Abstract
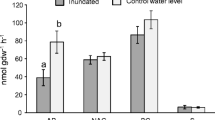
Coastal freshwater and brackish wetlands are exposed to pulses of saltwater during times of reduced freshwater flows (i.e., dry seasons, droughts), periodic storm surges, and increased tidal extent associated with rising seas. The effects of saltwater pulses on belowground processing rates of detrital organic matter as mediated by microbial activities are uncertain. Our objectives were to quantify whether and how pulses of saltwater (i) change soil porewater physicochemistry, (ii) change soil microbial extracellular enzyme activities, and (iii) change root litter breakdown over time in freshwater and brackish marshes. From 2014 to 2016, we simulated saltwater intrusion as monthly in situ pulsed additions of artificial seawater in experimental dosing chambers (1.4 m diameter) within freshwater and brackish marshes of Everglades National Park. At monthly intervals, we collected soil porewater chemistry, and measured microbial extracellular enzymes, elemental stoichiometric ratios, and breakdown rates (k) of incubated (0–30 cm depth) root litter and compared these responses over time. Saltwater pulses increased sulfate and nitrogen concentrations in porewater at the freshwater site. However, saltwater pulses generally decreased porewater constituents (e.g., dissolved organic carbon, dissolved nitrogen and phosphorus species) at the brackish site. One saltwater pulse increased root litter k by 1.25-fold in the brackish marsh. However, long-term (740 days) k in brackish wetlands, and both short- and long-term k in freshwater wetlands, were not affected by 24 monthly pulses of saltwater. Enzyme activities fluctuated with time and did not respond to multiple saltwater pulses. Our results suggest that detrital organic matter stocks and associated soil microbial activities are relatively resistant to single and multiple (n = 24) pulses of saltwater.







Similar content being viewed by others
References
Alef, K., and P. Nannipieri. 1995. Methods in applied soil microbiology and biochemistry (No. 631.46 M592ma). Academic Press.
Allison, S.D., and P.M. Vitousek. 2004. Extracellular enzyme activities and carbon chemistry as drivers of tropical plant litter decomposition. Biotropica 36 (3): 285–296.
Anderson, D.R., and K.P. Burnham. 2002. Avoiding pitfalls when using information-theoretic methods. The Journal of Wildlife Management, pp.: 912–918.
Ardón, M., J.L. Morse, B.P. Colman, and E.S. Bernhardt. 2013. Drought-induced saltwater incursion leads to increased wetland nitrogen export. Global Change Biology 19 (10): 2976–2985.
Atkinson, M.J., and C. Bingman. 1997. Elemental composition of commercial seasalts. Journal of Aquariculture and Aquatic Sciences 8 (2): 39–43.
Burns, R.G. 1982. Enzyme activity in soil: Location and a possible role in microbial ecology. Soil Biology and Biochemistry 14 (5): 423–427.
Chambers, L.G., K.R. Reddy, and T.Z. Osborne. 2011. Short-term response of carbon cycling to salinity pulses in a freshwater wetland. Soil Science Society of America Journal 75 (5): 2000–2007.
Chambers, L.G., S.E. Davis, T.G. Troxler, and J.A. Entry. 2015. Sea level rise in the Everglades: Plant-soil-microbial feedbacks in response to changing physical conditions. Microbiology of the Everglades Ecosystem: 89–112.
Chambers, L.G., R. Guevara, J.N. Boyer, T.G. Troxler, and S.E. Davis. 2016. Effects of salinity and inundation on microbial community structure and function in a mangrove peat soil. Wetlands 36 (2): 361–371.
Chambers, L.G., H.E. Steinmuller, and J.L. Breithaupt. 2019. Toward a mechanistic understanding of “peat collapse” and its potential contribution to coastal wetland loss. Ecology: e02720.
Chapin, F.S., G.M. Woodwell, J.T. Randerson, E.B. Rastetter, G.M. Lovett, D.D. Baldocchi, et al. 2006. Reconciling carbon-cycle concepts, terminology, and methods. Ecosystems 9 (7): 1041–1050.
