylation in germ cells initiates from the formation of pro-spermatogonia or gonocytes. Although DNA methylation is acquired during the prenatal mitotic arrest of the gonocytes, de novo and maintenance of methylation occur only during mitosis of spermatogonia and meiotic prophase I, whereas maintenance methylation appears only during mitosis [87] (Table 3). The global erasure of DNA methylation also occurs during early embryonic development [88, 89].
Schematic diagram reveals the expression of DNA methylation profiles in mammalian spermatogenesis. Bimodal DNA methylation patterns in male germ cell development. PGCs are derived from the epiblast at E6.5 and migrate to the genital ridge. During migration, the epigenetic marks are widely erased. After erasure of the DNA methylation marks, reestablishment of the male germ cell DNA patterns initiates from prospermatogonia to entering meiosis. After fertilization, DNA patterns are broadly erased by active demethylation, whereas the imprinted genes are maintained by DNMT1 activity
DNMT3-like (DNMT3L) is involved in the maintenance of DNA methylation in stem cells during the quiescent state or during self-renewal of SSCs, whereas DNMT3a and DNMT3b are not involved in this process. In addition to its role in self-renewal, DNA methylation of SSCs may be required for the transition from SSCs to differentiated spermatogonia. DNMT3a and DNMT3b transcripts remain at the highest level in type A spermatogonia compared with other types of male germ cells [90]. Studies into the roles of DNA methyltransferases in SSC differentiation in mice are useful for gaining a better understanding of the underlying biological principles and for the development of new therapies.
Expression of DNMT1, DNMT3a, and DNMT3b is upregulated in leptotene and zygotene spermatocytes during meiosis and spermatogenesis [91]. DNMT1 is present in non-proliferative round spermatids, whereas DNMT3a and DNMT3b maintain the methylation patterns through the de novo methylation pathways, although the roles of DNMT1 in round spermatids remain to be solved. The role of ten–eleven translocation methylcytosine dioxygenase (TET1) has not been elucidated in spermatogenesis, albeit it plays a significant role as a meiotic initiator in oocytes [92]. It remains to be determined whether the biological function of TET1 in spermatogenesis is similar to that in oocytes [92]. In contrast to mouse, human DNMT1, DNMT3a, and DNMT3b are expressed in pachytene spermatocytes [94]. However, in both mice and humans, DNMT1, DNMT3a and DNMT3b are highly expressed in round spermatids [93, 94]. DNMT1 is present in non-proliferative round spermatids, whereas DNMT3a and DMMT3b are expressed after the establishment of the paternal methylation pattern. Thus, DNMT3a2 and DNMT3b may play a role in the de novo methylation pathways, although the role of DNMT1 in round spermatids remains to be solved.
In addition to DNA methylation and demethylation, global changes in histone modifications, such as a decrease in histone H3K9 dimethylation and an increase in histone H3K27 trimethylation, occur in the PGC genome [95, 96]. Although the significance of the global changes in histone modifications remains unclear, it is likely that the alteration is required for the acquisition of potency in the terminal products. A better knowledge on the epigenetic profile during germ cell development is crucial for understanding the underlying biological mechanisms, and thus for developing suitable culture techniques for germ cells, which, in turn, are major prerequisites for developing new therapies with germ cells.
Micro-RNAs (miRNAs) in meiotic and post-meiotic cells
A conditional knockout of Dicer 1 in mice disrupts meiotic and post-meiotic development by decreasing the number of mouse SSCs and by blocking differentiation [97, 98]. In addition, loss of Dicer1 resulted in male infertility in mice [99]. Sertoli cell-specific deletion of Dicer severely impairs sperm competence and leads to male infertility due to the absence of mature spermatozoa and testicular degeneration [97]. Germ cell-specific deletion of Dicer 1 leads to overexpression of genes for meiotic sex chromosome inactivation, to increased spermatocyte apoptosis, and to defects in chromatin organization, the elongation and nuclear shaping of spermatids [100]. These effects suggest that Dicers are crucial for the meiotic and haploid phases of spermatogenesis (Table 3).
MiR-34c expression is up-regulated in spermatocytes and round spermatids trigger apoptosis [101]. This process is at least partially mediated by targeting transcription factor ATF-1 [102]. Thus, miR34c is critical for germ cell development. MiR-469 has been shown to target transition protein 2 (TP2) and protamine mRNAs to be repressed in pachytene spermatocytes and round spermatids [103]. MiR-122a also controls the degradation of TP2 mRNA cleavage [104], and miR-18 can directly target heat shock factor 2 mRNA at the spermatogenesis stage [105].
Collectively, miRNAs play essential roles by regulating each step of male germ cell development, including mitosis, meiosis, and spermatogenesis in rodents. Nevertheless, it remains to be defined which miRNAs are required for the three major stages of spermatogenesis in humans, including spermatogonia, pachytene spermatocytes, and round spermatids [106]. A better understanding these processes may provide new targets for the treatment of male infertility.
In vitro gametogenesis from bovine iPSCs and production of genetically modified (GM) cattle from transgenic iPSCs
Bovine iPSCs established in our laboratory exhibited characteristics similar to those of mESCs with regard to gene expression, transcription factor dependency, and active signaling molecules [36, 37]. Expression of pluripotency markers, including OCT4, NANOG, SOX2, STAT3, c-MYC, KLF4, TERT, and DNMT3A, is maintained in bovine iPSCs (Table 3). Mouse ESCs and iPSCs expressed SSEA-1, but not SSEA-4, whereas human ESCs and iPSCs expressed SSEA-4, but not SSEA-1 (Table 3). Morphology and expression of the SSEA antigens in bovine iPSCs resembled those of mouse ESCs and iPSCs rather than those of human ESCs and iPSCs. Bovine iPSCs express both SSEA-1 and SSEA-4. SSEA-1 expression has been observed in both bovine and equine embryonic stem-like cells [107–109]. The conditions reported by Hayashi et al. [19] may be useful for purifying PGC-like cells from bovine iPSCs (Fig. 1). The availability of functional in vitro culture system is promising for improving breeding of farm animals. The selection process for stud sires aiming to obtaining genetically improved progeny in animal breeding is very expensive and time-consuming. The use of fertile sperm cells derived from iPSCs established from the tissues of neonatal bull calves may be a promising economical option. In addition, stem cell therapies may be useful for restoring fertility in elite bull sires that are unable to produce semen because of physical damage or disease of the testicular somatic environment.
