Abstract
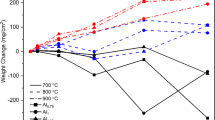
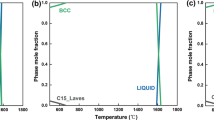
Oxidation of a Ni–16Mo–7Cr–4Fe superalloy containing various yttrium concentrations (0.00, 0.05, 0.12, 0.21 and 0.43 wt%) was undertaken in air at 1273 K for times up to 250 h. The nature and the structure of the oxide scales were investigated by synchrotron radiation techniques, TEM, SEM, XPS, etc. The oxidation kinetics of the alloys containing a low Cr content of 7 wt% sectionally obeyed the parabolic law. The oxidation rate of the alloy in the steady-state stage was reduced by about a factor of 30 by the micro-addition of 0.05 wt% yttrium. Yttrium microalloying greatly enhanced the selective oxidation of chromium and promoted the formation of a compact inner Cr2O3-enriched layer, which can inhibit the outward diffusion of oxidizable elements, especially the volatile-oxide-forming-element Mo, and remarkably improve the adhesion of the oxide scale to the matrix. The oxide scale of the alloy containing 0.05 wt% Y had a thin duplex structure: an outer NiO/NiFe2O4 layer and an inner Cr2O3/YCrO3/spinel oxide layer. In comparison, the oxide scale of the Y-free alloy and the alloys containing excess Y roughly had a thick triple-layer structure: an outmost NiO/NiFe2O4 layer, an intermediate Mo0.84Ni0.16/Cr2O3/spinel oxide layer and an inner Cr2O3/spinel oxide layer. Increasing the concentration of Y in solid solution and reducing the amount of Y-bearing compound are helpful to optimize the effect of Y on improving the oxidation resistance of the alloy.











Similar content being viewed by others
References
J. W. Koger, in ORNL/TM-4189 (Oak Ridge National Laboratory, 1972).
J. H. DeVan, in ORNL/TM-2021, Vol. 1 (Oak Ridge National Laboratory, 1969).
W. R. Huntley and P. A. Gnadt, ORNL-TM-3863 (Oak Ridge National Laboratory (operated by Union Carbide Corporation for the US Atomic Energy Commission), Oak Ridge, Tennessee, 1973).
J. X. Fan, J. X. Zhang, Y. L. Lu, Z. J. Li, X. T. Zhou and P. Huai, Chinese Journal of Rare Metals 39, 399–405 (2015).
J. X. Fan, Y. L. Lu, Z. J. Li, J. S. Dong, J. X. Zhang, X. T. Zhou and P. Huai, Rare Metal Materials and Engineering 44, 1953–1958 (2015).
T. Liu, J. S. Dong, H. Li, Z. J. Li, X. T. Zhou and L. H. Lou, Chinese Journal of Materials Research 28, 895–900 (2014).
L. E. McNeese, in ORNL-5047 (Oak Ridge National Laboratory, 1975), p. 125–150.
L. E. McNeese, in ORNL-5132 (Oak Ridge National Laboratory, 1976), p. 1–15.
S. X. Wang, C. Li, B. J. Xiong, X. B. Tian and S. Q. Yang, Applied Surface Science 257, 5826–5830 (2011).
J. T. Lu, S. L. Zhu and F. H. Wang, Oxidation of Metals 76, 67–82 (2011).
R. G. Ding, O. A. Ojo and M. C. Chaturvedi, Intermetallics 15, 1504–1510 (2007).
J. H. Luan, Z. B. Jiao, G. Chen and C. T. Liu, Journal of Alloys and Compounds 602, 235–240 (2014).
D. Monceau, D. Oquab, C. Estournes, M. Boidot, S. Selezneff, Y. Thebault and Y. Cadoret, Surface & Coatings Technology 204, 771–778 (2009).
R. Vilar, E. C. Santos, P. N. Ferreira, N. Franco and R. C. da Silva, Acta Materialia 57, 5292–5302 (2009).
K. L. Wang, Q. B. Zhang, M. L. Sun, X. G. Wei and Y. M. Zhu, Applied Surface Science 174, 191–200 (2001).
E. Bullock, C. Lea and M. McLean, Philosophical Transactions of the Royal Society A Mathematical, Physical and Engineering Sciences 295, 332 (1980).
C. G. Hsu and J. M. Pan, Analyst 110, 1245–1248 (1985).
A. Sato, H. Harada, Y. Koizumi, T. Kobayashi and H. Imai, Journal of the Japan Institute of Metals 70, 380–383 (2006).
F. H. Stott, G. C. Wood and J. G. Fountain, Oxidation of Metals 14, 135–146 (1980).
P. A. Vozzella and D. A. Condit, Analytical Chemistry 60, 2497–2500 (1988).
C. B. Xiao and Y. F. Han, Scripta Materialia 41, 1217–1221 (1999).
P. J. Zhou, J. J. Yu, X. F. Sun, H. R. Guan, X. M. He and Z. Q. Hu, Materials Science and Engineering A 551, 236–240 (2012).
