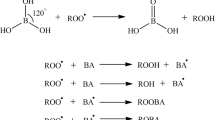
Abstract
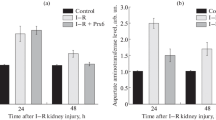
To explain the mechanism of renal injury caused by liver ischemia-reperfusion, we investigated biochemical and morphological changes in the liver and kidney in rats. After reperfusion following 60 min of liver ischemia, numerous changes were found. The level of serum transaminases and lipid peroxide formation in the liver tissue increased significantly. Electron microscopic studies revealed that most of the hepatocytes had swollen mitochondria and clumping of the nuclear chromatin. The sinusoidal endothelium was disrupted and the sinusoidal lumen was filled with numerous erythrocytes. Blood endotoxin concentration, plasma lipid peroxide levels, and serum β-glucuronidase activities were significantly higher than in the control group. Biochemical and morphological renal injury was also observed. Tissue lipid peroxide levels increased in both the kidney and the liver. Microscopic examination revealed damage to the renal tubules, including interstitial edema, dilatation of the lumen, and granular casts derived from necrotic cells in the proximal convoluted tubule. The levels of urinary N-acetyl-β-d-glucosaminidase (NAG) in the liver ischemia-reperfusion group were also higher than in the control group. These results suggest that the renal injury was caused by an increase in endotoxin, lipid peroxide, and lysosomal enzymes in the blood following the liver injury induced by the ischemia-reperfusion.
Similar content being viewed by others
References
Adkison D, Höllwarth ME, Benoit JN, Parks DA, McCord JM, Granger DN (1986) Role of free radicals in ischemia-reperfusion injury to the liver. Acta Physiol Scand (suppl) 548: 101–107
Arthur MJP, Bentley IS, Tanner AR, Saunders PK, Millward-Sadler GH, Wright R (1985) Oxygen-derived free radicals promote hepatic injury in the rat. Gastroenterology 89: 1114–1122
Atalla SL, Toledo-Pereyra LH, MacKenzie GH, Cederna JP (1985) Influence of oxygen-derived free radical scavengers on ischemic livers. Transplantation 40: 584–590
Egashira T, Nagai T, Kinba Y, Murayama F, Goto S, Kudo Y, Sudo S, Kono T, Yamanaka Y (1991) An injury of the liver caused by ischemia-reperfusion in rat liver. Folia Pharmacol Japon 97: 339–350
Epstein M, Berk DP, Hollenberg NK, Adams DF, Chalmers TC, Abrams HL, Merrill JP (1970) Renal failure in the patient with cirrhosis. Am J Med 49: 175–185
Evrin PE, Peterson PA, Wide L, Berggard I (1971) Radioimmunoassay of β2-microglobulin in human biological fluids. Scand J Clin Lab Invest 28: 439–443
Fishman WH, Kato K, Anstiss CL, Green S (1967) Human serum β-glucuronidase; its measurement and some of its properties. Clin Chim Acta 15: 435–447
Fridovich I (1983) Superoxide radical: an endogenous toxicant. Annu Rev Pharmacol Toxicol 23: 239–257
Glenn TM, Lefer AM, Beardsley AC, Ferguson WW, Lopez-Rasi AM, Serate TS, Morris JR, Wangensteen SL (1972) Circulatory responses to splanchnic lysosomal hydrolases in the dog. Ann Surg 176: 120–127
Heptinstall RH (1974) Acute renal failure. In: Heptinstall RH (ed) Pathology of the kidney, vol II. Little Brown Boston, pp 781–820
Janoff A, Weissmann G, Zweifach BW, Thomas L (1962) Pathogenesis of experimental shock. IV. Studies on lysosomes in normal and tolerant animals subjected to lethal trauma and endotoxemia. J Exp Med 116: 451–466
Lefer AM (1970) Role of a myocardial depressant factor in the pathogenesis of circulatory shock. Fed Proc 29: 1836–1847
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193: 265–275
McCord JM (1985) Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med 312: 159–163
McCord JM, Roy RS (1982) The pathophysiology of superoxide: roles in inflammation and ischemia. Can J Physiol Pharmacol 60: 1346–1352
Mead JF (1976) Free radical mechanisms of lipid damage and consequences for cellular membranes. In: Prior WA (ed) Free radicals in biology, Vol. 1. Academic Press New York, pp 51–68
Miyazawa T, Saeki R, Inaba H (1989) Detection of chemiluminescence in lipid peroxidation in biological systems and its application to HPLC. J Biolumin Chemilumin 4: 475–478
Morin JP, Viotte G, Vandewalle A, Van Hoof F, Tulkens P, Fillastre JP (1980) Gentamicin-induced nephrotoxicity: a cell biology approach. Kidney Int 18: 583–590
Nagai T, Egashira T, Yamanaka Y (1990) Effect of bifemelane hydrochloride on an injury of the liver caused by ischemia-reperfusion in rats. Jpn J Pharmacol 52: 383–385