Several attempts have been made to establish germ line-competent bovine ESCs or iPSCs [108–111]; however, so far teratoma formation with derivatives of the three germ layers has not been observed, although it has been confirmed for goat ESCs [112]. Recently, we demonstrated that gene expression could be silenced in bovine iPSCs by using small interfering RNA against p21Cip1, which resulted in the reduced expression of the target genes [36], suggesting the possibility of gene targeting with bovine iPSCs.
Spermatozoa may be useful as vectors for producing GM animals [113–116]. It could be a valuable option in the cattle industry to use spermatids differentiated from genetically modified iPSCs to produce transgenic animals by transplantation into the testes of recipient bull calves or by injecting them into bovine oocytes. We propose to produce transgenic animals by using sperm-like cells differentiated from transgenic iPSCs via in vitro fertilization or ICSI. Bovine SSCs could successfully be propagated in the presence of LIF, epidermal growth factor or fibroblast growth factor 2; however, no full spermatogenesis was established from SSCs transplanted into recipient mouse testis [117]. Complete spermatogenesis has been obtained from autologous transfer of bovine SSCs [47, 48, 118]. Thus, the methodologies described above need significant improvements, and cell-based approaches in livestock reproduction are a challenging task. The derivation of PSCs in livestock is promising for the development of novel disease-resistance strategy, cell or organ therapies, drug screening, and human disease models. It is also important for increasing the efficiency of the livestock industry. For example, dairy manufacturers could derive protein-rich milk from GM cows and thereby reduce the cost of cheese production.
The rapidly emerging DNA nucleases such as ZFNs, TALEN, and CRISPR/Cas may provide additional new options for producing livestock species with targeted genetic modifications with novel traits useful for application in agriculture and biomedicine [119]. There is no doubt that the application of genetic modifications and PSC techniques will improve our understanding of the dynamics of gametogenesis and reproductive biology in general, and will play an important role in the development of novel therapeutic treatments in humans and other mammalian species.
Conclusions
Over the past decade, revolutionary progress has been made in the derivation and characterization of germ cells from various types of stem cells. SSC transplantation in non-human primates is now compatible with functional spermatogenesis in infertile testes after chemotherapy, clearly showing the possibility of using human SSCs from tissue biopsies of adolescent male patients to obtain functional germ cells prior to treatment with high-dose chemotherapy. However, transplantation of human ESC-derived gametes may be associated with incompatibilities of the immune systems, although the testicles constitute an immune-privileged site. Therefore, iPSCs may be a suitable option for supplying sufficient numbers of autologous cells. Differentiated spermatid-like cells from human iPSCs have been unable to fertilize human oocytes until now. More feasible and safer systems must be established in animal models, including large domestic livestock species, to improve the low efficiency of current differentiation protocols and cell viability. From both the academic and therapeutic point of view, in vitro differentiation models using PSCs are highly promising areas. The self-renewal capacity and the pluripotency of stem cells may be valuable in preserving individual genomes and modifying germ lines.
Abbreviations
- AR:
-
Androgen receptor
- bFGF:
-
Basic fibroblast growth factor
- BMP:
-
Bone morphogenic protein
- DNMT3:
-
DNA methyltransferase 3
- DNMT3L:
-
DNA methyltransferase 3-like
- E:
-
Embryonic
- EBs:
-
Embryoid bodies
- ESCs:
-
Embryonic stem cells
- EpiSCs:
-
Epiblast stem cells
- FACS:
-
Fluorescence-activated cell sorting
- GM:
-
Genetically modified
- GDNF:
-
Glial cell line-derived neurotrophic factor
- hESCs:
-
Human embryonic stem cells
- hiESCs:
-
Human induced embryonic stem cells
- hiPSCs:
-
Human induced pluripotent stem cells
- ICM:
-
Inner cell mass
- ICSI:
-
Intracytoplasmic sperm injection
- iPSCs:
-
Induced pluripotent stem cells
- LIF:
-
Leukemia inhibitory factor
- mESCs:
-
Mouse embryonic stem cells
- MEHP:
-
Mono-(2-ethylhexyl) phthalate
- miRNA:
-
Micro-RNA
- Mvh:
-
Mouse vase homologue
- PGCs:
-
Primordial germ cells
- PSCs:
-
Pluripotent stem cells
- SSCs:
-
Spermatogonial stem cells
- SSEA-1:
-
Stage specific antigen-1
- SSEA-4:
-
Stage specific antigen-4
- TET1:
-
Ten–eleven translocation methylcytosine dioxygenase
- TP2:
-
Transition protein 2
References
Schlegel PN (2009) Evaluation of male infertility. Minerva Ginecol 61(4):261–283
Easley CA, Simerly CR, Schatten G (2013) Stem cell therapeutic possibilities: future therapeutic options for male-factor and female-factor infertility? Reprod Biomed Online 27(1):75–80. doi:10.1016/j.rbmo.2013.03.003
Saito S, Lin YC, Murayama Y, Hashimoto K, Yokoyama KK (2012) Human amnion-derived cells as a reliable source of stem cells. Curr Mol Med 12(10):1340–1349
Boiani M, Scholer HR (2005) Regulatory networks in embryo-derived pluripotent stem cells. Nat Rev Mol Cell Biol 6(11):872–884. doi:10.1038/nrm1744