Charles, S.P., J.S. Kominoski, T.G. Troxler, E.E. Gaiser, S. Servais, B.J. Wilson, et al. 2019. Experimental saltwater intrusion drives rapid soil elevation and carbon loss in freshwater and brackish Everglades marshes. Estuaries and Coasts 42 (7): 1868–1881.
Chröst, R.J. 1991. Environmental control of the synthesis and activity of aquatic microbial ectoenzymes. In Microbial enzymes in aquatic environments, 29–59. New York, NY: Springer.
Craft, C. 2007. Freshwater input structures soil properties, vertical accretion, and nutrient accumulation of Georgia and US tidal marshes. Limnology and Oceanography 52 (3): 1220–1230.
Craft, C.B., and C.J. Richardson. 2008. Soil characteristics of the Everglades peatland. In Everglades experiments, 59–72. New York, NY: Springer.
Davis, S.E., III, and D.L. Childers. 2007. Importance of water source in controlling leaf leaching losses in a dwarf red mangrove (Rhizophora mangle L.) wetland. Estuarine, Coastal and Shelf Science 71 (1–2): 194–201.
Davis, S.E., III, C. Coronado-Molina, D.L. Childers, and J.W. Day Jr. 2003. Temporally dependent C, N, and P dynamics associated with the decay of Rhizophora mangle L. leaf litter in oligotrophic mangrove wetlands of the Southern Everglades. Aquatic Botany 75 (3): 199–215.
Davis, S.E., III, D.L. Childers, and G.B. Noe. 2006. The contribution of leaching to the rapid release of nutrients and carbon in the early decay of wetland vegetation. Hydrobiologia 569 (1): 87–97.
Day, J.W., G.P. Kemp, D.J. Reed, D.R. Cahoon, R.M. Boumans, J.M. Suhayda, and R. Gambrell. 2011. Vegetation death and rapid loss of surface elevation in two contrasting Mississippi delta salt marshes: The role of sedimentation, autocompaction and sea-level rise. Ecological Engineering 37 (2): 229–240.
Dessu, S.B., R.M. Price, T.G. Troxler, and J.S. Kominoski. 2018. Effects of sea-level rise and freshwater management on long-term water levels and water quality in the Florida Coastal Everglades. Journal of Environmental Management 211: 164–176.
Dick, R.P. 1994. Soil enzyme activities as indicators of soil Quality1. Defining soil quality for a sustainable environment: 107–124.
Faulkner, S.P., W.H. Patrick Jr., and R.P. Gambrell. 1989. Field techniques for measuring wetland soil parameters. Soil Science Society of America Journal 53 (3): 883–890.
Freeman, C., G. Liska, N.J. Ostle, M.A. Lock, B. Reynolds, and J. Hudson. 1996. Microbial activity and enzymic decomposition processes following peatland water table drawdown. Plant and Soil 180 (1): 121–127.
Freeman, C., N. Ostle, and H. Kang. 2001. An enzymic ‘latch’ on a global carbon store. Nature 409 (6817): 149.
Helton, A.M., M. Ardón, and E.S. Bernhardt. 2015. Thermodynamic constraints on the utility of ecological stoichiometry for explaining global biogeochemical patterns. Ecology Letters 18 (10): 1049–1056.
Herbert, E.R., P. Boon, A.J. Burgin, S.C. Neubauer, R.B. Franklin, M. Ardón, K.N. Hopfensperger, L.P. Lamers, and P. Gell. 2015. A global perspective on wetland salinization: Ecological consequences of a growing threat to freshwater wetlands. Ecosphere 6 (10): 1–43.
Herbert, E.R., J. Schubauer-Berigan, and C.B. Craft. 2018. Differential effects of chronic and acute simulated seawater intrusion on tidal freshwater marsh carbon cycling. Biogeochemistry: 1–18.
Hoorens, B., R. Aerts, and M. Stroetenga. 2003. Does initial litter chemistry explain litter mixture effects on decomposition? Oecologia 137 (4): 578–586.
