Summary
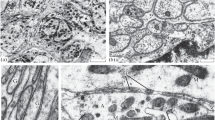
After cutting a neck connective of Schistocerca gregaria, only 2% of the axons on each side of the lesion degenerate. The remainder show reactive changes, which last for approximately one week at 28° C. There is no morphological change in either of the pro/mesothoracic connectives after injury to the neck connective. Phagocytes invade the stumps, but attack only degenerating cells, and are absent by Day 7.
Regeneration from the connective stumps begins a week after injury; a functional link may be formed by Day 10, but by Day 23 the new connective cannot function adequately for the locust's survival, if the undamaged connective is then cut.
The chief morphological changes in the reactive axoplasm are increases in the number of mitochondria, neurotubules, vesicles and vacuoles. These changes appear to be a local response, and not to be influenced by the neuron cell bodies. Some glial cytoplasm (presumably enucleated), degenerates rapidly after injury, and replacement begins by Day 5. Tracheoles, never seen in normal connectives appear in the reactive connective from Days 3–8, this is interpreted as a migration from the ganglion in response to oxygen deficiency in the connective.
The results are discussed in relationship to previous work.
Similar content being viewed by others
References
Ashhurst, D. E.: The connective tissues of insects. Ann. Rev. Entomol. 13, 45–74 (1968).
Blümcke, S., u. H. R. Niedorf: Fluoreszenmikroskopische und elektronenmikroskopische Untersuchungen an regenerierenden adrenergischen Nervenfasern. Z. Zellforsch. 68, 724–732 (1965).
Bodenstein, D.: Studies on nerve regeneration in Periplaneta americana. J. exp. Zool. 136, 89–116 (1957).
Boulton, P. S., and C. H. F. Rowell: Structure and function of the extraneural sheath in insects. Nature (Lond.) 217, 379–380 (1968).
Bullock, T. A., and G. A. Horridge: Structure and function in the nervous systems of invertebrates. San Francisco: Freeman 1965.
Coggeshall, R. E., and S. W. Fawcett: The fine structure of the CNS of the leech Hirudo medicinalis. J. Neurophysiol. 27, 229–289 (1964).
Delphin, F.: The histology and possible function of the neurosecretory cells in the ventral ganglia of Schistocerca gregaria Forskal (Orthoptera: Acrididae). Trans. roy. ent. Soc., Lond. 117, 167–214 (1965).
Estable, C., W. Acosta, and J. R. Sotelo: An electron microscope study of the regenerating nerve fibres. Z. Zellforsch. 46, 387–399 (1957).
Farley, R. D., and N. S. Milburn: Structure and function of the giant fibre system in the cockroach Periplaneta americana. J. Insect. Physiol. 15, 457–476 (1969).
Guthrie, D. M.: Regenerative growth in insect nerve axons. J. Insect Physiol. 8, 79–92 (1962).
—: The regeneration of motor axons in an insect. J. Insect Physiol. 13, 1593–1611 (1967).
Hess, A.: Experimental anatomical studies of pathways in the severed central nerve cord of the cockroach. J. Morph. 103, 479–502 (1958c).
—: The fine structure of degenerating nerve fibres, their sheaths and their terminations in the CNS of the cockroach (Periplaneta americana). J. biophys. biochem. Cytol. 7, 339–344 (1960).
Horridge, G. A.: Pitch discrimination in locusts. Proc. roy. Soc. B 155, 218–231 (1961).
Hoy, R. R., G. D. Bittner, and D. Kennedy: Regeneration in crustacean motoneurons: evidence for axonal fusion. Science 156, 251–252 (1967).
Hudson, G., A. Lazarow, and J. F. Hartmann: A quantitative electron microscopic study of mitochondria in motor neurones following axonal section. Exp. Cell Res. 24, 440–456 (1961).
Jacklet, J. W., and M. J. Cohen: Nerve regeneration: correlation of electrical, histological and behavioural events. Science 156, 1640–1643 (1967).
Lampert, P. W.: A comparative electron microscopic study of reactive, degenerating, regenerating and dystrophic axons. J. Neuropath. exp. Neurol. 26, 345–368 (1967).
—, and M. Cressman: Axonal regeneration in the dorsal columns of the spinal cord of adult rats. An electron microscopic study. Lab. Invest. 13, 825–839 (1964).
Lane, N. J.: The thoracic ganglia of the grasshopper, Melanoplus differentialis: Fine structure of the perineurium and neuroglia with special reference to the intracellular distribution of phosphatases. Z. Zellforsch. 86, 293–312 (1968).
Melamed, J., and O. Trujillo-Cenóz: Electronmicroscopic observations on the reactional changes occuring in insect nerve fibres after transection. Z. Zellforsch. 59, 851–856 (1963).
Pellegrino De Iraldi, A., and E. De Robertis: The neurotubular system of the axon and the origin of granulated and non-granulated vesicles in regenerating nerves. Z. Zellforsch. 87, 330–344 (1968).