Fujiwara Y, Komiya T, Kawabata H, Sato M, Fujimoto H, Furusawa M, Noce T (1994) Isolation of a DEAD-family protein gene that encodes a murine homolog of Drosophila vasa and its specific expression in germ cell lineage. Proc Natl Acad Sci USA 91(25):12258–12262
Oatley JM, Brinster RL (2008) Regulation of spermatogonial stem cell self-renewal in mammals. Annu Rev Cell Dev Biol 24:263–286. doi:10.1146/annurev.cellbio.24.110707.175355
Kanatsu-Shinohara M, Inoue K, Lee J, Yoshimoto M, Ogonuki N, Miki H, Baba S, Kato T, Kazuki Y, Toyokuni S, Toyoshima M, Niwa O, Oshimura M, Heike T, Nakahata T, Ishino F, Ogura A, Shinohara T (2004) Generation of pluripotent stem cells from neonatal mouse testis. Cell 119(7):1001–1012. doi:10.1016/j.cell.2004.11.011
Guan K, Nayernia K, Maier LS, Wagner S, Dressel R, Lee JH, Nolte J, Wolf F, Li M, Engel W, Hasenfuss G (2006) Pluripotency of spermatogonial stem cells from adult mouse testis. Nature 440(7088):1199–1203. doi:10.1038/nature04697
Conrad S, Renninger M, Hennenlotter J, Wiesner T, Just L, Bonin M, Aicher W, Buhring HJ, Mattheus U, Mack A, Wagner HJ, Minger S, Matzkies M, Reppel M, Hescheler J, Sievert KD, Stenzl A, Skutella T (2008) Generation of pluripotent stem cells from adult human testis. Nature 456(7220):344–349. doi:10.1038/nature07404
Mizrak SC, Chikhovskaya JV, Sadri-Ardekani H, van Daalen S, Korver CM, Hovingh SE, Roepers-Gajadien HL, Raya A, Fluiter K, de Reijke TM, de la Rosette JJ, Knegt AC, Belmonte JC, van der Veen F, de Rooij DG, Repping S, van Pelt AM (2010) Embryonic stem cell-like cells derived from adult human testis. Hum Reprod 25(1):158–167. doi:10.1093/humrep/dep354
Evans MJ, Kaufman MH (1981) Establishment in culture of pluripotential cells from mouse embryos. Nature 292(5819):154–156
Brons IG, Smithers LE, Trotter MW, Rugg-Gunn P, Sun B, de Sousa Chuva, Lopes SM, Howlett SK, Clarkson A, Ahrlund-Richter L, Pedersen RA, Vallier L (2007) Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature 448(7150):191–195. doi:10.1038/nature05950
Tesar PJ, Chenoweth JG, Brook FA, Davies TJ, Evans EP, Mack DL, Gardner RL, McKay RD (2007) New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature 448(7150):196–199. doi:10.1038/nature05972
Toyooka Y, Tsunekawa N, Akasu R, Noce T (2003) Embryonic stem cells can form germ cells in vitro. Proc Natl Acad Sci USA 100(20):11457–11462. doi:10.1073/pnas.1932826100
Geijsen N, Horoschak M, Kim K, Gribnau J, Eggan K, Daley GQ (2004) Derivation of embryonic germ cells and male gametes from embryonic stem cells. Nature 427(6970):148–154. doi:10.1038/nature02247
Nayernia K, Nolte J, Michelmann HW, Lee JH, Rathsack K, Drusenheimer N, Dev A, Wulf G, Ehrmann IE, Elliott DJ, Okpanyi V, Zechner U, Haaf T, Meinhardt A, Engel W (2006) In vitro-differentiated embryonic stem cells give rise to male gametes that can generate offspring mice. Dev Cell 11(1):125–132. doi:10.1016/j.devcel.2006.05.010
Hayashi K, Ohta H, Kurimoto K, Aramaki S, Saitou M (2011) Reconstitution of the mouse germ cell specification pathway in culture by pluripotent stem cells. Cell 146(4):519–532. doi:10.1016/j.cell.2011.06.052
Clark AT, Bodnar MS, Fox M, Rodriquez RT, Abeyta MJ, Firpo MT, Pera RA (2004) Spontaneous differentiation of germ cells from human embryonic stem cells in vitro. Hum Mol Genet 13(7):727–739. doi:10.1093/hmg/ddh088
Teramura T, Takehara T, Kawata N, Fujinami N, Mitani T, Takenoshita M, Matsumoto K, Saeki K, Iritani A, Sagawa N, Hosoi Y (2007) Primate embryonic stem cells proceed to early gametogenesis in vitro. Cloning Stem Cells 9(2):144–156. doi:10.1089/clo.2006.0070
Tilgner K, Atkinson SP, Golebiewska A, Stojkovic M, Lako M, Armstrong L (2008) Isolation of primordial germ cells from differentiating human embryonic stem cells. Stem Cells 26(12):3075–3085. doi:10.1634/stemcells.2008-0289
Bucay N, Yebra M, Cirulli V, Afrikanova I, Kaido T, Hayek A, Montgomery AM (2009) A novel approach for the derivation of putative primordial germ cells and sertoli cells from human embryonic stem cells. Stem Cells 27(1):68–77. doi:10.1634/stemcells.2007-1018
Fukunaga N, Teramura T, Onodera Y, Takehara T, Fukuda K, Hosoi Y (2010) Leukemia inhibitory factor (LIF) enhances germ cell differentiation from primate embryonic stem cells. Cell Reprogram 12(4):369–376. doi:10.1089/cell.2009.0097
Marques-Mari AI, Lacham-Kaplan O, Medrano JV, Pellicer A, Simon C (2009) Differentiation of germ cells and gametes from stem cells. Human Reprod Update 15(3):379–390. doi:10.1093/humupd/dmp001
Nichols J, Smith A (2009) Naive and primed pluripotent states. Cell Stem Cell 4(6):487–492. doi:10.1016/j.stem.2009.05.015
Takahashi K, Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126(4):663–676. doi:10.1016/j.cell.2006.07.024
Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S (2007) Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131(5):861–872. doi:10.1016/j.cell.2007.11.019
Eguizabal C, Montserrat N, Vassena R, Barragan M, Garreta E, Garcia-Quevedo L, Vidal F, Giorgetti A, Veiga A, Izpisua Belmonte JC (2011) Complete meiosis from human induced pluripotent stem cells. Stem Cells 29(8):1186–1195. doi:10.1002/stem.672
Kee K, Angeles VT, Flores M, Nguyen HN, Reijo Pera RA (2009) Human DAZL, DAZ and BOULE genes modulate primordial germ-cell and haploid gamete formation. Nature 462(7270):222–225. doi:10.1038/nature08562
