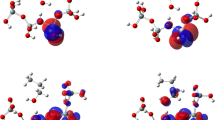
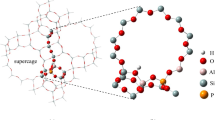
Abstract
Dehydration reactions are important in the petroleum and petrochemical industries, especially for the feedstock production. In this work, the catalytic activity of zeolites with different acidities for the dehydration of ethanol to ethylene and diethylether is investigated by density functional calculations on cluster models of three isomorphous B, Al, and Ga substituted H-ZSM-5 zeolites. Both unimolecular and bimolecular mechanisms are investigated. Detailed reaction profiles for the dehydration reaction, assuming either a stepwise or a concerted mechanism, were calculated by using the ONIOM(MP2:M06-2X) + SCREEP method. The adsorption energies of ethanol are −21.6, −28.1, and −27.7 kcal mol−1 on H-[B]-ZSM-5, H-[Al]-ZSM-5, and H-[Ga]-ZSM-5 zeolites, respectively. The activation energies for the rate-determining step of the unimolecular concerted mechanism for the ethylene formation are 48.5, 42.6, and 43.6 kcal mol−1 on H-[B]-ZSM-5, H-[Al]-ZSM-5, and H-[Ga]-ZSM-5 zeolites, respectively. The activation energies for the ethoxy formation as the rate-determining step for the bimolecular formation of diethylether are 42.3, 40.0, and 41.1 kcal mol−1 on H-[B]-ZSM-5, H-[Al]-ZSM-5, and H-[Ga]-ZSM-5 zeolites, respectively. The results indicate that the catalytic activities for the dehydration of ethanol decrease in the order H-[Al]-ZSM-5 ~ H-[Ga]-ZSM-5 > H-[B]-ZSM-5. Besides the acid strength, the zeolite framework affects the reaction by stabilizing the reaction intermediates, leading to more stable adsorption complexes and lower activation barriers.
Graphical abstract
















Similar content being viewed by others
Data availability
N/A.
Code availability
N/A.
References
Corma A (2003) State of the art and future challenges of zeolites as catalysts. J Catal 216:298–312. https://doi.org/10.1016/S0021-9517(02)00132-X
Smit B, Maesen TLM (2008) Towards a molecular understanding of shape selectivity. Nature 451:671–678. https://doi.org/10.1038/nature06552
Corma A, Iborra S, Velty A (2007) Chemical routes for the transformation of biomass into chemicals. Chem Rev 107:2411–2502. https://doi.org/10.1021/cr050989d
Bhan A, Iglesia E (2008) A link between reactivity and local structure in acid catalysis on zeolites. Acc Chem Res 41:559–567. https://doi.org/10.1021/ar700181t
Fan D, Dai DJ, Wu HS (2013) Ethylene formation by catalytic dehydration of ethanol with industrial considerations. Materials 6:101–115. https://doi.org/10.3390/ma6010101
Morschbacker A (2009) Bio-ethanol based ethylene. Polym Rev 49:79–84. https://doi.org/10.1080/15583720902834791
Takahara I, Saito M, Inaba M, Murata K (2005) Dehydration of ethanol into ethylene over solid acid catalysts. Catal Lett 105:249–252. https://doi.org/10.1007/s10562-005-8698-1
Tret’yakov VF, Makarfi YI, Tret’yakov KV, Frantsuzova NA, Talyshinskii RM (2010) The catalytic conversion of bioethanol to hydrocarbon fuel: a review and study. Catal Ind 2:402–420. https://doi.org/10.1134/S2070050410040161
Straathof AJJ (2014) Transformation of biomass into commodity chemicals using enzymes or cells. Chem Rev 114:1871–1908. https://doi.org/10.1021/cr400309c
Haw JF, Xu T (1998) NMR studies of solid acidity. Adv Catal 42:115–180. https://doi.org/10.1016/S0360-0564(08)60628-8
Lamberti C, Groppo E, Spoto G, Bordiga S, Zecchina A (2007) Infrared spectroscopy of transient surface species. Adv Catal 51:1–74. https://doi.org/10.1016/S0360-0564(06)51001-6