Nagai T, Egashira T, Yamanaka Y, Kohno M (1991) The protective effect of glycyrrhizin against injury of the liver caused by ischemiareperfusion. Arch Environ Contam Toxicol 20: 432–436
Nagai T, Egashira T, Kudo Y, Yamanaka Y, Shimada T (1992) Attenuation of dysfunction in the ischemia-reperfused liver by Glycyrrhizin. Jpn J Pharmacol 58: 209–218
Nakamura M, Shimada T, Fujimori O (1980) Ultrastructual studies on the pancreatic polypeptide cell of the rat with special reference to pancreatic regional differences and changes induced by alloxan diabetes. Acta Anat 108: 193–201
Nakano M, Kimura H, Hara M, Kuroiwa M, Kato M, Totsune K, Yoshikawa T (1991) A highly sensitive method for determining both Mn- and Cu-Zn superoxide dismutase activities in tissues and blood cells. Anal Biochem 187: 277–280
Nishigaki I, Hagihara M, Hiramatsu M, Izawa Y, Yagi K (1980) Effect of thermal injury on lipid peroxide levels of rat. Biochem Med 24: 185–189
Noto A, Ogawa Y, Mori S, Yoshioka M, Kitakaze T, Hori T, Nakamura M (1983) Simple, rapid spectrophotometry of urinary N-acetylβ-d-glucosaminidase, with use of a new chromogenic substrate. Clin Chem 29: 1713–1716
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95: 351–358
Oyanagui Y (1984) Reevaluation of assay methods and establishment of kit for superoxide dismutase activity. Anal Biochem 142: 290–296
Peeters-Joris C, Vandevoorde A-M, Baudhuin P (1975) Subcellular localization of superoxide dismutase in rat liver. Biochem J 150: 31–39
Peterson PA, Evrin P-E, Berggard I (1969) Differentiation of glomerular, tubular, and normal proteinuria: Determinations of urinary excretion of β2-microglobulin, albumin, and total protein. J Clin Invest 48: 1189–1198
Richman AV, Gerber LI, Balis JU (1980) Peritubular capillaries. A major target site of endotoxin-induced vascular injury in the primate kidney. Lab Invest 43: 327–332
Roders MK, Glende EA, Recknagel RO (1978) NADPH-dependent microsomal lipid peroxidation and the problem of pathological action at a distance. New data on induction of red cell damage. Biochem Pharmacol 27: 437–443
Schlesinger P, Rodman JS, Frey M, Lang S, Stahl P (1976) Clearance of lysosomal hydrolases following intravenous infusion. The role of liver in the clearance of β-glucuronidase and N-acetyl-β-d-glucosaminidase. Arch Biochem Biophys 177: 606–614
Slater TF (1984) Free-radical mechanisms in tissue injury. Biochem J 222: 1–15
Takayama F, Egashira T, Kudo Y, Yamanaka Y (1992) Chemiluminescence-HPLC assay of phosphatidylcholine hydroperoxide generated by ischemia-reperfusion in the liver of rats. Biochem Pharmacol 44: 2412–2414
Tamura H, Obayashi T, Takagi K, Tanaka S, Nakahara C, Kawai T (1982) Perchloric acid treatment of human blood for quantitative endotoxin assay using synthetic chromogenic substrate for horseshoe crab clotting enzyme. Thromb Res 27: 51–57
Tappel AL (1973) Lipid peroxidation damage to cell components. Fed Proc 32: 1870–1874
Wilkinson SP, Arroyo V, Gazzard BG, Moodie H, Williams R (1974a) Relation of renal impairment and haemorrhagic diathesis to endotoxaemia in fulminant hepatic failure. Lancet 30: 521–524
Wilkinson SP, Blendis LM, Williams R (1974b) Frequency and type of renal and electrolyte disorders in fulminant hepatic failure. BMJ 2: 186–189
Wilkinson SP, Arroyo VA, Moodie H, Blendis LM, Williams R (1976) Abnormalities of sodium excretion and other disorders of renal function in fulminant hepatic failure. Gut 17: 501–505
Wilkinson SP, Moodie H, Arroyo VA, Williams R (1977) Frequency of renal impairment in paracetamol overdose compared with other causes of acute liver damage. J Clin Pathol 30: 141–143
Wilkinson SP, Hirst D, Day DW, Williams R (1978) Spectrum of renal tubular damage in renal failure secondary to cirrhosis and fulminant hepatic failure. J Clin Pathol 31: 101–107
Yajima Y, Fukuda I, Otsuki M, Suzuki H, Ota S, Ishii M, Mori K, Goto Y (1985) Endotoxemia in liver diseases: Detection by a quantitative assay using chromogenic substrate with perchloric acid pretreatment. Tohoku J Exp Med 147: 411–419
Yamamoto Y, Brodsky MH, Baker JC, Ames BN (1987) Detection and characterization of lipid hydroperoxides at picomole levels by highperformance liquid chromatography. Anal Biochem 160: 7–13
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kudo, Y., Egashira, T., Takayama, F. et al. Investigation of the renal injury caused by liver ischemia-reperfusion in rats. Arch Toxicol 67, 502–509 (1993). https://doi.org/10.1007/BF01969922
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01969922