Panula S, Medrano JV, Kee K, Bergstrom R, Nguyen HN, Byers B, Wilson KD, Wu JC, Simon C, Hovatta O, Reijo Pera RA (2011) Human germ cell differentiation from fetal- and adult-derived induced pluripotent stem cells. Hum Mol Genet 20(4):752–762. doi:10.1093/hmg/ddq520
Easley CA, Phillips BT, McGuire MM, Barringer JM, Valli H, Hermann BP, Simerly CR, Rajkovic A, Miki T, Orwig KE, Schatten GP (2012) Direct differentiation of human pluripotent stem cells into haploid spermatogenic cells. Cell Rep 2(3):440–446. doi:10.1016/j.celrep.2012.07.015
Anway MD, Cupp AS, Uzumcu M, Skinner MK (2005) Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science 308(5727):1466–1469. doi:10.1126/science.1108190
Rajpert-De Meyts E (2006) Developmental model for the pathogenesis of testicular carcinoma in situ: genetic and environmental aspects. Hum Reprod Update 12(3):303–323. doi:10.1093/humupd/dmk006
Tiido T, Rignell-Hydbom A, Jonsson B, Giwercman YL, Rylander L, Hagmar L, Giwercman A (2005) Exposure to persistent organochlorine pollutants associates with human sperm Y: X chromosome ratio. Hum Reprod 20(7):1903–1909. doi:10.1093/humrep/deh855
Casals-Casas C, Desvergne B (2011) Endocrine disruptors: from endocrine to metabolic disruption. Annu Rev Physiol 73:135–162. doi:10.1146/annurev-physiol-012110-142200
Fisher JS (2004) Environmental anti-androgens and male reproductive health: focus on phthalates and testicular dysgenesis syndrome. Reproduction 127(3):305–315. doi:10.1530/rep.1.00025
Wang SW, Wang SS, Wu DC, Lin YC, Ku CC, Wu CC, Chai CY, Lee JN, Tsai EM, Lin CL, Yang RC, Ko YC, Yu HS, Huo C, Chuu CP, Murayama Y, Nakamura Y, Hashimoto S, Matsushima K, Jin C, Eckner R, Lin CS, Saito S, Yokoyama KK (2013) Androgen receptor-mediated apoptosis in bovine testicular induced pluripotent stem cells in response to phthalate esters. Cell Death Dis 4:e907. doi:10.1038/cddis.2013.420
Lin YC, Kuo KK, Wuputra K, Lin SH, Ku CC, Yang YH, Wang SW, Wang SW, Wu DC, Wu CC, Chai CY, Lin CL, Lin CS, Kajitani M, Miyoshi H, Nakamura Y, Hashimoto S, Matsushima K, Jin C, Huang SK, Saito S, Yokoyama KK (2014) Bovine induced pluripotent stem cells are more resistant to apoptosis than testicular cells in response to mono-(2-ethylhexyl) phthalate. Int J Mol Sci 15(3):5011–5031. doi:10.3390/ijms15035011
Easley CA, Gradner JM, Moser A, Rickman CA, Mcrachin ZT, Merritt MM, Hansen JM, Caudle WM (2015) Assessing reproductive toxicity of two environmental toxicants with a novel in vitro human spermatogenic model. Stem Cell Res 14:347–355
Brinster RL, Avarbock MR (1994) Germline transmission of donor haplotype following spermatogonial transplantation. Proc Natl Acad Sci USA 91(24):11303–11307
Brinster RL, Zimmermann JW (1994) Spermatogenesis following male germ-cell transplantation. Proc Natl Acad Sci USA 91(24):11298–11302
Ogawa T, Dobrinski I, Avarbock MR, Brinster RL (2000) Transplantation of male germ line stem cells restores fertility in infertile mice. Nat Med 6(1):29–34. doi:10.1038/71496
Brinster RL (2002) Germline stem cell transplantation and transgenesis. Science 296(5576):2174–2176. doi:10.1126/science.1071607
Kubota H, Brinster RL (2006) Technology insight: in vitro culture of spermatogonial stem cells and their potential therapeutic uses. Nat Clin Pract Endocrinol Metab 2(2):99–108. doi:10.1038/ncpendmet0098
Brinster RL (2007) Male germline stem cells: from mice to men. Science 316(5823):404–405. doi:10.1126/science.1137741
Oatley JM, Brinster RL (2006) Spermatogonial stem cells. Methods Enzymol 419:259–282. doi:10.1016/S0076-6879(06)19011-19014
Mikkola M, Sironen A, Kopp C, Taponen J, Sukura A, Vilkki J, Katila T, Andersson M (2006) Transplantation of normal boar testicular cells resulted in complete focal spermatogenesis in a boar affected by the immotile short-tail sperm defect. Reprod Domest Anim 41(2):124–128. doi:10.1111/j.1439-0531.2006.00651.x
Izadyar F, Den Ouden K, Stout TA, Stout J, Coret J, Lankveld DP, Spoormakers TJ, Colenbrander B, Oldenbroek JK, Van der Ploeg KD, Woelders H, Kal HB, De Rooij DG (2003) Autologous and homologous transplantation of bovine spermatogonial stem cells. Reproduction 126(6):765–774
Honaramooz A, Behboodi E, Megee SO, Overton SA, Galantino-Homer H, Echelard Y, Dobrinski I (2003) Fertility and germline transmission of donor haplotype following germ cell transplantation in immunocompetent goats. Biol Reprod 69(4):1260–1264. doi:10.1095/biolreprod.103.018788
Herrid M, Olejnik J, Jackson M, Suchowerska N, Stockwell S, Davey R, Hutton K, Hope S, Hill JR (2009) Irradiation enhances the efficiency of testicular germ cell transplantation in sheep. Biol Reprod 81(5):898–905. doi:10.1095/biolreprod.109.078279
Kim Y, Turner D, Nelson J, Dobrinski I, McEntee M, Travis AJ (2008) Production of donor-derived sperm after spermatogonial stem cell transplantation in the dog. Reproduction 136(6):823–831. doi:10.1530/REP-08-0226
Kirk AD, Burkly LC, Batty DS, Baumgartner RE, Berning JD, Buchanan K, Fechner JH Jr, Germond RL, Kampen RL, Patterson NB, Swanson SJ, Tadaki DK, TenHoor CN, White L, Knechtle SJ, Harlan DM (1999) Treatment with humanized monoclonal antibody against CD154 prevents acute renal allograft rejection in nonhuman primates. Nat Med 5(6):686–693. doi:10.1038/9536
Geens M, Goossens E, De Block G, Ning L, Van Saen D, Tournaye H (2008) Autologous spermatogonial stem cell transplantation in man: current obstacles for a future clinical application. Hum Reprod Update 14(2):121–130. doi:10.1093/humupd/dmm047
