Abstract
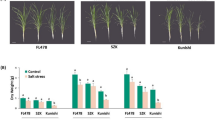
The effects of treatment with nitric oxide donor sodium nitroprusside (SNP, 0.5 mM) on salt tolerance of wild type (Col–0) Arabidopsis thaliana plants and Arabidopsis thaliana plants transformed with the bacterial salicylate hydroxylase gene (NahG) were compared. Basic salt tolerance level (200 mM NaCl) was higher in NahG transformants. Under salt stress conditions, these plants showed higher activity levels for antioxidant enzymes as well as higher content of sugars and anthocyanins. The treatment with NO donor induced salt tolerance in the plants of both genotypes, which could be observed as less strong growth inhibition, reduced oxidative damage, and preservation of chlorophyll pool in leaves. After the exposure to salt stress, the activity of both superoxide dismutase and guaiacol peroxidase was higher in SNP-treated wild type plants and NahG transformants than in the nontreated plants. After the imposition of salt stress, proline content in leaves of the wild type plants treated with the nitric oxide donor was lower than in the leaves of the nontreated plants. In contrast, SNP treatment of NahG transformants led to a significant increase in the proline content in leaves under the salt stress conditions. Conclusions have been made that wild type Col-0 plants and NahG transformants differ in how their systems of protection against salt stress are activated and that nitric oxideinduced mobilization of protection systems in A. thaliana may not require the presence of salicylate.
Similar content being viewed by others
References
Dmitriev, A.P., Signal role of nitric oxide in plants, Tsitol. Genet., 2004, vol. 38, no. 4, pp. 67–75.
Zhang, Y., Wang, L., Liu, Y., Zhang, Q., Wei, Q., and Zhang, W., Nitric oxide enhances salt tolerance in maize seedlings through increasing activities of protonpump and Na+/H+ antiport in the tonoplast, Planta, 2006, vol. 224, no. 3, pp. 545–555.
Siddiqui, M.H., Al-Whaibi, M.H., and Basalah, M.O., Role of nitric oxide in tolerance of plants to abiotic stress, Protoplasma, 2011, vol. 248, no. 3, pp. 447–455.
Hayat, Q., Hayat, S., Irfan, M., and Ahmad, A., Effect of exogenous salicylic acid under changing environment: a review, Environ. Exp. Bot., 2010, vol. 68, no. 1, pp. 14–25.
Hara, M., Furukawa, J., Sato, A., Mizoguchi, T., and Miura, K., Abiotic stress and role of salicylic acid in plants, Abiotic Stress Responses in Plants, Ahmad, P. and Prasad, M.N.V., Eds., New York: Springer, 2012, pp. 235–251.
Krasylenko, Y.A., Yemets, A.I., Sheremet, Y.A., and Blume, Ya.B., Nitric oxide as a critical factor for perception of UV-B irradiation by microtubules in Arabidopsis, Physiol. Plant., 2012, vol. 145, no. 4, pp. 505–515.
Sawada, H., Shim, I., and Usui, K., Induction of benzoic acid 2-hydroxylase and salicylic acid biosynthesis— modulation by salt stress in rice seedlings, Plant Sci., 2006, vol. 171, no. 2, pp. 263–270.
Uchida, A., Jagendorf, A.T., Hibino, T., Takabe, T., and Takabe, T., Effects of hydrogen peroxide and nitric oxide on both salt and heat stress tolerance in rice, Plant Sci., 2002, vol. 163, no. 3, pp. 515–523.
Karpets, Yu.V., Kolupaev, Yu.E., Yastreb, T.O., and Dmitriev, O.P., Possible pathways of heat resistance induction in plant cells by exogenous nitrogen oxide, Cytol. Genet., 2012, vol. 46, no. 6, pp. 354–359.
Kolupaev, Yu.E., Yastreb, T.O., Shvidenko, N.V., and Karpets, Yu.V., Induction of heat resistance of wheat coleoptiles by salicylic and succinic acids: connection of the effect with the generation and neutralization of reactive oxygen species, Appl. Biochem. Microbiol., 2012, vol. 48, no. 5, pp. 500–505.
Mur, L.A.J., Kenton, P., Atzorn, R., Miersch, O., and Wasternack, C., The outcomes of concentration-specific interactions between salicylate and jasmonate signaling include synergy, antagonism, and oxidative stress leading to cell death, Plant Physiol., 2006, vol. 140, no. 1, pp. 249–262.
Tewari, R.K. and Paek, K.Y., Salicylic acid-induced nitric oxide and ROS generation stimulate ginsenoside accumulation in Panax ginseng roots, J. Plant Growth Regul., 2011, vol. 30, no. 4, pp. 396–404.
