Abstract
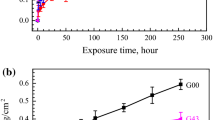
In the present study, the effect of adding yttrium to alloys is investigated. The microstructure of the cast Fe-25Cr-4Al-0.5Y alloy used in the study shows that the vttrium is present in different shapes and sizes as the intermetallic phase, (Fe, Cr)4(Al, Y), previously unreported in the literature. Upon oxidation in dry oxygen in the 1100–1200 °C temperature range, a columnar, fine-grained (0.5–1 μm) α-Al2O3 scale is formed which grows predominantly by inward oxygen grain-boundary transport. The intermetallic phase, during incorporation into the oxide scale, is converted into Y3Al5O12, the chromium and iron from the intermetallic diffusing back into the metal matrix. The Y3Al5O12 phase saturates the oxide scale with yttrium, which segregates to oxide grain boundaries. The microstructural features of the oxide scale resemble those of the scale formed on the yttria-dispersed alloy we investigated earlier. The improved adherence of the oxide scale is a consequence of yttrium doping, which facilitates the formation of a fine-grained scale in which oxide growth stresses can be relieved by diffusional plastic flow. Further, yttrium suppresses Al transport in the oxide scale and prevents Al2O3 nucleation within the scale, a process which can generate compressive stresses in the scale. The yttrium doping in the oxide scale is somewhat more efficient when it is present as a dispersoid in the metal.
Similar content being viewed by others
References
E. J. Febten,J. Electrochem. Soc. 108, 490 (1961).
J. M. Francis and W. H. Whitlow,Corr. Sci. 5, 701 (1965).
G. C. Wood and J. Boustead,Corr. Sci. 8, 719 (1968).
J. E. Antill and K. A. Peakall,J. Iron Steel Inst. 205, 1136 (1967).
A. M. Beltran,Cobalt 46, 3 (1970).
J. M. Francis and J. A. Jutson,Corr. Sci. 8, 445 (1968).
J. K. Tien and F. S. Pettit,Met. Trans. 3, 1587 (1972).
I. A. Kvernes,Oxid. Met. 6, 45 (1973).
J. D. Kuenzly and D. L. Douglass,Oxid. Met. 8, 139 (1974).
A. Kumar, M. Nasrallah, and D. L. Douglass,Oxid. Met. 8, 227 (1974).
F. A. Golightly, F. H. Stott, and G. C. Wood,Oxid. Met. 10, 163 (1976).
F. A. Golightly, F. H. Stott, and G. C. Wood,J. Electrochem. Soc. 126, 1035 (1979).
I. M. Allam, D. P. Whittle, and J. Stringer,Oxid. Met. 12, 35 (1978).
J. C. Pivin, D. Delaunay, C. Roques-Carmes, A. M. Huntz, and P. Lacombe,Corr. Sci. 20, 351 (1980).
D. Delaunay and A. M. Huntz,J. Mat. Sci. 17, 2027 (1982).
C. S. Giggins and F. S. Pettit,Met. Trans. 2, 1071 (1971).
D. P. Whittle, M. E. El-Dashan, and J. Stringer,Corr. Sci. 17, 879 (1977).
J. Stringer, B. A. Wilcox, and R. I. Jaffee,Oxid. Met. 5, 11 (1972).
G. R. Wallwork and A. Z. Hed.Oxid. Met. 3, 229 (1971).
J. Stringer and I. G. Wright,Oxid. Met. 5, 59 (1952).
J. Stringer, A. Z. Hed, G. R. Wallwork, and B. A. Wilcox,Corr. Sci. 12, 625 (1972).
H. M. Fowler and B. A. Wilcox,Corr. Sci. 17, 253 (1977).
H. H. Davis, H. C. Graham, and I. A. Kvernes,Oxid. Met. 3, 431 (1971).
I. G. Wright and B. A. Wilcox,Met. Trans. 5, 957 (1974).
A. U. Seybolt,Corr. Sci. 6, 263 (1966).
M. S. Seltzer, A. A. Wilcox, and J. Stringer,Met. Trans. 3, 2391 (1972).
I. M. Allam, D. P. Whittle, and J. Stringer,Met. Trans. 13, 381 (1979).
H. T. Michels,Met. Trans. 7A, 379 (1976).
O. T. Goncel, D. P. Whittle, and J. Stringer,Corr. Sci. 19, 305 (1979).
I. G. Wright and J. Stringer,Metal. 6, 65 (1973).
D. P. Whittle and J. Stringer,Phil. Trans. Roy Soc. Lond. A295, 309 (1980).
T. A. Ramanarayanan, M. Raghavan, and R. Petkovic-Luton,J. Electrochem. Soc. 131, 923 (1984).
M. Raghavan, T. Ramanarayanan, R. Petkovic-Luton, and J. C. Scanlon,Met. Trans., to appear.
M. Raghavan, J. W. Steeds, and R. Petkovic-Luton,Met. Trans. 13A, 1953 (1982).
J. L. Smialek and R. Gibala, NASA Techn. Memo. 79259 (July 1979).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ramanarayanan, T.A., Raghavan, M. & Petkovic-Luton, R. Metallic yttrium additions to high temperature alloys: Influence on Al2O3 scale properties. Oxid Met 22, 83–100 (1984). https://doi.org/10.1007/BF00656898
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00656898