Abstract
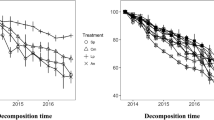
We tested the hypothesis that interactions in litter mixtures (expressed as the difference between observed and expected decomposition rates) are greater when the component species differ more in their initial litter chemistry. Thereto, we collected freshly senesced leaf litter from a wide range of species from an old field and woodland vegetation, and a fen ecosystem in The Netherlands. Litterbags with either mono-specific litter (20 and 15 species), or litter mixtures (50 and 42 species pairs) of randomly drawn combinations of two representatives from different plant functional types were incubated for 20, 35 and 54 weeks in a purpose-built decomposition bed (woodland/old field) or in the field (fen). Species showed a wide range of decomposition rates. For the woodland/old field species, initial litter C and P concentrations were significantly correlated with litter decomposition rate. For the fen species, litter phenolics concentration was correlated with decomposition rate. If the Sphagnum species were left out of the analyses, initial litter P and phenolics concentration both showed a significant relationship, albeit only with the remaining mass after 1 year. Differences between observed and expected decomposition were often considerable in individual litter mixtures. Regression analysis showed that such differences were not related to the differences in litter chemistry of the component species. Furthermore, litter mixtures containing species with very different initial litter chemistry did not show stronger interaction when tested against litter mixtures containing chemically similar litter types. From these observations we conclude that the difference in initial single litter chemistry parameters of the component is not a useful concept to explain interactions in litter mixtures.


Similar content being viewed by others
References
Aerts R (1997) Climate, leaf litter chemistry and leaf litter decomposition in terrestrial ecosystems: a triangular relationship. Oikos 79:439–449
Aerts R, De Caluwe H (1997) Nutritional and plant-mediated controls on leaf litter decomposition of Carex species. Ecology 78:244–260
Anderson JM, Hetherington SL (1999) Temperature, nitrogen availability and mixture effects on the decomposition of heather [Calluna vulgaris (L.) Hull] and bracken [Pteridium aquilinum (L.) Kuhn] litters. Funct Ecol 13(Suppl 1):116–124
Blair JM, Parmelee RW, Beare MH (1990) Decay rates, nitrogen fluxes and decomposer communities in single and mixed-species foliar litter. Ecology 71:1976–1985
Briones MJI, Ineson, P (1996) Decomposition of Eucalyptus leaves in litter mixtures. Soil Biol Biochem 28:1381–1388
Cadisch G, Giller KE (eds) (1997) Driven by nature, plant litter quality and decomposition. CAB International, Wallingford
Cornelissen JHC (1996) An experimental comparison of leaf decomposition rates in a wide range of temperate plant species and types. J Ecol 84:573–582
Craine JM, Berin DM, Reich PB, Tilman DG, Knops JMH (1999) Measurement of leaf longevity of 14 species of grasses and forbs using a novel approach. New Phytol 142:475–481
Finzi AC, Canham CD (1998) Non-additive effects of litter mixtures on net N mineralization in a southern New England forest. For Ecol Manage 105:129–136
Harrison AF (1971) The inhibitory effect of oak leaf litter tannins on the growth of fungi, in relation to litter decomposition. Soil Biol Biochem 3:167–172
Hättenschwiler S, Vitousek PM (2000) The role of polyphenols in terrestrial ecosystem nutrient cycling. Trends Ecol Evol 15:238–243
Johnson LC, Damman AWH (1993) Decay and its regulation in Sphagnum peatlands. Adv Bryol 5:249–296
Klemmedson JO (1992) Decomposition and nutrient release from mixtures of gambel oak and ponderosa pine leaf litter. For Ecol Manage 47:349–361
McTiernan KB, Ineson P, Coward PA (1997) Respiration and nutrient release from tree leaf litter mixtures. Oikos 78:527–538
Melillo JM, Aber JD, Muratore JF (1982) Nitrogen and lignin control of hardwood leaf litter decomposition dynamics. Ecology 63:621–626