P. J. Zhou, J. J. Yu, X. F. Sun, H. R. Guan and Z. Q. Hu, Scripta Materialia 57, 643–646 (2007).
P. Moulin, A. M. Huntz and P. Lacombe, Acta Metallurgica 28, 1295–1300 (1980).
Y. D. Zhang, C. Zhang, H. Lan, P. Y. Hou and Z. G. Yang, Corrosion Science 53, 1035–1043 (2011).
F. C. Nunes, J. Dille, J. L. Delplancke and L. H. de Almeida, Scripta Materialia 54, 1553–1556 (2006).
T. B. Massalski, H. Okamoto and P. R. Subramanian, Binary Alloy Phase Diagrams Vol. 2 (ASM International, Willian W. Scott, 1986).
Z. Zhang, J. Wang, E.-H. Han and W. Ke, Corrosion Science 53, 3623–3635 (2011).
S. E. Ziemniak and M. Hanson, Corrosion Science 48, 498–521 (2006).
A. A. Hermas, Corrosion Science 50, 2498–2505 (2008).
P. Stefanov, D. Stoychev, I. Valov, A. Kakanakova-Georgieva and T. Marinova, Materials Chemistry and Physics 65, 222–225 (2000).
H. Sun, X. Q. Wu and E. H. Han, Corrosion Science 51, 2565–2572 (2009).
A. Machet, A. Galtayries, S. Zanna, L. Klein, V. Maurice, P. Jolivet, M. Foucault, P. Combrade, P. Scott and P. Marcus, Electrochimica Acta 49, 3957–3964 (2004).
N. S. McIntyre, T. E. Rummery, M. G. Cook and D. Owen, Journal of the Electrochemical Society 123, 1164–1170 (1976).
N. S. McIntyre, D. G. Zetaruk and D. Owen, Journal of the Electrochemical Society 126, 750–760 (1979).
Q. Zhang, R. Tang, K. J. Yin, X. Luo and L. F. Zhang, Corrosion Science 51, 2092–2097 (2009).
C. D. Wagner, W. M. Riggs, L. E. Davis, J. F. Moulder and G. E. Muilenberg, Handbook of X-Ray Photoelectron Spectroscopy, (Perkin-Elmer Co., Minneapolis, 1979).
J. B. Yan, Y. M. Gao, L. Liang, Z. Z. Ye, Y. F. Li, W. Chen and J. J. Zhang, Corrosion Science 53, 329–337 (2011).
V. D. Divya, S. S. K. Balam, U. Ramamurty and A. Paul, Scripta Materialia 62, 621–624 (2010).
R. A. Rapp, Metallurgical Transactions B 15B, 195–212 (1984).
P. Y. Hou, in Shreir’s Corrosion (2010), p. 195–239.
G. C. Wood and F. H. Stott, Materials Science and Technology 3, 519–530 (1987).
E. A. Brandes and G. B. Brook (eds.), Smithells Metals Reference Book, 7th ed, (Butterworth-Heinemann Ltd., Oxford, 1992).
M. Sennour, L. Marchetti, F. Martin, S. Perrin, R. Molins and M. Pijolat, Journal of Nuclear Materials 402, 147–156 (2010).
S. Tei, M. Hirotoshi, S. Tatsuya, F. Yasuhiko and K. Kiyoshi, Journal of Plasma and Fusion Research 78, 3–4 (2002).
G. R. Smolik, D. A. Petti and S. T. Schuetz, Journal of Nuclear Materials 283–287, 1458–1462 (2000).
D. P. Moon, Materials Science and Technology 5, 754–764 (1989).
P. Y. Hou, Materials Science Forum 696, 39–44 (2011).
X. L. Li, S. M. He, X. T. Zhou, P. Huai, Z. J. Li, A. G. Li and X. H. Yu, Journal of Nuclear Materials 464, 342–345 (2015).
Y. Saito, B. Önay and T. Maruyama, Journal de Physique IV Colloque 03, 217–230 (1993).
T. Amanoa, H. Isobe, N. Sakai and T. Shishido, Journal of Alloys and Compounds 344, 394–400 (2002).
D. P. Whittle and J. Stringer, Philosophical Transactions of the Royal Society of London Series A 295, 309–329 (1980).
Acknowledgements
This work is financially supported by the National Natural Science Foundation of China (Nos. 51371189, 11775292, 51801227) and the National key research and development program of China (2016YFB0700404). The authors also wish to thank beamlines BL15U1 and BL14B1 (Shanghai Synchrotron Radiation Facility) for providing the beam time and help during experiments.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, X., He, S., Liang, J. et al. High-Temperature Oxidation Behavior and Oxide Scale Structure of Yttrium-Modified Ni–16Mo–7Cr–4Fe Superalloy at 1273 K. Oxid Met 92, 67–88 (2019). https://doi.org/10.1007/s11085-019-09914-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11085-019-09914-0