Abstract
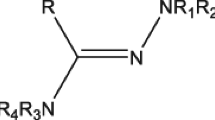
New piperazinyl amidrazones have been synthesized by direct interaction of the corresponding aryl hydrazones with the appropriate piperazine. On the basis of preliminary screening data for these new compounds, the antitumor activity of 1-(4-methylpiperazin-1-yl)-1,2-propandione 1-[2-(4-chlorophenyl)hydrazone] and 1-(4-ethylpiperazin-1-yl)-1,2-propandione 1-[2-(4-chlorophenyl)hydrazone] was evaluated. Mean 50% growth inhibition (GI 50), 50% cell killing (LC 50), and total growth inhibition (TGI) for both compounds were calculated on the basis of data obtained from 55 test cell lines. Mean GI 50 is significantly lower for the methylpiperazine derivative (4.81 μM) compared with that of the ethylpiperazine derivative (4.92 μM) (p > 0.01); however, mean TGI is not measurably different (p > 0.1) for both compounds (4.52 and 4.52 μM, respectively). Both compounds exhibit substantial antitumor activity against a number of cell lines at 4 μM concentration. It was found that the methylpiperazine derivative is more potent against leukemia cell lines (mean GI 50 = 4.73 μM and mean TGI = 4.36 μM), whereas the ethylpiperazine derivative is more potent against CNS cell lines (GI 50 = 4.68 μM and mean TGI = 4.37 μM). Cancers of the breast are least susceptible to methylpiperazine derivative activity compared with all other cell lines (mean GI 50 = 4.91 μM). Melanomas and renal cancers are least susceptible to the ethylpiperazine derivative activity as compared with other cancer types (mean GI 50 = 5.06 μM). Cyclic voltammetry has been employed to probe the electrochemical oxidation and reduction of the piperazinyl amidrazones at glassy carbon electrodes in dimethylformamide containing tetramethylammonium tetrafluoroborate.
Graphical abstract



Similar content being viewed by others
References
MacCoss M, Baillie TA (2004) Science 303:1810
Hanahan D, Weinberg RA (2000) Cell 100:57
Giamas G, Stebbing J, Vorgias CE, Knippschild U (2007) Pharmacogenomics 8:1005
Khalaj A, Adibpour N, Shahverdi AR, Daneshtalab M (2004) Eur J Med Chem 39:699
Broekkamp CLE, Leysen D, Peeters BWMM, Pinder RM (1995) J Med Chem 38:4615
Naito H, Ohsuki S, Atsumi R, Minami M, Mochizuki M, Hirotani K, Kumazawa E, Ejima A (2005) Chem Pharm Bull 53:153
Ibarra M, Hong E, Villalobos-Molina RJ (2000) J Auton Pharmacol 20:139
Jiang X-H, Song Y-L, Long Y-Q (2004) Bioorg Med Chem Lett 14:3675
Yoon J, Yoo EA, Kim J-Y, Pae AN, Rhim H, Park W-K, Kong JY, Choo H-YP (2005) Bioorg Med Chem 16:5405
Vu CB, Peng B, Kumaravel G, Smits G, Jin X, Phadke D, Engber T, Huang C, Reilly J, Tam S, Grant D, Hetu G, Chen L, Zhang J, Petter RC (2004) J Med Chem 47:4291
Tollefson GD, Lancaster SP, Montague-Clouse J (1991) Psychopharmacol Bull 27:163
Rotzinger S, Fang J, Baker GB (1998) Drug Metab Dispos 26:572
Wilson DM, Termin AP, Mao L, Ramirez-Weinhouse MM, Molteni V, Grootenhuis PDJ, Miller K, Keim S, Wise GJ (2002) Med Chem 45:2123
Oh YS, Yun M, Hwang SY, Hong S, Shin Y, Lee K, Yoon KH, Yoo YJ, Kim DS, Lee SH, Lee YH, Park HD, Lee CH, Lee SK, Kim S (1998) Bioorg Med Chem Lett 8:631
Lee K, Hwaqng SY, Hong S, Hong CY, Lee C-S, Shin Y, Kim S, Yun M, Yoo YJ, Kang M, Oh YS (1998) Bioorg Med Chem 6:869
Lee K, Jung W-H, Park CW, Park HD, Lee SH, Kwon OH (2002) Bioorg Med Chem Lett 12:1017
Clemens F, Drutkowski G, Wiese M, Frohberg P (2002) Biochim Biophys Acta 1549:88
Goyal RNJ (1992) Sci Ind Res 51:948
Becker HGO, Görmar G, Timpe H-JJ (1970) J Prakt Chem 312:610
Tabaković I, Laćan M, Damoni S (1976) Electrochim Acta 21:621
Barbey G, Delahaye D, Lamant M, Caullet C (1980) Electrochim Acta 25:1273
Limacher LL, Delay FD, Bédert N, Tissot P (1989) Helv Chim Acta 72:1383
Okimoto M, Nagata Y, Takahasi Y (2003) Bull Chem Soc Jpn 76:1447
Okimoto M, Takahasi Y, Kakuchi T (2003) Synthesis 13:2057
Mamatha GP, Sherigara BS, Mahadevan KMJ (2003) Chem Sci 119:267
Hussein AQ, El-Abadelah MM, Al-Adhami KH, Abushamleh AS (1989) Heterocycles 29:1163
Marple LW (1967) Anal Chem 39:844
Manning CW, Purdy WC (1970) Anal Chim Acta 51:124
Hall JL, Jennings PW (1976) Anal Chem 48:2026
Vieira KL, Peters DGJ (1985) Electroanal Chem 196:93
Acknowledgments
Financial support provided by Hashemite University is gratefully acknowledged. R. J. A.-J. thanks Deutsche Forschungsgemeinschaft (DFG) for a research fellowship for visiting faculty. High-resolution mass analyses were performed in the Indiana University Mass Spectrometry Facility; the HRMS system was purchased with funds provided by the National Institutes of Health grant no. 1S10RR016657-01.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Abdel-Jalil, R.J., El Momani, E.Q., Hamad, M. et al. Synthesis, antitumor activity, and electrochemical behavior of some piperazinyl amidrazones. Monatsh Chem 141, 251–258 (2010). https://doi.org/10.1007/s00706-009-0241-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-009-0241-4