Murphy J, Riley JP (1962) A modified single solution method for the determination of phosphate in natural waters. Anal Chim Acta 27:31–36
Peréz-Harguindeguy N, Díaz S, Cornelissen JHC, Vendramini F, Cabido M, Castellanos A (2000) Chemistry and toughness predict leaf litter decomposition rates over a wide spectrum of functional types and taxa in central Argentina. Plant Soil 218:21–30
Poorter H, Villar R (1997) The fate of acquired carbon in plants: chemical composition and construction costs. In: Bazzaz FA, Grace J (eds) Plant resource allocation. Academic Press, San Diego, pp. 39–72
Prescott CE, Zabek LM, Staley CL, Kabzems R (2000) Decomposition of broadleaf and needle litter in forests of British Columbia: influences of litter type, forest type, and litter mixtures. Can J For Res 30:1742–1750
Quested HM, Press MC, Callaghan TV, Cornelissen JHC (2002) The hemiparasitic angiosperm Bartsia alpina has the potential to accelerate decomposition in sub-arctic communities. Oecologia 130:88–95
Robinson CH, Kirkham JB, Littlewood R (1999) Decomposition of root mixtures from high arctic plants: a microcosm study. Soil Biol Biochem 31:1101–1108
Scheffer RA, van Logtestijn RSP, Verhoeven JTA (2001) Decomposition of Carex and Sphagnum litter in two mesotrophic fens differing in dominant plant species. Oikos 92:44–54
Seastedt TR (1984) The role of microarthropods in decomposition and mineralisation processes. Annu Rev Entomol 29:25–46
Smith AJE (1978) The moss flora of Britain and Ireland, 1st edn. Cambridge University Press, Cambridge
Swift MJ, Heal OW, Anderson JM (1979) Decomposition in terrestrial ecosystems. Studies in Ecology 5. Blackwell Scientific, Oxford
Taylor BR, Parsons WFJ, Parkinson D (1989) Decomposition of Populus tremuloides leaf litter accelerated by addition of Alnus crispa litter. Can J For Res 19:674–679
Van Breemen N (1995) How Sphagnum bogs down other plants. Trends Ecol Evol 10:270–275
Van der Meijden R (1990) Heukels’ Flora van Nederland, 21st edition. Wolters-Noordhoff, Groningen
Van der Putten WH, Mortimer SR, Hedlund K, Van Dijk C, Brown VK, Leps J, Rodriguez-Barrueco C, Roy J, Len TAD, Gormsen D, Korthals GW, Lavorel S, Regina IS, Smilauer P (2000) Plant species diversity as a driver of early succession in abandoned fields: a multi-site approach. Oecologia 124:91–99
Verhoeven JTA, Liefveld WM (1995) The ecological significance of organochemical compounds in Sphagnum. Acta Bot Neerl 46:117–130
Verhoeven JTA, Koerselman W, Beltman B (1988) The vegetation of fens in relation to their hydrology and nutrient dynamics: a case study. In: Symoens JJ (ed) Vegetation of inland waters. Kluwer Academic, Dordrecht, pp 249–282
Wardle DA, Bonner KI, Nicholson KS (1997) Biodiversity and plant litter: experimental evidence which does not support the view that enhanced species richness improves ecosystem function. Oikos 79:247–258
Waterman PG, Mole S (1994) Analysis of phenolic plant metabolites. Methods in Ecology. Blackwell Scientific, Oxford
Acknowledgements
The authors would like to thank R. Broekman for help with the chemical analyses, and P. van Bodegom and H. Cornelissen for their constructive comments on an earlier version of this manuscript. The authors would further like to thank W. van der Putten for permission to collect plant litter at the CLUE experimental field site at ‘De Mossel’.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
List of species (ordered into functional groups) used in the experiment. Litter C, N, P and total phenolics concentrations are given for each species, as well as the average fraction remaining mass at the end of the experiment. Nomenclature follows Van der Meijden (1990) for vascular plants and Smith (1978) for Sphagna (Table 5).
Rights and permissions
About this article
Cite this article
Hoorens, B., Aerts, R. & Stroetenga, M. Does initial litter chemistry explain litter mixture effects on decomposition?. Oecologia 137, 578–586 (2003). https://doi.org/10.1007/s00442-003-1365-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-003-1365-6