Jackson, C.R., and S.C. Vallaire. 2009. Effects of salinity and nutrients on microbial assemblages in Louisiana wetland sediments. Wetlands 29 (1): 277–287.
Jackson, C.R., C.M. Foreman, and R.L. Sinsabaugh. 1995. Microbial enzyme activities as indicators of organic matter processing rates in a Lake Erie coastal wetland. Freshwater Biology 34 (2): 329–342.
Kang, H., and C. Freeman. 1999. Phosphatase and arylsulphatase activities in wetland soils: Annual variation and controlling factors. Soil Biology and Biochemistry 31 (3): 449–454.
Kearns, P.J., J.H. Angell, E.M. Howard, L.A. Deegan, R.H. Stanley, and J.L. Bowen. 2016. Nutrient enrichment induces dormancy and decreases diversity of active bacteria in salt marsh sediments. Nature Communications 7: 12881.
Liu, Z., and C. Lee. 2007. The role of organic matter in the sorption capacity of marine sediments. Marine Chemistry 105 (3): 240–257.
Liu, X., A. Ruecker, B. Song, J. Xing, W.H. Conner, and A.T. Chow. 2017. Effects of salinity and wet–dry treatments on C and N dynamics in coastal-forested wetland soils: Implications of sea level rise. Soil Biology and Biochemistry 112: 56–67.
Manning, D.W., A.D. Rosemond, J.S. Kominoski, V. Gulis, J.P. Benstead, and J.C. Maerz. 2015. Detrital stoichiometry as a critical nexus for the effects of streamwater nutrients on leaf litter breakdown rates. Ecology 96 (8): 2214–2224.
McIvor, C.C., J.A. Ley, and R.D. Bjork. 1994. Changes in freshwater inflow from the Everglades to Florida bay including effects on biota and biotic processes: A review, 117–146. Everglades: The ecosystem and its restoration.
McKee, K.L., and E.D. Seneca. 1982. The influence of morphology in determining the decomposition of two salt marsh macrophytes. Estuaries 5 (4): 302–309.
McKee, K.L., I.A. Mendelssohn, and M.W. Hester. 1988. Reexamination of pore water sulfide concentrations and redox potentials near the aerial roots of Rhizophora mangle and Avicennia germinans. American Journal of Botany 75: 1352–1359.
McKee, K.L. 2011. Biophysical controls on accretion and elevation change in Caribbean mangrove ecosystems. Estuarine, Coastal and Shelf Science 91 (4): 475–483.
Mendelssohn, I.A., B.K. Sorrell, H. Brix, H.H. Schierup, B. Lorenzen, and E. Maltby. 1999. Controls on soil cellulose decomposition along a salinity gradient in a Phragmites australis wetland in Denmark. Aquatic Botany 64 (3–4): 381–398.
Mitsch, W.J., and J.G. Gosselink. 2007. Wetlands. 4th ed.
Morrissey, E.M., J.L. Gillespie, J.C. Morina, and R.B. Franklin. 2014. Salinity affects microbial activity and soil organic matter content in tidal wetlands. Global Change Biology 20 (4): 1351–1362.
Neubauer, S.C., R.B. Franklin, and D.J. Berrier. 2013. Saltwater intrusion into tidal freshwater marshes alters the biogeochemical processing of organic carbon. Biogeosciences 10 (12): 8171.
Newman, S., H. Kumpf, J.A. Laing, and W.C. Kennedy. 2001. Decomposition responses to phosphorus enrichment in an Everglades (USA) slough. Biogeochemistry 54 (3): 229–250.
Nyman, J.A., R.J. Walters, R.D. Delaune, and W.H. Patrick Jr. 2006. Marsh vertical accretion via vegetative growth. Estuarine, Coastal and Shelf Science 69 (3–4): 370–380.
Odum, W.E., E.P. Odum, and H.T. Odum. 1995. Nature’s pulsing paradigm. Estuaries 18 (4): 547.