Durner, J., Wendehenne, D., and Klessig, D.F., Defense gene induction in tobacco by nitric oxide, cyclic GMP and cyclic ADP-ribose, Proc. Natl. Acad. Sci. U. S. A., 1998, vol. 95, no. 17, pp. 10328–10333.
Song, F. and Goodman, R.M., Activity of nitric oxide is dependent on, but is partially required for function of, salicylic acid in the signaling pathway in tobacco systemic acquired resistance, Mol. Plant–Microbe Interact., 2001, vol. 14, no. 12, pp. 1458–1462.
Chun, H.J., Park, H.C., Koo, S.C., Lee, J.H., Park, C.Y., Choi, M.S., Kang, C.H., Baek, D., Cheong, Y.H., Yun, D.-J., Chung, W.S., Cho, M.J., and Kim, M.C., Constitutive expression of mammalian nitric oxide synthase in tobacco plants triggers disease resistance to pathogens, Mol. Cells, 2012, vol. 34, no. 5, pp. 463–471.
Kovacs, I., Durner, J., and Lindermayr, C., Crosstalk between nitric oxide and glutathione is required for NONEXPRESSOR OFPATHOGENESISRELATED GENES 1 (NPR1)-dependent defense signaling in Arabidopsis thaliana, New Phytol., 2015, vol. 208, no. 3, pp. 860–872.
Klessig, D.F., Durner, J., Noad, R., Navarre, D.A., Wendehenne, D., Kumar, D., Zhou, J.M., Shah, J., Zhang, S., Kachroo, P., Trifa, Y., Pontier, D., Lam, E., and Silva, H., Nitric oxide and salicylic acid signaling in plant defense, Proc. Natl. Acad. Sci. U. S. A., 2000, vol. 97, no. 16, pp. 8849–8855.
Liu, S., Dong, Y., Xu, L., and Kong, J., Effects of foliar applications of nitric oxide and salicylic acid on saltinduced changes in photosynthesis and antioxidative metabolism of cotton seedlings, Plant Growth Regul., 2014, vol. 73, no. 1, pp. 67–78.
Borsani, O., Valpuesta, V., and Botella, M.A., Evidence for a role of salicylic acid in the oxidative damage generated by NaCl and osmotic stress in Arabidopsis seedlings, Plant Physiol., 2001, vol. 126, no. 3, pp. 1024–1030.
He, Q., Zhao, S., Ma, Q., Zhang, Y., Huang, L., Li, G., and Hao, L., Endogenous salicylic acid levels and signaling positively regulate Arabidopsis response to polyethylene glycol-simulated drought stress, J. Plant Growth Regul., 2014, vol. 33, no. 4, pp. 871–880.
Cao, Y., Zhan, Z.W., Xue, L.W., Du, J.B., Shang, J., Xu, F., Yuan, S., and Lin, H.H., Lack of salicylic acid in Arabidopsis protects plants against moderate salt stress, Z. Naturforsch., A: Phys. Sci., 2009, vol. 64, nos. 3–4, pp. 231–238.
Yastreb, T.O., Kolupaev, Yu.E., Karpets, Yu.V., and Dmitriev, A.P., Effect of nitric oxide donor on salt resistance of Arabidopsis jin1 mutants and wild-type plants, Russ. J. Plant Physiol., 2017, vol. 64, no. 2, pp. 207–214.
Gaffney, T., Friedrich, L., Vernooij, B., Negrotto, D., Nye, G., Uknes, S., Ward, E., Kessmann, H., and Ryals, J., Requirement of salicylic acid for the induction of systemic acquired resistance, Science, 1993, vol. 261, no. 5122, pp. 754–756.
Gibeaut, D.M., Hulett, J., Cramer, G.R., and Seemann, J.R., Maximal biomass of Arabidopsis thaliana using a simple, low-maintenance hydroponic method and favorable environmental conditions, Plant Physiol., 1997, vol. 115, no. 2, pp. 317–319.
Yastreb, T.O., Kolupaev, Yu.E., Shvidenko, N.V., Lugovaya, A.A., and Dmitriev, A.P., Salt stress response in Arabidopsis thaliana plants with defective jasmonate signaling, Appl. Biochem. Microbiol., 2015, vol. 51, no. 4, pp. 451–454.
Shlyk, A.A., Measuring of chlorophylls and carotenoids in extracts of green leaves, in Biokhimicheskie metody v fiziologii rastenii (Biochemical Methods in Plant Physiology), Pavlinova, O.A., Ed., Moscow, Nauka. 1971.
Karpets, Yu.V., Kolupaev, Yu.E., Lugovaya, A.A., and Oboznyi, A.I., Effect of jasmonic acid on the pro-/ antioxidant system of wheat coleoptiles as related to hyperthermia tolerance, Russ. J. Plant Physiol., 2014, vol. 61, no. 3, pp. 339–346.