Wang W, Seiler M, Hunger M (2001) Role of surface methoxy species in the conversion of methanol to dimethyl ether on acidic zeolites investigated by in situ stopped-flow MAS NMR spectroscopy. J Phys Chem B 105:12553–12558. https://doi.org/10.1021/jp0129784
Batchu R, Galvita VV, Alexopoulos K, Glazneva TS, Poelman H, Reyniers MF, Marin GB (2020) Ethanol dehydration pathways in H-ZSM-5: insights from temporal analysis of products. Catal Today 355:822–831. https://doi.org/10.1016/j.cattod.2019.04.018
Wang Z, O’Dell LA, Zeng X, Liu C, Zhao S, Zhang W, Gaborieau M, Jiang Y, Huang J (2019) Insight into three-coordinate aluminum species on ethanol-to-olefin conversion over ZSM-5 zeolites. Angew Chem Int Ed 58:18061–18068. https://doi.org/10.1002/anie.201910987
Wu CY, Wu HS (2017) Ethylene formation from ethanol dehydration using ZSM-5 catalyst. ACS Omega 2:4287–4296. https://doi.org/10.1021/acsomega.7b00680
Bukowski BC, Bates JS, Gounder R, Greeley J (2018) First principles, microkinetic, and experimental analysis of Lewis acid site speciation during ethanol dehydration on Sn-beta zeolites. J Catal 365:261–276. https://doi.org/10.1016/j.jcat.2018.07.012
Sarve DT, Singh SK, Ekhe JD (2020) Kinetic and mechanistic study of ethanol dehydration to diethyl ether over Ni-ZSM-5 in a closed batch reactor. React Kinet Mech Catal 131:261–281. https://doi.org/10.1007/s11144-020-01847-z
Kondo JN, Nishioka D, Yamazaki H, Kubota J, Domen K, Tatsumi T (2010) Activation energies for the reaction of ethoxy species to ethene over zeolites. J Phys Chem C 114:20107–20113. https://doi.org/10.1021/jp107082t
Kondo JN, Ito K, Yoda E, Wakabayashi F, Domen K (2005) An ethoxy intermediate in ethanol dehydration on Brønsted acid sites in zeolite. J Phys Chem B 109:10969–10972. https://doi.org/10.1021/jp050721q
Kunz LY, Bu L, Knott BC, Liu C, Nimlos MR, Assary RS, Curtiss LA, Robichaud DJ, Kim S (2019) Theoretical determination of size effects in zeolite-catalyzed alcohol dehydration. Catalysts 9:700. https://doi.org/10.3390/catal9090700
Costa RJ, Castro EAS, Politi JRS, Gargano R, Martins JBL (2019) Methanol, ethanol, propanol, and butanol adsorption on H-ZSM-5 zeolite: an ONIOM study. J Mol Model 25:34. https://doi.org/10.1007/s00894-018-3894-2
Lee CC, Gorte RJ, Farneth WE (1997) Calorimetric study of alcohol and nitrile adsorption complexes in H-ZSM-5. J Phys Chem B 101:3811–3817. https://doi.org/10.1021/jp970711s
Kim S, Robichaud DJ, Beckham GT, Paton RS, Nimlos MR (2015) Ethanol dehydration in HZSM-5 studied by density functional theory: evidence for a concerted process. J Phys Chem A 119:3604–3614. https://doi.org/10.1021/jp513024z
Alexopoulos K, John M, Van Der Borght K, Galvita V, Reyniers MF, Marin GB (2016) DFT-based microkinetic modeling of ethanol dehydration in H-ZSM-5. J Catal 339:173–185. https://doi.org/10.1016/j.jcat.2016.04.020
Nguyen CM, Reyniers MF, Marin GB (2010) Theoretical study of the adsorption of C1–C4 primary alcohols in H-ZSM-5. Phys Chem Chem Phys 12:9481–9493. https://doi.org/10.1039/c000503g
Nguyen CM, Reyniers MF, Marin GB (2015) Adsorption thermodynamics of C1–C4 alcohols in H-FAU, H-MOR, H-ZSM-5, and H-ZSM-22. J Catal 322:91–103. https://doi.org/10.1016/j.jcat.2014.11.013
Alexopoulos K, Lee MS, Liu Y, Zhi Y, Liu Y, Reyniers MF, Marin GB, Glezakou VA, Rousseau R, Lercher JA (2016) Anharmonicity and confinement in zeolites: structure, spectroscopy, and adsorption free energy of ethanol in H-ZSM-5. J Phys Chem C 120:7172–7182. https://doi.org/10.1021/acs.jpcc.6b00923
Zhou X, Wang C, Chu Y, Xu J, Wang Q, Qi G, Zhao X, Feng N, Deng F (2019) Observation of an oxonium ion intermediate in ethanol dehydration to ethene on zeolite. Nat Commun 10:1961. https://doi.org/10.1038/s41467-019-09956-7