Schlatt S, Ehmcke J, Jahnukainen K (2009) Testicular stem cells for fertility preservation: preclinical studies on male germ cell transplantation and testicular grafting. Pediatr Blood Cancer 53(2):274–280. doi:10.1002/pbc.22002
Hermann BP, Sukhwani M, Lin CC, Sheng Y, Tomko J, Rodriguez M, Shuttleworth JJ, McFarland D, Hobbs RM, Pandolfi PP, Schatten GP, Orwig KE (2007) Characterization, cryopreservation, and ablation of spermatogonial stem cells in adult rhesus macaques. Stem Cells 25(9):2330–2338. doi:10.1634/stemcells.2007-0143
Hermann BP, Sukhwani M, Hansel MC, Orwig KE (2010) Spermatogonial stem cells in higher primates: are there differences from those in rodents? Reproduction 139(3):479–493. doi:10.1530/REP-09-0255
Hermann BP, Sukhwani M, Salati J, Sheng Y, Chu T, Orwig KE (2011) Separating spermatogonia from cancer cells in contaminated prepubertal primate testis cell suspensions. Hum Reprod 26(12):3222–3231. doi:10.1093/humrep/der343
Hermann BP, Sukhwani M, Winkler F, Pascarella JN, Peters KA, Sheng Y, Valli H, Rodriguez M, Ezzelarab M, Dargo G, Peterson K, Masterson K, Ramsey C, Ward T, Lienesch M, Volk A, Cooper DK, Thomson AW, Kiss JE, Penedo MC, Schatten GP, Mitalipov S, Orwig KE (2012) Spermatogonial stem cell transplantation into rhesus testes regenerates spermatogenesis producing functional sperm. Cell Stem Cell 11(5):715–726. doi:10.1016/j.stem.2012.07.017
Boyer LA, Lee TI, Cole MF, Johnstone SE, Levine SS, Zucker JP, Guenther MG, Kumar RM, Murray HL, Jenner RG, Gifford DK, Melton DA, Jaenisch R, Young RA (2005) Core transcriptional regulatory circuitry in human embryonic stem cells. Cell 122(6):947–956. doi:10.1016/j.cell.2005.08.020
Hochedlinger K, Yamada Y, Beard C, Jaenisch R (2005) Ectopic expression of Oct-4 blocks progenitor-cell differentiation and causes dysplasia in epithelial tissues. Cell 121(3):465–477. doi:10.1016/j.cell.2005.02.018
Loh YH, Wu Q, Chew JL, Vega VB, Zhang W, Chen X, Bourque G, George J, Leong B, Liu J, Wong KY, Sung KW, Lee CW, Zhao XD, Chiu KP, Lipovich L, Kuznetsov VA, Robson P, Stanton LW, Wei CL, Ruan Y, Lim B, Ng HH (2006) The Oct4 and Nanog transcription network regulates pluripotency in mouse embryonic stem cells. Nat Genet 38(4):431–440. doi:10.1038/ng1760
van den Berg DL, Snoek T, Mullin NP, Yates A, Bezstarosti K, Demmers J, Chambers I, Poot RA (2010) An Oct4-centered protein interaction network in embryonic stem cells. Cell Stem Cell 6(4):369–381. doi:10.1016/j.stem.2010.02.014
Oatley JM, Avarbock MR, Telaranta AI, Fearon DT, Brinster RL (2006) Identifying genes important for spermatogonial stem cell self-renewal and survival. Proc Natl Acad Sci USA 103(25):9524–9529. doi:10.1073/pnas.0603332103
Chambers I, Silva J, Colby D, Nichols J, Nijmeijer B, Robertson M, Vrana J, Jones K, Grotewold L, Smith A (2007) Nanog safeguards pluripotency and mediates germline development. Nature 450(7173):1230–1234. doi:10.1038/nature06403
Lin YC, Murayama Y, Hashimoto K, Nakamura Y, Lin CS, Yokoyama KK, Saito S (2014) Role of tumor suppressor genes in the cancer-associated reprogramming of human induced pluripotent stem cells. Stem Cell Res Ther 5(2):58. doi:10.1186/scrt447
Sato T, Yokonishi T, Komeya M, Katagiri K, Kubota Y, Matoba S, Ogonuki N, Ogura A, Yoshida S, Ogawa T (2012) Testis tissue explantation cures spermatogenic failure in c-Kit ligand mutant mice. Proc Natl Acad Sci USA 109(42):16934–16938. doi:10.1073/pnas.1211845109
Griswold MD, Oatley JM (2013) Concise review: defining characteristics of mammalian spermatogenic stem cells. Stem Cells 31(1):8–11. doi:10.1002/stem.1253
Yang S, Ping P, Ma M, Li P, Tian R, Yang H, Liu Y, Gong Y, Zhang Z, Li Z, He Z (2014) Generation of haploid spermatids with fertilization and development capacity from human spermatogonial stem cells of cryptorchid patients. Stem Cell Rep 3(4):663–675. doi:10.1016/j.stemcr.2014.08.004
Lyche JL, Gutleb AC, Bergman A, Eriksen GS, Murk AJ, Ropstad E, Saunders M, Skaare JU (2009) Reproductive and developmental toxicity of phthalates. J Toxicol Environ Health Part B 12(4):225–249. doi:10.1080/10937400903094091
Jurewicz J, Hanke W (2011) Exposure to phthalates: reproductive outcome and children health. A review of epidemiological studies. Int J Occup Med Environ Health 24(2):115–141. doi:10.2478/s13382-011-0022-2
Sjoberg P, Lindqvist NG, Ploen L (1986) Age-dependent response of the rat testes to di(2-ethylhexyl) phthalate. Environ Health Perspect 65:237–242
Awal MA, Kurohmaru M, Ishii M, Andriana BB, Kanai Y, Hayashi Y (2004) Mono-(2-ethylhexyl) phthalate (MEHP) induces spermatogenic cell apoptosis in guinea pig testes at prepubertal stage in vitro. Int J Toxicol 23(6):349–355. doi:10.1080/10915810490901985
Lambrot R, Muczynski V, Lecureuil C, Angenard G, Coffigny H, Pairault C, Moison D, Frydman R, Habert R, Rouiller-Fabre V (2009) Phthalates impair germ cell development in the human fetal testis in vitro without change in testosterone production. Environ Health Perspect 117(1):32–37. doi:10.1289/ehp.11146
Angenard G, Muczynski V, Coffigny H, Pairault C, Duquenne C, Frydman R, Habert R, Rouiller-Fabre V, Livera G (2010) Cadmium increases human fetal germ cell apoptosis. Environ Health Perspect 118(3):331–337. doi:10.1289/ehp.0900975