Bates, L.S., Walden, R.P., and Tear, G.D., Rapid determination of free proline for water stress studies, Plant Soil, 1973, vol. 39, no. 1, pp. 205–207.
Kefu, Z., Hai, F., San, Z., and Jie, S., Study on the salt and drought tolerance of Suaeda salsa and Kalanchoe claigremontiana under isoosmotic salt and water stress, Plant Sci., 2003, vol. 165, no. 4, pp. 837–844.
Kolupaev, Yu.E., Ryabchun, N.I., Vayner, A.A., Yastreb, T.O., and Oboznyi, A.I., Antioxidant enzyme activity and osmolyte content in winter cereal seedlings under hardening and cryostress, Russ. J. Plant Physiol., 2015, vol. 62, no. 4, pp. 499–506.
Pietrini, F. and Massacci, A., Leaf anthocyanin content changes in Zea mays L. grown at low temperature: significance for the relationship between the quantum yield of PSII and the apparent quantum yield of CO2 assimilation, Photosynth. Res., 1998, vol. 58, no. 3, pp. 213–219.
Merzlyak, M.N., Pogosyan, S.I., Yuferova, S.G., and Shevyreva, V.A., The use of 2-thiobarbituric acid in the study of lipid peroxidation in plant tissues, Biol. Nauki, 1978, no. 9, pp. 86–94.
Mur, L.A., Mandon, J., Persijn, S., Cristescu, S.M., Moshkov, I.E., Novikova, G.V., Hall, M.A., Harren, F.J., Hebelstrup, K.H., and Gupta, K.J., Nitric oxide in plants: an assessment of the current state of knowledge, AoB Plants, 2013, vol. 5, pls052. doi 10.1093/aobpla/pls052
Carcia, A.B., Engler, J.A., Iyer, S., Gerats, T., Van Montagu, M., and Caplan, A.B., Effects of osmoprotectants upon NaCl stress in rice, Plant Physiol., 1997, vol. 115, no. 1, pp. 159–169.
Khlestkina, E.K., The adaptive role of flavonoids: emphasis on cereals, Cereal Res. Commun., 2013, vol. 41, pp. 185–198.
Kavi Kishor, P.B. and Sreenivasulu, N., Is proline accumulation per se correlated with stress tolerance or is proline homeostasis a more critical issue?, Plant, Cell Environ., 2014, vol. 37, no. 2, pp. 300–311.
Soshinkova, T.N., Radyukina, N.L., Korolkova, D.V., and Nosov, A.V., Proline and functioning of the antioxidant system in Thellungiella salsuginea plants and cultured cells subjected to oxidative stress, Russ. J. Plant Physiol., 2013, vol. 60, no. 1, pp. 41–54.
Neill, S.O. and Gould, K.S., Anthocyanins in leaves: light attenuators or antioxidants?, Funct. Plant Biol., 2003, vol. 30, no. 8, pp. 865–873.
Qiu, Q.S., Barkla, B.J., Vera-Estrella, R., Zhu, J.-K., and Schumaker, K.S., Na+/H+-exchange activity in the plasma membrane of Arabidopsis, Plant Physiol., 2003, vol. 132, no. 2, pp. 1041–1052.
Grun, S., Lindermayr, C., Sell, S., and Durner, J., Nitric oxide and gene regulation in plants, J. Exp. Bot., 2006, vol. 57, no. 3, pp. 507–516.
Neill, S., Bright, J., Desikan, R., Hancock, J., Harrison, J., and Wilson, I., Nitric oxide evolution and perception, J. Exp. Bot., 2008, vol. 59, no. 1, pp. 25–35.
Besson-Bard, A., Pugin, A., and Wendehenne, D., New insights into nitric oxide signaling in plants, Annu. Rev. Plant Biol., 2008, vol. 59, pp. 21–39.
Courtois, C., Besson, A., Dehan, J., Bourque, S., Dobrowolska, G., Pugin, A., and Wendehenne, D., Nitric oxide signalling in plants: interplays with Ca2+ and protein kinases, J. Exp. Bot., 2008, vol. 59, no. 2, pp. 155–163.
Wilson, I.D., Neill, S.J., and Hancock, J.T., Nitric oxide synthesis and signalling in plants, Plant, Cell Environ., 2008, vol. 31, no. 5, pp. 622–631.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © T.O. Yastreb, Yu.V. Karpets, Yu.E. Kolupaev, A.P. Dmitriev, 2017, published in Tsitologiya i Genetika, 2017, Vol. 51, No. 2, pp. 73–82.
About this article
Cite this article
Yastreb, T.O., Karpets, Y.V., Kolupaev, Y.E. et al. Induction of salt tolerance in salicylate-deficient NahG Arabidopsis transformants using the nitric oxide donor. Cytol. Genet. 51, 134–141 (2017). https://doi.org/10.3103/S0095452717020086
Received:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S0095452717020086