—, and G. Jaim Etcheverry: Granulated vesicles in retinal synapses and neurons. Z. Zellforsch. 81, 283–296 (1967).
Pipa, R. L.: Insect neurometamorphosis. III. Nerve cord shortening in a moth, Galleria mellonella. (L) may be accomplished by humoral potentiation of neuroglial motility. J. exp. Zool. 164, 47–60 (1967).
—, and P. S. Woolever: Insect neurometamorphosis. II. The fine structure of perineurial connective tissue, adipohemocytes, and the shortening ventral nerve cord of a moth, Galleria mellonella (L.). Z. Zellforsch. 68, 80–101 (1965).
Reynolds, E. S.: The use of lead citrate at high pH as an electronopaque stain in electron microscopy. J. Cell Biol. 17, 208–212 (1963).
Rowell, C. H. F., and A. E. Dorey: The number and size of axons in the thoracic connectives of the desert locust Schistocerca gregaria Forsk. Z. Zellforsch. 83, 288–294 (1967).
-, and J. M. McKay: An acridid auditory interneuron. I. Functional connections and response to single sounds. J. exp. Biol. (1969) (in press).
Satija, R. C.: Histological and experimental study of nervous pathways in the brain and thoracic nerve cord of Locusta migratoria migratoriodes R. and F. Res. Bull. Panjab Univ. Sci. 137, 13–32 (1958).
Slautterback, D. B.: Cytoplasmic microtubules. 1. Hydra. J. Cell Biol. 18, 367–388 (1963).
Smith, D. S., and J. E. Treherne: Functional aspects of the organisation of the insect nervous system. In: Advances in insect physiology, vol. 1, p. 401–484 (eds J. W. Beament, J. E. Treherne and V. B. Wigglesworth). New York and London: Academic Press 1963.
Thé, G. De: Cytoplasmic microtubules in different animal cells. J. Cell Biol. 23, 265–276 (1964).
Treherne, J. E., and S. H. P. Maddrell: Axonal function and ionic regulation in the CNS of a phytophagous insect Carausius morosus. J. exp. Biol. 47, 235–247 (1967).
Usherwood, P. N. R.: Responses of insect muscle to denervation. 1. Resting potential changes. J. Insect Physiol. 9, 247–255 (1963).
Vowles, D. W.: The structure and connexions of the corpora pedunculata in bees and ants. Quart. J. micr. Sci. 96, 239–255 (1955).
Waxman, S. G.: Micropinocytotic Invaginations in the axolemma of peripheral nerves. Z. Zellforsch. 86, 571–573 (1968).
Webster, H. de F.: Transient, focal accumulation of axonal mitochondria during the early stages of Wallerian degeneration. J. Cell Biol. 12, 361–384 (1962).
Wettstein, R., and J. R. Sotelo: Electronmicroscopic study on the regenerative process of peripheral nerves of mice. Z. Zellforsch. 59, 708–730 (1963).
Wigglesworth, V. B.: Growth and regeneration in the tracheal sytem of an insect Rhodnius prolixus (Hemiptera). Quart. J. micr. Sci. 95, 115–137 (1954).
—: The histology of the nervous system of an insect, Rhodnius prolixus (Hemiptera). Quart. J. micr. Sci. 100, 285–298 (1959a).
—: The role of the epidermal cells in the “migration” of tracheoles in Rhodnius prolixus (Hemiptera). J. exp. Biol. 36, 632–640 (1959b).
—: The nutrition of the central nervous system in the cockroach Periplaneta americana L. The role of perineurium and glial cells in the mobilisation of reserves. J. exp. Biol. 37, 500–512 (1960).
—: Cytological changes in the fat body of Rhodnius during starvation, feeding and oxygen want. J. Cell Sci. 2, 243–256 (1967).
Wolfe, D. E., and J. G. Nicholls: Uptake of radioactive glucose and its conversion to glycogen by neurons and glial cells in the leech CNS. J. Neurophysiol. 30, 1593–1609 (1967).
Zelená, J.: Bidirectional movements of mitochondria along axons of an isolated nerve segment. Z. Zellforsch. 92, 186–196 (1968).
—, L. Lubinska, and J. E. Gutmann: Accumulation of organelles at the ends of interrupted axons. Z. Zellforsch. 91, 200–219 (1968).
Author information
Authors and Affiliations
Additional information
This work was supported by a “Study and Serve” grant from the British Government, and a grant from the Worshipful Company of Goldsmiths.
I wish to acknowledge the help and advice given to me by Dr. C. H. F. Rowell.
Rights and permissions
About this article
Cite this article
Boulton, P.S. Degeneration and regeneration in the insect central nervous system. I. Z. Zellforsch. 101, 98–118 (1969). https://doi.org/10.1007/BF00335588
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00335588