Muczynski V, Cravedi JP, Lehraiki A, Levacher C, Moison D, Lecureuil C, Messiaen S, Perdu E, Frydman R, Habert R, Rouiller-Fabre V (2012) Effect of mono-(2-ethylhexyl) phthalate on human and mouse fetal testis: in vitro and in vivo approaches. Toxicol Appl Pharmacol 261(1):97–104. doi:10.1016/j.taap.2012.03.016
Angenard G, Muczynski V, Coffigny H, Duquenne C, Frydman R, Habert R, Livera G, Rouiller-Fabre V (2011) In vitro effects of Uranium on human fetal germ cells. Reprod Toxicol 31(4):470–476. doi:10.1016/j.reprotox.2010.12.058
Zou K, Yuan Z, Yang Z, Luo H, Sun K, Zhou L, Xiang J, Shi L, Yu Q, Zhang Y, Hou R, Wu J (2009) Production of offspring from a germline stem cell line derived from neonatal ovaries. Nat Cell Biol 11(5):631–636. doi:10.1038/ncb1869
Kossack N, Meneses J, Shefi S, Nguyen HN, Chavez S, Nicholas C, Gromoll J, Turek PJ, Reijo-Pera RA (2009) Isolation and characterization of pluripotent human spermatogonial stem cell-derived cells. Stem Cells 27(1):138–149. doi:10.1634/stemcells.2008-0439
White YA, Woods DC, Takai Y, Ishihara O, Seki H, Tilly JL (2012) Oocyte formation by mitotically active germ cells purified from ovaries of reproductive-age women. Nat Med 18(3):413–421. doi:10.1038/nm.2669
Zhang H, Zheng W, Shen Y, Adhikari D, Ueno H, Liu K (2012) Experimental evidence showing that no mitotically active female germline progenitors exist in postnatal mouse ovaries. Proc Natl Acad Sci USA 109(31):12580–12585. doi:10.1073/pnas.1206600109
Nagano MC (2007) In vitro gamete derivation from pluripotent stem cells: progress and perspective. Biol Reprod 76(4):546–551. doi:10.1095/biolreprod.106.058271
Hubner K, Fuhrmann G, Christenson LK, Kehler J, Reinbold R, De La Fuente R, Wood J, Strauss JF 3rd, Boiani M, Scholer HR (2003) Derivation of oocytes from mouse embryonic stem cells. Science 300(5623):1251–1256. doi:10.1126/science.1083452
West FD, Machacek DW, Boyd NL, Pandiyan K, Robbins KR, Stice SL (2008) Enrichment and differentiation of human germ-like cells mediated by feeder cells and basic fibroblast growth factor signaling. Stem Cells 26(11):2768–2776. doi:10.1634/stemcells.2008-0124
Hayashi Y, Saitou M, Yamanaka S (2012) Germline development from human pluripotent stem cells toward disease modeling of infertility. Fertil Steril 97(6):1250–1259. doi:10.1016/j.fertnstert.2012.04.037
Valli H, Phillips BT, Shetty G, Byrne JA, Clark AT, Meistrich ML, Orwig KE (2014) Germline stem cells: toward the regeneration of spermatogenesis. Fertil Steril 101(1):3–13. doi:10.1016/j.fertnstert.2013.10.052
Seisenberger S, Andrews S, Krueger F, Arand J, Walter J, Santos F, Popp C, Thienpont B, Dean W, Reik W (2012) The dynamics of genome-wide DNA methylation reprogramming in mouse primordial germ cells. Mol Cell 48(6):849–862. doi:10.1016/j.molcel.2012.11.001
Popp C, Dean W, Feng S, Cokus SJ, Andrews S, Pellegrini M, Jacobsen SE, Reik W (2010) Genome-wide erasure of DNA methylation in mouse primordial germ cells is affected by AID deficiency. Nature 463(7284):1101–1105. doi:10.1038/nature08829
Santos F, Peters AH, Otte AP, Reik W, Dean W (2005) Dynamic chromatin modifications characterise the first cell cycle in mouse embryos. Developmental biology 280(1):225–236. doi:10.1016/j.ydbio.2005.01.025
Mayer W, Niveleau A, Walter J, Fundele R, Haaf T (2000) Demethylation of the zygotic paternal genome. Nature 403(6769):501–502. doi:10.1038/35000654
Oswald J, Engemann S, Lane N, Mayer W, Olek A, Fundele R, Dean W, Reik W, Walter J (2000) Active demethylation of the paternal genome in the mouse zygote. Curr Biol 10(8):475–478
Shirakawa T, Yaman-Deveci R, Tomizawa S, Kamizato Y, Nakajima K, Sone H, Sato Y, Sharif J, Yamashita A, Takada-Horisawa Y, Yoshida S, Ura K, Muto M, Koseki H, Suda T, Ohbo K (2013) An epigenetic switch is crucial for spermatogonia to exit the undifferentiated state toward a Kit-positive identity. Development 140(17):3565–3576. doi:10.1242/dev.094045
Oakes CC, La Salle S, Smiraglia DJ, Robaire B, Trasler JM (2007) Developmental acquisition of genome-wide DNA methylation occurs prior to meiosis in male germ cells. Dev Biol 307(2):368–379. doi:10.1016/j.ydbio.2007.05.002
Yamaguchi S, Hong K, Liu R, Shen L, Inoue A, Diep D, Zhang K, Zhang Y (2012) Tet1 controls meiosis by regulating meiotic gene expression. Nature 492(7429):443–447. doi:10.1038/nature11709
La Salle S, Trasler JM (2006) Dynamic expression of DNMT3a and DNMT3b isoforms during male germ cell development in the mouse. Dev Biol 296(1):71–82. doi:10.1016/j.ydbio.2006.04.436
Marques CJ, Joao Pinho M, Carvalho F, Bieche I, Barros A, Sousa M (2011) DNA methylation imprinting marks and DNA methyltransferase expression in human spermatogenic cell stages. Epigenetics 6(11):1354–1361. doi:10.4161/epi.6.11.17993
Seki Y, Hayashi K, Itoh K, Mizugaki M, Saitou M, Matsui Y (2005) Extensive and orderly reprogramming of genome-wide chromatin modifications associated with specification and early development of germ cells in mice. Dev Biol 278(2):440–458. doi:10.1016/j.ydbio.2004.11.025
Hajkova P, Ancelin K, Waldmann T, Lacoste N, Lange UC, Cesari F, Lee C, Almouzni G, Schneider R, Surani MA (2008) Chromatin dynamics during epigenetic reprogramming in the mouse germ line. Nature 452(7189):877–881. doi:10.1038/nature06714
Papaioannou MD, Pitetti JL, Ro S, Park C, Aubry F, Schaad O, Vejnar CE, Kuhne F, Descombes P, Zdobnov EM, McManus MT, Guillou F, Harfe BD, Yan W, Jegou B, Nef S (2009) Sertoli cell Dicer is essential for spermatogenesis in mice. Dev Biol 326(1):250–259. doi:10.1016/j.ydbio.2008.11.011