Chu CTW, Chang CD (1985) Isomorphous substitution in zeolite frameworks. 1. Acidity of surface hydroxyls in [B]-, [Fe]-, [Ga]-, and [Al]-ZSM-5. J Phys Chem 89:1569–1571. https://doi.org/10.1021/j100255a005
Jungsuttiwong S, Lomratsiri J, Limtrakul J (2011) Characterization of acidity in [B], [Al], and [Ga] isomorphously substituted ZSM-5: embedded DFT/UFF approach. Int J Quantum Chem 111:2275–2282. https://doi.org/10.1002/qua.22531
Deka RC, Vetrivel R, Pal S (1999) Application of hard-soft acid-base principle to study Brönsted acid sites in zeolite clusters: a quantum chemical study. J Phys Chem A 103:5978–5982. https://doi.org/10.1021/jp984267k
Yuan SP, Wang JG, Li YW, Jiao H (2002) Brønsted acidity of isomorphously substituted ZSM-5 by B, Al, Ga, and Fe. Density functional investigations. J Phys Chem A 106:8167–8172. https://doi.org/10.1021/jp025792t
Yuan SP, Wang JG, Li YW, Jiao H (2004) Density functional investigations into the siting of Fe and the acidic properties of isomorphously substituted mordenite by B, Al, Ga and Fe. J Mol Struct (Thoechem) 674:267–274. https://doi.org/10.1016/S0166-1280(03)00463-9
Wang Y, Yang G, Zhou D, Bao X (2004) Density functional theory study of chemical composition influence on the acidity of H-MCM-22 zeolite. J Phys Chem B 108:18228–18233. https://doi.org/10.1021/jp049384w
Wang Y, Zhou D, Yang G, Miao S, Liu X, Bao X (2004) A DFT study on isomorphously substituted MCM-22 zeolite. J Phys Chem A 108:6730–6734. https://doi.org/10.1021/jp0376875
Patet RE, Koehle M, Lobo RF, Caratzoulas S, Vlachos DG (2017) General acid-type catalysis in the dehydrative aromatization of furans to aromatics in H-[Al]-BEA, H-[Fe]-BEA, H-[Ga]-BEA, and H-[B]-BEA zeolites. J Phys Chem C 121:13666–13679. https://doi.org/10.1021/acs.jpcc.7b02344
Wannapakdee W, Suttipat D, Dugkhuntod P, Yutthalekha T, Thivasasith A, Kidkhunthod P, Nokbin S, Pengpanich S, Limtrakul J, Wattanakit C (2019) Aromatization of C5 hydrocarbons over Ga-modified hierarchical HZSM-5 nanosheets. Fuel 236:1243–1253. https://doi.org/10.1016/j.fuel.2018.09.093
Derouane EG, Chang CD (2000) Confinement effects in the adsorption of simple bases by zeolites. Microporous Mesoporous Mater 35–36:425–433. https://doi.org/10.1016/S1387-1811(99)00239-5
Boekfa B, Choomwattana S, Khongpracha P, Limtrakul J (2009) Effects of the zeolite framework on the adsorptions and hydrogen-exchange reactions of unsaturated aliphatic, aromatic, and heterocyclic compounds in ZSM-5 zeolite: a combination of perturbation theory (MP2) and a newly developed density functional theory (M06–2X) in ONIOM scheme. Langmuir 25:12990–12999. https://doi.org/10.1021/la901841w
Zhao Y, Truhlar DG (2008) The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor Chem Acc 120:215–241. https://doi.org/10.1007/s00214-007-0310-x
Boekfa B, Pantu P, Probst M, Limtrakul J (2010) Adsorption and tautomerization reaction of acetone on acidic zeolites: the confinement effect in different types of zeolites. J Phys Chem C 114:15061–15067. https://doi.org/10.1021/jp1058947
Klinyod S, Boekfa B, Pornsatitworakul S, Maihom T, Jarussophon N, Treesukol P, Wattanakit C, Limtrakul J (2019) Theoretical and experimental study on the 7-hydroxy-4-methylcoumarin synthesis with H-beta zeolite. ChemistrySelect 4:10660–10667. https://doi.org/10.1002/slct.201902596
Kongpatpanich K, Nanok T, Boekfa B, Probst M, Limtrakul J (2011) Structures and reaction mechanisms of glycerol dehydration over H-ZSM-5 zeolite: a density functional theory study. Phys Chem Chem Phys 13:6462–6470. https://doi.org/10.1039/c0cp01720e
