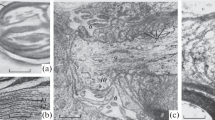
Inverted phase contrast microscopy was used to study living undamaged frog sciatic nerve fibers and fibers subjected to different degrees of injury. Swelling of myelin incisures (MI) (Schmidt–Lanterman clefts) was found to occur in a manner similar to that observed for changes in nodes of Ranvier and to depend on swelling of the Schwann cell (SC) perikaryon. This was found to be a single process, which could be combined with nonspecific changes in nerve fiber myelin. Swelling of MI, nodes of Ranvier, and SC perikarya on exposure to mechanical trauma and hypotonic solution occurred without any change in the external diameter of fibers, as a result of marked local axon thinning. Electron microscopic studies of axon cytoskeletal structures showed that this was not merely local narrowing – a significant role was played by increases in the distribution density of the cytoskeletal components of the axoplasm (by 200–275%). These reversible reactive rearrangements to myelin fibers suggest a new type of interaction between axons and SC, with a mechanism of reversible translocation of the liquid fraction of the axoplasm into the cytoplasm of gliocytes.
Similar content being viewed by others
References
V. N. Bykov, Morphofunctional Characteristics of the Sciatic Nerve in Health and Liver Failure: Auth. Abstr. of Master’s Thesis in Med. Sci., St. Petersburg (2003).
V. I. Zhikhorev, Forensic Medical Disorganization of Cardiac Nerve Structures in Various Types of Death: Auth. Abstr. of Master’s Thesis in Med. Sci., Moscow (2007).
E. Zapryanova, O. S. Sotnikov, S. S. Sergeeva, et al., “Axon reactions precede demyelination in experimental models of multiple sclerosis,” Morfologiya, 122, No. 5, 54–59 (2002).
D. V. Rusanova, Patterns and Mechanisms of Peripheral Nerve Lesions on Exposure to Metallic Mercury and a Complex of Toxic Substances: Auth. Abstr. of Master’s Thesis in Med. Sci., Irkutsk (2007).
V. V. Semchenko and S. S. Stepanov, “The neuroglia,” in: Handbook in Histology [in Russian], SpetsLit, St. Petersburg (2011), pp. 519–532.
O. S. Sotnikov, Dynamics of the Structures of Living Neurons [in Russian], Nauka, Leningrad (1985).
G. Allt, “The node of Ranvier in experimental allergic neuritis: an electron microscope study,” J. Neurocytol., 4, No. 1, 63–76 (1975).
V. Bay and A. M. Butt, “Relationship between glial potassium regulation and axon excitability: a role for glial Kir4.1 channels,” Glia, 60, No. 4, 651–660 (2012).
S. R. Cajal, “Sobre un nuevo proceder de impregnacion de la neuroglia y sus resultados en los centros nerviosos del hombre y animales,” Trab. Lab. Invest. Biol., 11, 219–237 (1913).
B. Casanova, M. C. Martínez-Bisbal, C. Valero, et al., “Evidence of Wallerian degeneration in normal appearing white matter in the early stages of relapsing-remitting multiple sclerosis: a HMRS study,” J. Neurol., 250, 22–28 (2003).
A. D. Gaudet, P. G. Popovich, and M. S. Ramer, “Wallerian degeneration: gaining perspective on inflammatory events after peripheral nerve injury,” J. Neuroinflammation, 8, 110 (2011).
Y. Kawagashira, H. Koike, M. Tomita, et al., “Morphological progression of myelin abnormalities in IgM-monoclonal gammopathy of undetermined significance anti-myelin-associated glycoprotein neuropathy,” J. Neuropathol. Exp. Neurol., 69, 1143–1157 (2010).
D. Kilinc, G. Gallo, and K. A. Barbee, “Mechanical membrane injury induces axonal beading through localized activation of calpain,” Exp. Neurol., 219, 553–561 (2009).
G. Malkinson and M. E. Spira, “Clustering of excess growth resources within leading growth cones underlies the recurrent ‘deposition’ of varicosities along developing neurites,” Exp. Neurol., 225, 140–153 (2010).
F. L. Mastaglia, W. I. McDonald, J. V. Watson, and K. Jogendran, “Effects of x-radiation on the spinal cord: an experimental study of the morphological changes in central nerve fibres,” Brain, 99, 101–122 (1976).
A. Nans, S. Einhaber, J. L. Salzer, et al., “Electron tomography of paranodal septate-like junctions and the associated axonal and glial cytoskeletons in the central nervous system,” J. Neurosci. Res., 89, 310–319 (2011).
K. Olmarker, C. Nordborg, K. Larsson, et al., “Ultrastructural changes in spinal nerve roots induced by autologous nucleus pulposus,” Spine, 21, 411–414 (1996).
B. R. Ransom and R. Fern, “Does astrocytic glycogen benefit axon function and survival in CNS white matter during glucose deprivation?” Glia, 21, 134–141 (1997).
R. J. Reynolds, G. L. Little, M. Lin, et al., “Imaging myelinated nerve fibers by confocal fluorescence microscopy: individual fibres in whole nerve trunks traced through multiple consecutive internodes,” J. Neurocytol., 23, 555–564 (1994).
S. S. Scherer, J. Arroyo, and E. Peles, “Functional organization of the nodes of Ranvier,” in: Myelin Biology and Disorders, Elsevier Academic Press, Amsterdam, Boston, etc. (2004),Vol. 1, pp. 89–107.
C. C. Speidel, “The experimental induction of visible structural changes in single nerve fibers in living frog tadpoles,” Cold Spring Harb. Symp. Quant. Biol., 4, 13–17 (1936).
M. Takahashi, B. Billups, D. Rossi, et al., “The role of glutamate transporters in glutamate homeostasis in the brain,” J. Exp. Biol., 200, No. 2, 401–409 (1997).
B. D. Trapp and G. J. Kidd, “Structure of the myelinated axon,” in: Myelin Biology and Disorders, Elsevier Academic Press, Amsterdam, Boston, etc. (2004), Vol. 1, pp. 3–28.
X. Yin, G. Kidd, K.-A. Nave, et al., “P0 protein is required for and can induce formation of Schmidt–Lantermann incisures in myelin internodes,” J. Neurosci., 28, 7068–7073 (2008).
R. C. Yu and R. P. Bunge, “Damage and repair of the peripheral myelin sheath and node of Ranvier after treatment with trypsin,” J. Cell Biol., 64, 1–14 (1975).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Translated from Morfologiya, Vol. 143, No. 2, pp. 35–42, March–April, 2013.
Rights and permissions
About this article
Cite this article
Kokurina, T.N., Sotnikov, O.S., Novakovskaya, S.A. et al. Interdependent Changes in Axons and Schwann Cells during Reactive Rearrangements of Myelinated Fibers. Neurosci Behav Physi 44, 528–535 (2014). https://doi.org/10.1007/s11055-014-9945-y
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11055-014-9945-y