Abstract
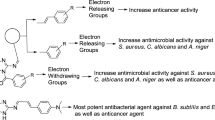
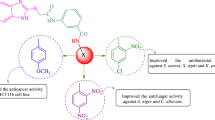
In this study, N-(substituted phenyl)-2/4-(1H-indol-3-ylazo)-benzamides (1–26) were synthesized and screened for their in vitro antibacterial (Gram positive; S. aureus, B. subtilis and Gram negative; E. coli) and antifungal (C. albicans and A. niger) activities. The antimicrobial activity results indicated that compound, 4-(1H-indol-3-ylazo)-N-(4-nitro-phenyl)-benzamide (12, pMICam = 1.61) was the most potent. In general, it was found that the synthesized compounds were bacteriostatic/fungistatic in action except fungicidal for A. niger. The synthesized compounds were also evaluated for their antiproliferative activity against human colon cancer (HCT116), murine leukemia (P388), and breast cancer (MCF7) cell lines. The antiproliferative study results demonstrated 4-(1H-indol-3-ylazo)-N-p-tolyl-benzamide (2, IC50 = 0.0003 μM/mL) and 4-(1H-indol-3-ylazo)-N-p-tolyl-benzamide (21, 0.0003 μM/mL) as lead compounds for the development of novel antiproliferative agents. The QSAR studies indicated the importance of topological parameters, Kier’s alpha second-order shape indice (κα2) and Wiener index (W) in describing the antimicrobial activity of the synthesized compounds.



Similar content being viewed by others
References
Abdel-Jalil RJ, Momani EQEl, Hamad M, Voelter W, Mubarak MS, Smith BH, Peters DG (2010) Synthesis, antitumor activity and electrochemical behavior of some piperazinyl amidrazones. Monatsh Chem 141:251–258
Bajaj S, Sambi SS, Madan AK (2005) Prediction of anti-inflammatory activity of N-arylanthranilic acids: computational approach using refined Zagreb Indices. Croat Chem Acta 78(2):165–174
Balaban AT (1982) Highly discriminating distance based topological indices. Chem Phys Lett 89:399–404
Cappucino JG, Sherman N (1999) Microbiology—a laboratory manual. Addison Wesley, Davis 263
Chiyanzu I, Clarkson C, Smith PJ, Lehman J, Gut J, Rosenthal PJ, Chibale K (2005) Design, synthesis and anti-plasmodial evaluation in vitro of new 4-aminoquinoline isatin derivatives. Bioorgan Med Chem 13:3249–3261
Cruz-Monteagudo M, Gonzalez-Diaz H, Aguero-Chapin G, Santana L, Borges F, Dominguez ER, Podda G, Uriarte E (2007) Computational chemistry development of a unified free energy Markov model for the distribution of 1300 chemicals to 38 different environmental or biological systems. J Comput Chem 28(11):1909–1923
Emami S, Falahati M, Banifafemi A, Shafiee A (2004) Stereoselective synthesis and antifungal activity of (Z)-trans-3-azolyl-2-methylchromanone oxime ethers. Bioorgan Med Chem 12:5881–5889
Furniss BS, Hannaford AJ, Smith PWG, Tatchell AR (1998) Vogel’s text book of practical organic chemistry. Addison Wesley, Davis, pp 34–35
Giaginis C, Tsantili-Kakoulidou A, Theocharis S (2009) Quantitative structure activity relationship (QSAR) methodology in forensic toxicology: modeling post mortem redistribution of structurally diverse drugs using multivariate statistics. Forensic Sci Int 190:9–15
Golbraikh A, Tropsha A (2002) Beware of q2! J Mol Graph Model 20:269–276
Gonzalez-Diaz H, Prado–Prado FJ (2008) Unified QSAR and network-based computational chemistry approach to antimicrobials, part 1: multispecies activity models for antifungals. J Comput Chem 29(4):656–667
Gonzalez-Diaz H, Vilar S, Santana L, Uriarte E (2007) Medicinal chemistry and bioinformatics—current trends in drugs discovery with networks topological indices. Curr Top Med Chem 7(10):1015–1029
Hansch C, Fujita T (1964) p–σ–π Analysis. A method for the correlation of biological activity and chemical structure. J Am Chem Soc 86:1616–1626
Hansch C, Leo A, Unger SH, Kim KH, Nikaitani D, Lien EJ (1973) “Aromatic” substituent constants for structure–activity correlations. J Med Chem 16(11):1207–1216
Ivachtchenko AV, Frolov EB, Mitkin OD, Tkachenko SE, Okun IM, Khvat AV (2010) Synthesis and biological activity of 5-styryl and 5-phenethyl-substituted 2,3,4,5-tetrahydro-1H-pyrido[4,3-b]indoles. Bioorg Med Chem Lett 20:78–82
Jacquemard U, Dias N, Lansiaux A, Bailly C, Loge C, Robert JM, Lozach O, Meijer L, Meroura JY, Routiera S (2008) Synthesis of 3,5-bis(2-indolyl)pyridine and 3-[(2-indolyl)-5-phenyl]-pyridine derivatives as CDK inhibitors and cytotoxic agents. Bioorgan Med Chem 16:4932–4953
Judge V, Narasimhan B, Ahuja M, Sriram D, Yogeeswari P, Clercq ED, Pannecouque C, Balzarini J (2012) Synthesis, antimycobacterial, antiviral, antimicrobial activity and QSAR studies of Isonicotinic acid-1-(substituted phenyl)-ethylidene/cycloheptylidene hydrazides. Med Chem Res 21(8):1935–1952
Kier LB, Hall LH (1976) Molecular connectivity in chemistry and drug research. Academic Press, New York