Olson, J.S. 1963. Energy storage and the balance of producers and decomposers in ecological systems. Ecology 44 (2): 322–331.
Pulford, I.D., and M.A. Tabatabai. 1988. Effect of waterlogging on enzyme activities in soils. Soil Biology and Biochemistry 20 (2): 215–219.
R Core Team (2017) R: A language and environment for statistical computing. In: R Foundation for statistical computing. Austria. URL, Vienna https://www.R-project.org/
Rejmánková, E., and D. Sirová. 2007. Wetland macrophyte decomposition under different nutrient conditions: Relationships between decomposition rate, enzyme activities and microbial biomass. Soil Biology and Biochemistry 39 (2): 526–538.
Romero, L.M., T.J. Smith III, and J.W. Fourqurean. 2005. Changes in mass and nutrient content of wood during decomposition in a South Florida mangrove forest. Journal of Ecology 93 (3): 618–631.
Rosenfeld, J.K. 1979. Ammonium adsorption in nearshore anoxic sediments. Limnology and Oceanography 24 (2): 356–364.
Ross, M.S., J.F. Meeder, J.P. Sah, P.L. Ruiz, and G.J. Telesnicki. 2000. The southeast saline Everglades revisited: 50 years of coastal vegetation change. Journal of Vegetation Science 11 (1): 101–112.
Saiya-Cork, K.R., R.L. Sinsabaugh, and D.R. Zak. 2002. The effects of long term nitrogen deposition on extracellular enzyme activity in an Acer saccharum forest soil. Soil Biology and Biochemistry 34 (9): 1309–1315.
Schimel, J.P., and M.N. Weintraub. 2003. The implications of exoenzyme activity on microbial carbon and nitrogen limitation in soil: A theoretical model. Soil Biology and Biochemistry 35 (4): 549–563.
Servais, S., J.S. Kominoski, S.P. Charles, E.E. Gaiser, V. Mazzei, T.G. Troxler, and B.J. Wilson. 2019. Saltwater intrusion and soil carbon loss: Testing effects of salinity and phosphorus loading on microbial functions in experimental freshwater wetlands. Geoderma 337: 1291–1300.
Sinsabaugh, R.L., and J.J. Follstad Shah. 2012. Ecoenzymatic stoichiometry and ecological theory. Annual Review of Ecology, Evolution, and Systematics 43: 313–343.
Sinsabaugh, R.L., R.K. Antibus, A.E. Linkins, C.A. McClaugherty, L. Rayburn, D. Repert, and T. Weiland. 1993. Wood decomposition: Nitrogen and phosphorus dynamics in relation to extracellular enzyme activity. Ecology 74 (5): 1586–1593.
Sinsabaugh, R.L., M.M. Carreiro, and S. Alvarez. 2002. Enzyme and microbial dynamics of litter decomposition. In Enzymes in the environment, activity, ecology, and applications, 249–265. New York, Basel: Marcel Dekker.
Sklar, F., C. McVoy, R. VanZee, D.E. Gawlik, K. Tarboton, D. Rudnick, S. Miao, and T. Armentano. 2002. The effects of altered hydrology on the ecology of the Everglades. In The Everglades, Florida Bay, and coral reefs of the Florida Keys: An ecosystem sourcebook. Florida: CRC Press, Boca Raton.
Solórzano, L., and J.H. Sharp. 1980. Determination of total dissolved phosphorus and particulate phosphorus in natural waters 1. Limnology and Oceanography 25 (4): 754–758.
Stachelek, J., S.P. Kelly, F.H. Sklar, C. Coronado-Molina, T. Troxler, and L. Bauman. 2018. In situ simulation of sea-level rise impacts on coastal wetlands using a flow-through mesocosm approach. Methods in Ecology and Evolution 9 (8): 1908–1915.
Tokarz, E., and D. Urban. 2015. Soil redox potential and its impact on microorganisms and plants of wetlands. Journal of Ecological Engineering 16 (3): 20–30.
