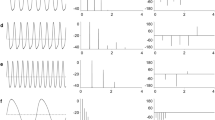
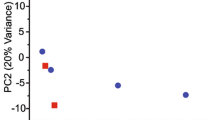
Summary
Sternopygus dariensis, a weakly electric gymnotiform fish, possesses sexually different electric organ discharges (EODs), with females emitting EODs at higher frequencies than males. This difference can be accounted for by the effects of gonadal steroids: laboratory experiments indicate that daily injections (0.25 to 20 μg/g body weight) of androgens lower discharge frequencies while injections of estrogens raise discharge frequencies. Clear changes in discharge frequencies can be noted over the course of days, and persist until several days after injections are terminated. These effects are noted irrespective of age or sex, and can be seen in both intact and gonadectomized animals. The discharge frequencies of these animals are established by a neuronal oscillator, the medullary pacemaker nucleus. Thus, the steroid-elicited changes in discharge frequencies are indicative of changes in the activity of the pacemaker nucleus. Steroids may be exerting their effects either through indirect effects upon those neurons which provide input to the pacemaker, or through direct effects upon the spontaneous depolarizations of the pacemaker itself.
Similar content being viewed by others
Abbreviations
- DHT :
-
5-α-dihydrotestosterone
- EOD :
-
electric organ discharge
References
Bass AH, Hopkins CD (1983) Hormonal control of sexual differentiation: Changes in electric organ discharge wave form. Science 220:971–974
Bennett MVL (1971) Electric organs. In: Hoar WS, Randall DH (eds) Fish physiology, vol 5. Academic Press, New York, pp 493–574
Bennett MVL, Pappas GD, Alijure E, Nakajima Y (1967) Physiology and ultrastructure of electrotonic junctions. II. Spinal and medullary electromotor nuclei in mormyrid fish. J Neurophysiol 30:180–208
Breedlove SM, Arnold AP (1980) Hormone accumulation in a sexually dimorphic nucleus of the rat spinal cord. Science 210:564–566
Bullock TH, Behrend K, Heiligenberg W (1975) Comparison of the jamming avoidance response in gymnotoid and gymnarchid electric fish: A case of convergent evolution and its sensory basis. J Comp Physiol 103:97–121
Dorner G, Kawakami M (1978) Hormones and Brain Development. Developments in endocrinology, vol 3. Elsevier/ North Holland Biomedical Press, Amsterdam New York Oxford
Edwards DA, Rowe FA (1975) Neural and endocrine control of aggressive behavior. In: Eleftherion BE, Sprott RL (eds) Hormonal correlates of behavior, vol 1. Plenum Press, New York, pp 275–303
Emmens CW, Martin L (1964) Anti-estrogens. In: Dorfman RI (ed) Methods in hormone research, vol III. Academic Press, New York, pp 81–125
Enger PS, Szabo T (1968) Effect of temperature on discharge rates of the electric organ of some gymnotids. Comp Biochem Physiol 27:625–627
Heiligenberg W (1977) Principles of electrolocation and jamming avoidance in electric fish. Springer, Berlin Heidelberg New York
Heiligenberg W, Finger T, Matsubara J, Carr C (1981) Input to the medullary pacemaker nucleus in the weakly electric fishEigenmannia (Sternopygidae, Gymnotiformes). Brain Res 211:418–423
Hopkins CD (1972) Sex differences in electric signaling in an electric fish. Science 176:1035–1037
Hopkins CD (1974a) Electric communication in the reproductive behavior ofSlernopygus macrurus (Gymnotoidei). Z Tierpsychol 35:518–535
Hopkins CD (1974b) Electric communication: Functions in the social behavior ofEigenmannia virescens. Behavior 50:270–305
Hopkins CD (1977) Electric communication. In: Sebeok T (ed) How animals communicate. Indiana Univ Press, Bloomington, pp 263–289
Kelley DB (1980) Auditory and vocal nuclei in the frog brain concentrate sex steroids. Science 207:553–555
Kelley DB, Morrell JI, Pfaff DW (1975) Autoradiographic localization of hormone concentrating cells in the brain of an amphibianXenopus laevis. J Comp Neurol 164:47–62
Kelley DB, Pfaff DW (1978) Generalizations from comparative studies on neuroanatomical and endocrine mechanisms of sexual behavior. In: Hutchison J (ed) Biological determinants of sexual behavior. Whiley, Chichester, England, pp 225–254
Kelly MJ, Moss RL, Dudley CA, Fawcett CP (1977) The specificity of the response of pre-optic septal area neurons to estrogens: 17 α vs 17 β estradiol and the response of extrahypothalamic neurons. Exp Brain Res 30:43–52
Kendrick KM, Drewett RF (1979) Testosterone reduces refractory period of stria terminalis neurons in the rat brain. Science 204:877–879
Lucker H, Kramer B (1981) Development of a sex difference in the preferred latency response in the weakly electric fish,Pollimyrus isidori (Cuvier et Valenciennes) (Mormyridae, Teleostei). Behav Ecol Sociobiol 9:103–109
Luine VN, Nottebohm F, Harding C, McCewen BS (1980) Androgen effects cholinergic enzymes in syringeal motor neurons and muscle. Brain Res 192:89–108
MacLusky NJ, Naftolin F (1981) Sexual differentiation of the central nervous system. Science 211:1294–1303
Matsubara J, Heiligenberg W (1978) How well do electric fish electrolocate under jamming? J Comp Physiol 125:285–290
Mazeaud NM, Mazeaud F, Donaldson EM (1977) Primary and secondary effects of stress in fish: Some new data with a general review. Trans Am Fish Soc 106(3):201–212
McCewen BS (1981) Neural gonadal steroid actions. Science 211:1303–1311
McCewen BS, Davis PG, Parsons B, Pfaff DW (1979) The brain as a target for steroid hormone action. Ann Rev Neurosci 2:65–112
Meyer JH, Zakon HH (1982) Androgens alter the tuning of electroreceptors. Science 217:635–637
Ozon R (1972) Androgens in fishes, amphibians, reptiles, and birds. In: Idler DR (ed) Steroids in nonmammalian vertebrates. Academic Press, New York London, pp 328–389
Sokal RR, RohIf FJ (1969) Biometrics. WH Freeman and Co, San Francisco
Teyler TJ, Vardaris RM, Lewis D, Rawitch AB (1980) Gonadal steroids: effects on excitability of hippocampal pyramidal cells. Science 209:1017–1019
Westby GW, Kirschbaum F (1977) Emergence and development of the electric organ discharge in the mormyrid fish,Pollimyrus isidori. I. The larval discharge. J Comp Physiol 122:251–271
Westby GW, Kirschbaum F (1981) Sex differences in the electric organ discharge ofEigenmannia virescens and the effect of gonadal maturation. In: Szabo T, Czeh G (eds) Adv Physiol Sci, vol 31. Sensory physiology of aquatic lower vertebrates. Pergamon Press, Budapest, pp 179–194
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Meyer, J.H. Steroid influences upon the discharge frequencies of a weakly electric fish. J. Comp. Physiol. 153, 29–37 (1983). https://doi.org/10.1007/BF00610339
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00610339