Kier LB, Hall LH (1999) The kappa indices for modeling molecular shape and flexibility. In: Devillers J, Balaban AT (eds) Topological indices and related descriptors in QSAR and QSPR. Gordon and Breach Science Publishers, Amsterdam, The Netherlands, pp 455–489
Kumar A, Narasimhan B, Kumar D (2007) Synthesis, antimicrobial, and QSAR studies of substituted benzamides. Bioorgan Med Chem 15:4113–4124
Kumar P, Narasimhan B, Sharma D, Judge V, Narang R (2009) Hansch analysis of substituted benzoic acid benzylidene/furan-2-yl-methylene hydrazides as antimicrobial agents. Eur J Med Chem 44:1853–1863
Kumar D, Judge V, Narang R, Sangwan S, Clercq ED, Balzarini J, Narasimhan B (2010) Benzylidene/2-chlorobenzylidene hydrazides: synthesis, antimicrobial activity, QSAR studies and antiviral evaluation. Eur J Med Chem 45:2806–2816
Lamotte Y, Martres P, Faucher N, Laroze A, Grillot D, Ancellin N, Saintillan Y, Beneton V, Gampe RT (2010) Synthesis and biological activities of novel indole derivatives as potent and selective PPARγ modulators. Bioorg Med Chem Lett 20:1399–1404
Laxmi SV, Rajitha B (2012) Synthesis and antimicrobial activity of newer indole semicarbazones. Med Chem Res 21(2):85–90
Lee C, Yao C, Huang S, Ko S, Tan YH, Lee-Chen G, Wang Y (2008) Novel 2-step synthetic indole compound 1,1,3-tri(3-indolyl)cyclohexane inhibits cancer cell growth in lung cancer cells and xenograft models. Cancer 113:815–825
Lee J, Attila C, Cirillo SLG, Cirillo JD, Wood TK (2009) Indole and 7-hydroxyindole diminish Pseudomonas aeruginosa virulence. Microb Biotechnol 2(1):75–90
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65(1–2):55–63
Narang R, Narasimhan B, Sharma S, Sriram D, Yogeeswari P, Clercq ED, Pannecouque C, Balzarini J (2012) Synthesis, antimycobacterial, antiviral, antimicrobial activity and QSAR studies of nicotinic acid benzylidene hydrazide derivatives. Med Chem Res 21(8):1557–1576
Narasimhan B, Judge V, Narang R, Ohlan S, Ohlan R (2007) Quantitative structure–activity relationship studies for prediction of antimicrobial activity of synthesized 2,4-hexadienoic acid derivatives. Bioorg Med Chem Lett 17:5836–5845
Ozkay Y, Tunali Y, Karaca H, Isikdag I (2010) Antimicrobial activity and a SAR study of some novel benzimidazole derivatives bearing hydrazones moiety. Eur J Med Chem 45:3293–3298
Ozturk A, Abdullah MI (2006) Toxicological effect of indole and its azo dye derivatives on some microorganisms under aerobic conditions. Sci Total Environ 358:137–142
Pharmacopoeia of India (2007) Controller of publications, Ministry of Health Department, Govt. of India, New Delhi, vol. I, p 37
Prado-Prado FJ, Gonzalez-Diaz H, Vega OMDL, Ubeira FM, Chou KC (2008) Unified QSAR approach to antimicrobials. Part 3: first multi-tasking QSAR model for Input-Coded prediction, structural back-projection, and complex networks clustering of antiprotozoal compounds. Bioorgan. Med Chem 16(11):5871–5880
Radwan MAA, Ragab EA, Sabry NM, El-Shenawy SM (2007) Synthesis and biological evaluation of new 3-substituted indole derivatives as potential anti-inflammatory and analgesic agents. Bioorgan Med Chem 15:3832–3841
Randic M (1975) On characterization of molecular branching. J Am Chem Soc 97:6609–6615
Randic M (1993) Comparative regression analysis: regression based on a single descriptor. Croat Chem Acta 66:289–312
Rodriguez-Arguelles MC, Lopez-Silva EC, Sanmartin J, Pelagatti P, Zani F (2005) Copper complexes of imidazole-2-, pyrrole-2- and indol-3-carbaldehyde thiosemicarbazones: inhibitory activity against fungi and bacteria. J Inorg Biochem 99:2231–2239
Strappaghetti G, Mastrini L, Lucacchini A, Giannaccini G, Betti L, Fabbrini L (2008) Synthesis and biological affinity of new imidazo- and indol-arylpiperazine derivatives: further validation of a pharmacophore model for α1-adrenoceptor antagonists. Bioorg Med Chem Lett 18:5140–5145
TSAR 3D Version 3.3, Oxford Molecular Limited, 2000
Velankar AD, Quintini G, Prabhu A, Weber A, Hunaeus G, Voland B, Wuest M, Orjeda C, Harel D, Varghese S, Gore V, Patil M, Gayke D, Herdemann M, Heit I, Zaliani A (2010) Synthesis and biological evaluation of novel (4 or 5-aryl)pyrazolyl-indoles as inhibitors of interleukin-2 inducible T-cell kinase (ITK). Bioorgan Med Chem 18:4547–4559
Wiener H (1947) Structural determination of paraffin boiling points. J Am Chem Soc 69:17–20
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kumar, H., Kumar, P., Narasimhan, B. et al. Synthesis, in vitro antimicrobial, antiproliferative, and QSAR studies of N-(substituted phenyl)-2/4-(1H-indol-3-ylazo)-benzamides. Med Chem Res 22, 1957–1971 (2013). https://doi.org/10.1007/s00044-012-0181-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-012-0181-0