Tully, K., K. Gedan, R. Epanchin-Niell, A. Strong, E.S. Bernhardt, T. BenDor, M. Mitchell, J. Kominoski, T.E. Jordan, S.C. Neubauer, and N.B. Weston. 2019. The invisible flood: The chemistry, ecology, and social implications of coastal saltwater intrusion. BioScience 69 (5): 368–378.
Valiela, I., S.D. Allen, R. Van Etten, D. Goehringer, and S. Volkmann. 1985. Decomposition in salt marsh ecosystems: The phases and major factors affecting disappearance of above-ground organic matter. Journal of Experimental Marine Biology and Ecology 89 (1): 29–54.
Vance, E.D., P.C. Brookes, and D.S. Jenkinson. 1987. An extraction method for measuring soil microbial biomass C. Soil Biology and Biochemistry 19 (6): 703–707.
Wanless, H.R., and B. Vlaswinkel. 2005. Coastal landscape and channel evolution affecting critical habitats at Cape Sable, Everglades National Park, Florida. Final report to Everglades National Park. Homestead: United States Department of the Interior.
Wdowinski, S., R. Bray, B.P. Kirtman, and Z. Wu. 2016. Increasing flooding hazard in coastal communities due to rising sea level: Case study of Miami Beach, Florida. Ocean & Coastal Management 126: 1–8.
Weston, N.B., R.E. Dixon, and S.B. Joye. 2006. Ramifications of increased salinity in tidal freshwater sediments: Geochemistry and microbial pathways of organic matter mineralization. Journal of Geophysical Research: Biogeosciences 111 (G1).
Weston, N.B., M.A. Vile, S.C. Neubauer, and D.J. Velinsky. 2011. Accelerated microbial organic matter mineralization following salt-water intrusion into tidal freshwater marsh soils. Biogeochemistry 102 (1–3): 135–151.
White, E., and D. Kaplan. 2017. Restore or retreat? Saltwater intrusion and water management in coastal wetlands. Ecosystem Health and Sustainability 3 (1).
White, D.A., and J.M. Trapani. 1982. Factors influencing disappearances of Spartina Alterniflora from litterbags. Ecology, pp.: 242–245.
Wilson, B.J., S. Servais, V. Mazzei, J.S. Kominoski, M. Hu, S.E. Davis, E. Gaiser, F. Sklar, L. Bauman, S. Kelly, and C. Madden. 2018. Salinity pulses interact with seasonal dry-down to increase ecosystem carbon loss in marshes of the Florida Everglades. Ecological Applications 28 (8): 2092–2108.
Zechmeister-Boltenstern, S., K.M. Keiblinger, M. Mooshammer, J. Peñuelas, A. Richter, J. Sardans, and W. Wanek. 2015. The application of ecological stoichiometry to plant–microbial–soil organic matter transformations. Ecological Monographs 85 (2): 133–155.
Acknowledgments
We thank R. Stolee, G. Cabral, K. Morales, and D. Segrera for field and laboratory assistance. We thank the Everglades Section of the South Florida Water Management District, and Everglades National Park for providing facilities support during this research.
Funding
Funding for this research was provided by the Florida Sea Grant (R/C-S-56). Additional funding and support were provided by the National Science Foundation award (DEB-1237517) to the Florida Coastal Everglades Long Term Ecological Research (FCE LTER) Program. S. Servais was supported by Florida Sea Grant (R/C-S-56), FCE LTER, and the Florida International University’s Dissertation Year Fellowship. This manuscript is contribution number 27 of the Sea Level Solutions Center and contribution number 948 from the Southeast Environmental Research Center in the Institute of Environment at Florida International University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Zhanfei Liu
Electronic Supplementary Material
ESM 1
(DOCX 68 kb)
Rights and permissions
About this article
Cite this article
Servais, S., Kominoski, J.S., Coronado-Molina, C. et al. Effects of Saltwater Pulses on Soil Microbial Enzymes and Organic Matter Breakdown in Freshwater and Brackish Coastal Wetlands. Estuaries and Coasts 43, 814–830 (2020). https://doi.org/10.1007/s12237-020-00708-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-020-00708-1