Astract
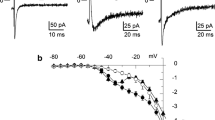
We investigated the electrical responses of Ca-activated K (KCa) currents induced by hypoxia and reduction or oxidation of the channel protein in pulmonary (PASMC) and ear (EASMC) arterial smooth muscle cells using the patch-clamp technique. In cell-attached patches, in the presence of a high K solution (containing 0.316 (μM Ca2+), the activity of KCa channels from PASMC was decreased (by 49±7% compared to control, pipette potential = −70 mV) by changing to a hypoxic solution (1 mM Na2S2O4, aeration with 100% N2 gas). EASMC channels did not respond to hypoxia. In order to investigate the possible mechanisms involved, using inside-out patches bathed symmetrically in 150 mM KCl, we applied redox couples to the intracellular side. Reducing agents, such as dithiothreitol (DDT, 5 mM), reduced glutathione, (GSH, 5 mM), and nicotinamide adenine dinucleotide reduced (NADH, 2 mM) decreased PASMC, but not EASMC, KCa channel activity. However, oxidizing agents such as 5,5′-dithio-bis(2-nitrobenzoic acid) (DTNB, 1 mM), oxidized glutathione (GSSG, 5 mM) and NAD (2 mM) increased KCa channel activity in both PASMC and EASMC. The increased activity due to oxidizing agents was restored by applying reducing agents. From these results, we could suggest that the basal redox state of the EASMC KCa channel is more reduced than that of the PASMC channel, since the response of KCa channels of the EASMC to intracellular reducing agents differs from that of the PASMC. This difference may be related to the different responses of PASMC and EASMC KCa channels to hypoxia.
Similar content being viewed by others
References
Archer SL, Huang J, Henry T, Peterson D, Wier EK (1993) A redox-based O2 sensor in rat pulmonary vasculature. Circ Res 73:1100–1112
Bennie RE, Packer CS, Powel DR, Jin N, Rhoades RA (1991) Biphasic contractile response of pulmonary artery to hypoxia. Am J Physiol 261:L156-L163
Biscoe TJ, Duchen MR (1990) Cellular basis of transduction in carotid chemoreceptors. Am J Physiol 258:L271-L278
Butler TM, Siegman MJ (1985) High-energy phosphate metabolism in vascular smooth muscle. Annu Rev Physiol 47:629–643
Carl A, Sanders KM (1989) Ca2+-activated K channels of canine colonic myocytes. Am J Physiol 257:C470-C480
Cornfield DN, Stevens T, McMurtry IF, Abman SH, Rodman DM (1994) Acute hypoxia causes membrane depolarization and calcium influx in fetal pulmonary artery smooth muscle cells. Am J Physiol 266:L469-L475
Fabiato A, Fabiato F (1979) Calculator programs for computing the composition of solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol (Paris) 75:463–505
Geisbuhler T, Altschuld RA, Trewyn RW, Ansel AZ, Lamka K, Brierley GP (1984) Adenine nucleotide metabolism and compartmentalization in isolated adult rat heart cells. Circ Res 54:536–546
Han J, Kim EY, Ho WK, Earm YE (1994) Modulation of the ATP-sensitive potassium channel by sulfhydryl redox in isolated rabbit ventricular myocytes. J Physiol (Lond) 477:24P
Islam MS, Berggren P, Larsson O (1993) Sulfhydryl oxidation induces rapid and reversible closure of the ATP-regulated K+ channel in the pancreatic β-cell FEBS Lett 319:128–132
Katz LA, Koretsky AP, Balaban GS (1987) Respiratory control in the glucose perfused heart: a 31P NMR and NADH fluorescence study. FEBS Lett 221:270–276
Lee SH, Earm YE (1994) Caffeine causes periodic oscillations of Ca2+-activated K+ current in pulmonary arterial smooth muscle cells. Pflugers Arch 426:189–198
Lee SH, Ho WK, Earm YE (1991) Effect of pH on calcium-activated K channels in pulmonary arterial smooth muscle cells of the rabbit. Korean J Physiol 25:17–26
Lee SH, Park MK, Ho WK, Earm YE (1993) Ca2+-activated K+ channel from rabbit pulmonary arterial smooth muscle cells: effect of intracellular pH, Na+, Mg2+ and ATP. In: Abstracts of 32nd Congress of the IUPS, Glasgow, U.K., 342.3/O
Lee SH, Park MK, So IS, Earm YE (1994) NADH and NAD modulate Ca2+-activated K channels in small pulmonary arterial smooth muscle cells of the rabbit. Pflügers Arch 427:378–380
Lipton SA (1993) Prospects for clinically tolerated NMDA antagonists: open-channel blockers and alternative redox states of nitric oxide. Trends Neurosci 16:527–532
Madden JA, Vadula MS, Kurup VP (1992) Effects of hypoxia and other vasoactive agents on pulmonary and cerebral artery smooth muscle cells. Am J Physiol 263:L384-L393
Minezaki KK, Suleiman MS, Chapman RA (1994) Changes in mitochondrial function induced in isolated guinea-pig ventricular myocytes by calcium overload. J Physiol (Lond) 476:459–471
Murray TR, Chen L, Marshall BE, Macarak EJ (1990) Hypoxic contraction of cultured pulmonary vascular smooth muscle cells. Am J Respir Cell Mol Biol 3:457–465
Nagasaka Y, Bhattacharya F, Nanjo S, Gropper MA, Staub NC (1984) Micropuncture measurement of long microvascular pressure profile during hypoxia in cats. Circ Res 54:90–95
Post JM, Hume JR, Archer SL, Weir EK (1992) Direct role of potassium channel inhibition in hypoxic pulmonary vasoconstriction. Am J Physiol 262:C882-C890
Robertson BE, Corry PR, Nye PCG, Kozlowski RZ (1992) Ca2+ and Mg-ATP activated potassium channels from rat pulmonary artery. Pflügers Arch 421:94–96
Smirnov SV, Robertson TP, Ward JPT, Aaronson PI (1994) Chronic hypoxia is associated with reduced delayed rectifier K+ current in rat pulmonary artery muscle cells. Am J Physiol 266: H365-H370
Sylvester JT, Harabin AL, Peake MD, Frank RS (1980) Vasodilator and constrictor responses to hypoxia in isolated pig lungs. J Appl Physiol 49:820–825
Tang LH, Aizenman E (1993) The modulation of N-methyl-D-aspartate receptors by redox and alkylating reagents in rat cortical neurons in vitro. J Physiol (Lond) 465:303–323
Yuan XJ, Tod ML, Rubin LJ, Blaustein MP (1990) Contrasting effects of hypoxia on tension in rat pulmonary and mesenteric arteries. Am J Physiol 259:H281-H289
Yuan XJ, Goldman WF, Tod ML, Rubin LJ, Blaustein MP (1993) Ionic currents in rat pulmonary and mesenteric arterial myocytes in primary culture and subculture. Am J Physiol 264: L107-L115
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Park, M.K., Lee, S.H., Lee, S.J. et al. Different modulation of Ca-activated K channels by the intracellular redox potential in pulmonary and ear arterial smooth muscle cells of the rabbit. Pflügers Arch 430, 308–314 (1995). https://doi.org/10.1007/BF00373904
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00373904