Injongkol Y, Maihom T, Choomwattana S, Boekfa B, Limtrakul J (2017) A mechanistic study of ethanol transformation into ethene and acetaldehyde on an oxygenated Au-exchanged ZSM-5 zeolite. RSC Adv 7:38052–38058. https://doi.org/10.1039/c7ra06313j
Paluka V, Maihom T, Warakulwit C, Srifa P, Boekfa B, Treesukol P, Poolmee P, Limtrakul J (2020) Density functional study of the effect of cation exchanged Sn-beta zeolite for the diels-alder reaction between furan and methyl acrylate. Chem Phys Lett 754:137743. https://doi.org/10.1016/j.cplett.2020.137743
Boekfa B, Pahl E, Gaston N, Sakurai H, Limtrakul J, Ehara M (2014) C-Cl bond activation on Au/Pd bimetallic nanocatalysts studied by density functional theory and genetic algorithm calculations. J Phys Chem C 118:22188–22196. https://doi.org/10.1021/jp5074472
Boekfa B, Treesukol P, Injongkol Y, Maihom T, Maitarad P, Limtrakul J (2018) The activation of methane on Ru, Rh, and Pd decorated carbon nanotube and boron nitride nanotube: a DFT study. Catalysts 8:190. https://doi.org/10.3390/catal8050190
Ketrat S, Maihom T, Treesukul P, Boekfa B, Limtrakul J (2019) Theoretical study of methane adsorption and C─H bond activation over Fe-embedded graphene: effect of external electric field. J Comput Chem 40:2819–2826. https://doi.org/10.1002/jcc.26058
Pueyo Bellafont N, Álvarez Saiz G, Viñes F, Illas F (2016) Performance of Minnesota functionals on predicting core-level binding energies of molecules containing main-group elements. Theor Chem Acc 135:1–9. https://doi.org/10.1007/s00214-015-1787-3
Gupta A, Boekfa B, Sakurai H, Ehara M, Priyakumar UD (2016) Structure, interaction, and dynamics of Au/Pd bimetallic nanoalloys dispersed in aqueous ethylpyrrolidone, a monomeric moiety of polyvinylpyrrolidone. J Phys Chem C 120:17454–17464. https://doi.org/10.1021/acs.jpcc.6b05097
Nimnual P, Tummatorn J, Boekfa B, Thongsornkleeb C, Ruchirawat S, Piyachat P, Punjajom K (2019) Construction of 5-aminotetrazoles via in situ generation of carbodiimidium ions from ketones promoted by TMSN3/TfOH. J Org Chem 84:5603–5613. https://doi.org/10.1021/acs.joc.9b00555
Khownium K, Romsaiyud J, Borwornpinyo S, Wongkrasant P, Pongkorpsakol P, Muanprasat C, Boekfa B, Vilaivan T, Ruchirawat S, Limtrakul J (2019) Turn-on fluorescent sensor for the detection of lipopolysaccharides based on a novel bispyrenyl terephtalaldehyde-bis-guanylhydrazone. New J Chem 43:7051–7056. https://doi.org/10.1039/c9nj00323a
Zhao P, Boekfa B, Nishitoba T, Tsunoji N, Sano T, Yokoi T, Ogura M, Ehara M (2020) Theoretical study on 31P NMR chemical shifts of phosphorus-modified CHA zeolites. Microporous Mesoporous Mater 294:109908. https://doi.org/10.1016/j.micromeso.2019.109908
Shetsiri S, Thivasasith A, Saenluang K, Wannapakdee W, Salakhum S, Wetchasat P, Nokbin S, Limtrakul J, Wattanakit C (2019) Sustainable production of ethylene from bioethanol over hierarchical ZSM-5 nanosheets. Sustainable Energy Fuels 3:115–126. https://doi.org/10.1039/c8se00392k
Knaeble W, Iglesia E (2016) Kinetic and theoretical insights into the mechanism of alkanol dehydration on solid Bronsted acid catalysts. The Journal of Physical Chemistry C 120:3371–3389. https://doi.org/10.1021/acs.jpcc.5b11127
Dapprich S, Komáromi I, Byun KS, Morokuma K, Frisch MJ (1999) A new ONIOM implementation in Gaussian98. Part I. The calculation of energies, gradients, vibrational frequencies and electric field derivatives. J Mol Struct (Thoechem) 461–462:1–21. https://doi.org/10.1016/S0166-1280(98)00475-8
Derouane EG (1998) Zeolites as solid solvents. J Mol Catal A: Chem 134:29–45. https://doi.org/10.1016/S1381-1169(98)00021-1