Papaioannou MD, Lagarrigue M, Vejnar CE, Rolland AD, Kuhne F, Aubry F, Schaad O, Fort A, Descombes P, Neerman-Arbez M, Guillou F, Zdobnov EM, Pineau C, Nef S (2011) Loss of Dicer in Sertoli cells has a major impact on the testicular proteome of mice. Mol Cellular Proteomics 10 (4):M900587MCP900200. doi:10.1074/mcp.M900587-MCP200
Maatouk DM, Loveland KL, McManus MT, Moore K, Harfe BD (2008) Dicer1 is required for differentiation of the mouse male germline. Biol Reprod 79(4):696–703. doi:10.1095/biolreprod.108.067827
Zimmermann C, Romero Y, Warnefors M, Bilican A, Borel C, Smith LB, Kotaja N, Kaessmann H, Nef S (2014) Germ cell-specific targeting of DICER or DGCR8 reveals a novel role for endo-siRNAs in the progression of mammalian spermatogenesis and male fertility. PLoS ONE 9(9):e107023. doi:10.1371/journal.pone.0107023
Romero Y, Meikar O, Papaioannou MD, Conne B, Grey C, Weier M, Pralong F, De Massy B, Kaessmann H, Vassalli JD, Kotaja N, Nef S (2011) Dicer1 depletion in male germ cells leads to infertility due to cumulative meiotic and spermiogenic defects. PLoS ONE 6(10):e25241. doi:10.1371/journal.pone.0025241
Liang X, Zhou D, Wei C, Luo H, Liu J, Fu R, Cui S (2012) MicroRNA-34c enhances murine male germ cell apoptosis through targeting ATF1. PLoS ONE 7(3):e33861. doi:10.1371/journal.pone.0033861
Dai L, Tsai-Morris CH, Sato H, Villar J, Kang JH, Zhang J, Dufau ML (2011) Testis-specific miRNA-469 up-regulated in gonadotropin-regulated testicular RNA helicase (GRTH/DDX25)-null mice silences transition protein 2 and protamine 2 messages at sites within coding region: implications of its role in germ cell development. J Biol Chem 286(52):44306–44318. doi:10.1074/jbc.M111.282756
Yu Z, Raabe T, Hecht NB (2005) MicroRNA Mirn122a reduces expression of the posttranscriptionally regulated germ cell transition protein 2 (Tnp2) messenger RNA (mRNA) by mRNA cleavage. Biol Reprod 73(3):427–433. doi:10.1095/biolreprod.105.040998
Bjork JK, Sandqvist A, Elsing AN, Kotaja N, Sistonen L (2010) miR-18, a member of Oncomir-1, targets heat shock transcription factor 2 in spermatogenesis. Development 137(19):3177–3184. doi:10.1242/dev.050955
Liu Y, Niu M, Yao C, Hai Y, Yuan Q, Liu Y, Guo Y, Li Z, He Z (2015) Fractionation of human spermatogenic cells using STA-PUT gravity sedimentation and their miRNA profiling. Sci Rep 5:8084. doi:10.1038/srep08084
Saito S, Ugai H, Sawai K, Yamamoto Y, Minamihashi A, Kurosaka K, Kobayashi Y, Murata T, Obata Y, Yokoyama K (2002) Isolation of embryonic stem-like cells from equine blastocysts and their differentiation in vitro. FEBS Lett 531(3):389–396
Saito S, Sawai K, Ugai H, Moriyasu S, Minamihashi A, Yamamoto Y, Hirayama H, Kageyama S, Pan J, Murata T, Kobayashi Y, Obata Y, Yokoyama KK (2003) Generation of cloned calves and transgenic chimeric embryos from bovine embryonic stem-like cells. Biochem Biophys Res Commun 309(1):104–113
Saito S, Liu B, Yokoyama K (2004) Animal embryonic stem (ES) cells: self-renewal, pluripotency, transgenesis and nuclear transfer. Hum Cell 17(3):107–115
Saito S, Strelchenko N, Niemann H (1992) Bovine embryonic stem cell like-cell lines cultured over several passages. Roux’s Arch Dev Biol 201:134–141
Talluri TR, Kumar D, Glage S, Garrels W, Ivics Z, Debowski K, Behr R, Niemann H, Kues WA (2015) Derivation and characterization of bovine induced pluripotent stem cells by transposon-mediated reprogramming. Cell Reprogram 17:131–140
Behboodi E, Bondareva A, Begin I, Rao K, Neveu N, Pierson JT, Wylie C, Piero FD, Huang YJ, Zeng W, Tanco V, Baldassarre H, Karatzas CN, Dobrinski I (2011) Establishment of goat embryonic stem cells from in vivo produced blastocyst-stage embryos. Mol Reprod Dev 78(3):202–211. doi:10.1002/mrd.21290
Brackett BG, Baranska W, Sawicki W, Koprowski H (1971) Uptake of heterologous genome by mammalian spermatozoa and its transfer to ova through fertilization. Proc Natl Acad Sci USA 68(2):353–357
Lavitrano M, Camaioni A, Fazio VM, Dolci S, Farace MG, Spadafora C (1989) Sperm cells as vectors for introducing foreign DNA into eggs: genetic transformation of mice. Cell 57(5):717–723
Brinster RL, Sandgren EP, Behringer RR, Palmiter RD (1989) No simple solution for making transgenic mice. Cell 59(2):239–241
Lavitrano M, Stoppacciaro A, Bacci ML, Forni M, Fioretti D, Pucci L, Di Stefano C, Lazzereschi D, Rughetti A, Ceretta S, Zannoni A, Rahimi H, Moioli B, Rossi M, Nuti M, Rossi G, Seren E, Alfani D, Cortesini R, Frati L (1999) Human decay accelerating factor transgenic pigs for xenotransplantation obtained by sperm-mediated gene transfer. Transpl Proc 31(1–2):972–974
Aponte PM, Soda T, Teerds KJ, Mizrak SC, van de Kant HJ, de Rooij DG (2008) Propagation of bovine spermatogonial stem cells in vitro. Reproduction 136(5):543–557. doi:10.1530/REP-07-0419
Oatley JM, Reeves JJ, McLean DJ (2004) Biological activity of cryopreserved bovine spermatogonial stem cells during in vitro culture. Biol Reprod 71(3):942–947. doi:10.1095/biolreprod.104.028894
Petersen B, Niemann H (2015) Molecular scissors and their application in genetically modified farm animals. Transgenic Res 24(3):381–396. doi:10.1007/s11248-015-9862-z
Hayashi K, Ogushi S, Kurimoto K, Shimamoto S, Ohta H, Saitou M (2012) Offspring from oocytes derived from in vitro primordial germ cell-like cells in mice. Science 338(6109):971–975. doi:10.1126/science.1226889
