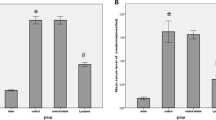
Oxidative kidney injury was compared in newborn and adult rats under conditions of ischemia/reperfusion and in experimental model of systemic inflammation induced by endotoxin (LPS of bacterial cell wall) administration. Oxidative stress in the kidney accompanied both experimental models, but despite similar oxidative tissue damage, kidney dysfunction in neonates was less pronounced than in adult animals. It was found that neonatal kidney has a more potent regenerative potential with higher level of cell proliferation than adult kidney, where the level proliferating cell antigen (PCNA) increased only on day 2 after ischemia/reperfusion. The pathological process in the neonatal kidney developed against the background of active cell proliferation, and, as a result, proliferating cells could almost immediately replace the damaged structures. In the adult kidney, regeneration of the renal tissue was activated only after significant loss of functional nephrons and impairment of renal function.
Similar content being viewed by others
References
Adachi S, Zelenin S, Matsuo Y, Holtback U. Cellular response to renal hypoxia is different in adolescent and infant rats. Pediatr. Res.2004;55(3):485-491.
Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P; Acute Dialysis Quality Initiative workgroup. Acute renal failure — definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit. Care.2004;8(4):R204-R212.
El-gammal AA, Ibrahim OY, Shaban SF, Dessouky AA. Postnatal development of the albino rat renal cortex (histological study). Egypt. J. Histol. 2010;33(4):745-756.
Fortenberry JD, Paden ML, Goldstein SL. Acute kidney injury in children: an update on diagnosis and treatment. Pediatr. Clin. North. Am. 2013;60(3):669-688.
Hoseini R, Otukesh H, Rahimzadeh N, Hoseini S. Glomerular function in neonates. Iran J. Kidney Dis. 2012;6(3):166-172.
Humphreys BD. Kidney injury, stem cells and regeneration. Curr. Opin. Nephrol. Hypertens. 2014;23(1):25-31.
Jiang JS, Chou HC, Yeh TF, Chen CM. Neonatal hyperoxia exposure induces kidney fibrosis in rats. Pediatr. Neonatol. 2015;56(4):235-241.
Nobakht M, Taki MT, Rezazadeh M, Torbaghan SS. Stages of development of renal glomeruli in the newborn rat kidney. Med. J. Islam. Repub. Iran. 1995;9(2):147-152.
Plotnikov EY, Chupyrkina AA, Pevzner IB, Isaev NK, Zorov DB. Myoglobin causes oxidative stress, increase of NO production and dysfunction of kidney’s mitochondria. Biochim. Biophys. Acta. 2009;1792(8):796-803.
Plotnikov EY, Morosanova MA, Pevzner IB, Zorova LD, Manskikh VN, Pulkova NV, Galkina SI, Skulachev VP, Zorov DB. Protective effect of mitochondria-targeted antioxidants in an acute bacterial infection. Proc. Natl Acad. Sci. USA. 2013;110(33):E3100-E3108.
Plotnikov EY, Kazachenko AV, Vyssokikh MY, Vasileva AK, Tcvirkun DV, Isaev NK, Kirpatovsky VI, Zorov DB. The role of mitochondria in oxidative and nitrosative stress during ischemia/reperfusion in the rat kidney. Kidney Int. 2007;72(12):1493-1502.
Rookmaaker MB, Joles JA. The nephron number counts — from womb to tomb. Nephrol. Dial. Transplant. 2013;28(6):1325-1328.
Saint-Faust M, Boubred F, Simeoni U. Renal development and neonatal adaptation. Am. J. Perinatol. 2014;31(9):773-780.
Stojanović VD, Vučković NM, Barišić NA, Srdić B, Doronjski AD, Peco Antić AE. Early biomarkers of renal injury and protective effect of erythropoietin on kidneys of asphyxiated newborn rats. Pediatr. Res. 2014;76(1):11-16.
Zahedi K, Wang Z, Barone S, Tehrani K, Yokota N, Petrovic S, Rabb H, Soleimani M. Identification of stathmin as a novel marker of cell proliferation in the recovery phase of acute ischemic renal failure. Am. J. Physiol. Cell. Physiol. 2004;286(5):C1203-C1211.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Byulleten’ Eksperimental’noi Biologii i Meditsiny, Vol. 165, No. 2, pp. 148-154, February, 2018
Rights and permissions
About this article
Cite this article
Pevzner, I.B., Pavlenko, T.A., Popkov, V.A. et al. Comparative Study of the Severity of Renal Damage in Newborn and Adult Rats under Conditions of Ischemia/Reperfusion and Endotoxin Administration. Bull Exp Biol Med 165, 189–194 (2018). https://doi.org/10.1007/s10517-018-4127-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10517-018-4127-5