Stefanovich EV, Truong TN (1996) Embedded density functional approach for calculations of adsorption on ionic crystals. J Chem Phys 104:2946–2955. https://doi.org/10.1063/1.471115
Allavena M, Seiti K, Kassab E, Ferenczy G, Ángyán JG (1990) Quantum-chemical model calculations on the acidic site of zeolites including Madelung-potential effects. Chem Phys Lett 168:461–467. https://doi.org/10.1016/0009-2614(90)85144-2
Maihom T, Boekfa B, Sirijaraensre J, Nanok T, Probst M, Limtrakul J (2009) Reaction mechanisms of the methylation of ethene with methanol and dimethyl ether over h-zsm-5: an ONIOM study. J Phys Chem C 113:6654–6662. https://doi.org/10.1021/jp809746a
Boekfa B, Pantu P, Limtrakul J (2008) Interactions of amino acids with H-ZSM-5 zeolite: an embedded ONIOM study. J Mol Struct 889:81–88. https://doi.org/10.1016/j.molstruc.2008.01.026
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnen Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam NJ, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09. Gaussian Inc, Wallingford
Freude D, Klinowski J, Hamdan H (1988) Solid-state NMR studies of the geometry of brønsted acid sites in zeolitic catalysts. Chem Phys Lett 149:355–362. https://doi.org/10.1016/0009-2614(88)85107-8
Muraoka K, Chaikittisilp W, Okubo T (2016) Energy analysis of aluminosilicate zeolites with comprehensive ranges of framework topologies, chemical compositions, and aluminum distributions. J Am Chem Soc 138:6184–6193. https://doi.org/10.1021/jacs.6b01341
Gagne OC, Hawthorne FC (2018) Bond-length distributions for ions bonded to oxygen: metalloids and post-transition metals: metalloids. Acta Crystallogr B Struct Sci Cryst Eng Mater 74:63–78. https://doi.org/10.1107/S2052520617017437
Cant NW, Hall WK (1972) Studies of the hydrogen held by solids. XXI. The interaction between ethylene and hydroxyl groups of a Y-zeolite at elevated temperatures. J Catal 25:161–172. https://doi.org/10.1016/0021-9517(72)90213-8
Acknowledgments
Nattida Maeboonruan acknowledges the Science Technology, Engineering, Mathematics (STEM) grant SCA-CO-2561-6176-TH. Bundet Boekfa acknowledges the Thailand Research Fund (TRF) grant MRG6080103. Support from the Kasetsart Research and Development Institute (KURDI) and the Faculty of Liberal Arts and Science Kasetsart University; the Thailand Graduate Institute of Science and Technology (TGIST); the National e-Science Infrastructure Consortium; the Graduate School of Kasetsart University; the Ministry of Higher Education, Science, Research and Innovation; and the Thai Agro Energy Public Company Limited is also acknowledged.
Funding
This work was supported by Science Technology, Engineering, Mathematics (STEM) grant SCA-CO-2561-6176-TH and the Thailand Research Fund (TRF) grant MRG6080103.
Author information
Authors and Affiliations
Contributions
Nattida Maeboonruan: investigation, methodology, and writing.
Bundet Boekfa: supervision, conceptualization, investigation, methodology, and writing.
Thana Maihom: conceptualization and investigation.
Piti Treesukol: conceptualization and writing.
Kanokwan Kongpatpanich: conceptualization and investigation.
Supawadee Namuangruk: conceptualization and investigation.
Michael Probst: conceptualization and writing.
Jumras Limtrakul: conceptualization and investigation.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Maeboonruan, N., Boekfa, B., Maihom, T. et al. Adsorption and dehydration of ethanol on isomorphously B, Al, and Ga substituted H-ZSM-5 zeolite: an embedded ONIOM study. J Mol Model 27, 354 (2021). https://doi.org/10.1007/s00894-021-04979-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-021-04979-8