Nakaki F, Hayashi K, Ohta H, Kurimoto K, Yabuta Y, Saitou M (2013) Induction of mouse germ-cell fate by transcription factors in vitro. Nature 501(7466):222–226. doi:10.1038/nature1241
Xie L, Lin L, Tang Q, Li W, Huang T, Huo X, Liu X, Jiang J, He G, Ma L (2015) Sertoli cell-mediated differentiation of male germ cell-like cells from human umbilical cord Wharton’s jelly-derived mesenchymal stem cells in an in vitro co-culture system. Eur J Med Res 20:9. doi:10.1186/s40001-014-0080-6
Duggal G, Heindryckx B, Warrier S, Taelman J, Van der Jeught M, Deforce D, Chuva de Sousa Lopes S, De Sutter P (2015) Exogenous supplementation of Activin A enhances germ cell differentiation of human embryonic stem cells. Mol Hum Reprod 21(5):410–423. doi:10.1093/molehr/gav004
Medrano JV, Ramathal C, Nguyen HN, Simon C, Reijo Pera RA (2012) Divergent RNA-binding proteins, DAZL and VASA, induce meiotic progression in human germ cells derived in vitro. Stem Cells 30(3):441–451. doi:10.1002/stem.1012
Durruthy Durruthy J, Ramathal C, Sukhwani M, Fang F, Cui J, Orwig KE, Reijo Pera RA (2014) Fate of induced pluripotent stem cells following transplantation to murine seminiferous tubules. Hum Mol Genet 23(12):3071–3084. doi:10.1093/hmg/ddu012
Ramathal C, Durruthy-Durruthy J, Sukhwani M, Arakaki JE, Turek PJ, Orwig KE, Reijo Pera RA (2014) Fate of iPSCs derived from azoospermic and fertile men following xenotransplantation to murine seminiferous tubules. Cell Rep 7(4):1284–1297. doi:10.1016/j.celrep.2014.03.067
Bao J, Li D, Wang L, Wu J, Hu Y, Wang Z, Chen Y, Cao X, Jiang C, Yan W, Xu C (2012) MicroRNA-449 and microRNA-34b/c function redundantly in murine testes by targeting E2F transcription factor-retinoblastoma protein (E2F-pRb) pathway. J Biol Chem 287(26):21686–21698. doi:10.1074/jbc.M111.32805
Yan N, Lu Y, Sun H, Tao D, Zhang S, Liu W, Ma Y (2007) A microarray for microRNA profiling in mouse testis tissues. Reproduction 134(1):73–79
Buchold GM, Coarfa C, Kim J, Milosavljevic A, Gunaratne PH, Matzuk MM (2010) Analysis of microRNA expression in the prepubertal testis. PLoS One 5(12):e15317. doi:10.1371/journal.pone.0015317
Ito T, Yagi S, Yamakuchi M (2010) MicroRNA-34a regulation of endothelial senescence. Biochem Biophys Res Commun 398(4):735–740. doi:10.1016/j.bbrc.2010.07.012
Vogt M, Munding J, Grüner M, Liffers ST, Verdoodt B, Hauk J, Steinstraesser L, Tannapfel A, Hermeking H (2011) Frequent concomitant inactivation of miR-34a and miR-34b/c by CpG methylation in colorectal, pancreatic, mammary, ovarian, urothelial, and renal cell carcinomas and soft tissue sarcomas. Virchows Arch 458(3):313–322. doi:10.1007/s00428-010-1030-5
Li M, Yu M, Liu C, Zhu H, He X, Peng S, Hua J (2013) miR-34c works downstream of p53 leading to dairy goat male germline stem-cell (mGSCs) apoptosis. Cell Prolif 46:223–231. doi:10.1002/jcb.24655
Yu M, Mu H, Niu Z, Chu Z, Zhu H, Hua J (2014) miR-34c enhances mouse spermatogonial stem cells differentiation by targeting Nanos2. J Cell Biochem 115(2):232–242. doi:10.1002/jcb.24655
Marcon E, Babak T, Chua G, Hughes T, Moens PB (2008) miRNA and piRNA localization in the male mammalian meiotic nucleus. Chromosome Res 16(2):243–260. doi:10.1007/s10577-007-1190-1196
Wu J, Bao J, Wang L, Hu Y, Xu C (2011) MicroRNA-184 downregulates nuclear receptor corepressor 2 in mouse spermatogenesis. BMC Dev Biol 11:64. doi:10.1186/1471-213X-11-64
Liu T, Huang Y, Liu J, Zhao Y, Jiang L, Huang Q, Cheng W, Guo L (2013) MicroRNA-122 influences the development of sperm abnormalities from human induced pluripotent stem cells by regulating TNP2 expression. Stem Cells Dev 22:1839–1850
McIver SC, Stanger SJ, Santarelli DM, Roman SD, Nixon B, McLaughlin EA (2012) A unique combination of male germ cell miRNAs coordinates gonocyte differentiation. PLoS One 7(4):e35553. doi:10.1371/journal.pone.0035553
Acknowledgments
The authors thank CC Ku for technical support and editing the manuscript. This work was partially supported by grants of Taiwan; National Science Council, MOST-104-2320-B-037-033-My2; MOST-103-2314-B-037-002; National Health Research Institutes, NHRI-Ex102-10109BI; NHRI-EX104-10416SI; Kaohsiung Medical University, KMY-DT-104001; KMU-TP103G00, KMU-TP103G03, KMU-TP103G04, KMU-TP103G05, KMU-TP103A04.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors have declared that they have no conflict of interest.
Additional information
The authors retracted this review article in agreement with the Editor-in-Chief. A part of the review, which was added during revision, had been duplicated from the following articles and was not adequately referred to in their manuscript:
1. MicroRNAs and DNA methylation as epigenetic regulators of mitosis, meiosis and spermiogenesis. Chencheng Yao, Yun Liu, Min Sun, Minghui Niu, Qingqing Yuan, Yanan Hai, Ying Guo, Zheng Chen, Jingmei Hou, Yang Liu and Zuping He. Reproduction 2015, 150: R25-R34
2. Germ line development: lessons learned from pluripotent stem cells. Ana M Martinez-Arroy, Jose V Medrano, Jose Remohi and Carlos Simon. Current Opinion in Genetics & Development 2014, 28:64-70
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Saito, S., Lin, YC., Murayama, Y. et al. RETRACTED ARTICLE: In vitro derivation of mammalian germ cells from stem cells and their potential therapeutic application. Cell. Mol. Life Sci. 72, 4545–4560 (2015). https://doi.org/10.1007/s00018-015-2020-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-015-2020-1