Abstract
The chemical reaction between CO2 and a blended Portland cement concrete, referred to as carbonation, can lead to reduced performance, particularly when concrete is exposed to elevated levels of CO2 (i.e., accelerated carbonation conditions). When slight changes in concrete mix designs or testing conditions are adopted, conflicting carbonation results are often reported. The RILEM TC 281-CCC ‘Carbonation of Concrete with Supplementary Cementitious Materials’ has conducted a critical analysis of the standardised testing methodologies that are currently applied to determine carbonation resistance of concrete in different regions. There are at least 17 different standards or recommendations being actively used for this purpose, with significant differences in sample curing, pre-conditioning, carbonation exposure conditions, and methods used for determination of carbonation depth after exposure. These differences strongly influence the carbonation depths recorded and the carbonation coefficient values calculated. Considering the importance of accurately determining carbonation potential of concrete, not just for predicting their durability performance, but also for determining the amount of CO2 that concrete can re-absorb during or after its service life, it is imperative to recognise the applicability and limitations of the results obtained from different tests. This will enable researchers and practitioners to adopt the most appropriate testing methodologies to evaluate carbonation resistance, depending on the purpose of the conclusions derived from such testing (e. g. materials selection, service life prediction, CO2 capture potential).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Chemical durability of concrete is often determined according to standardised accelerated testing methods that are intended to simulate the most damaging environmental scenarios that concrete structures will endure during their service life. In many instances such testing protocols are derived from empirical experience and can be far from accurate in simulating the in-service environmental exposure conditions of a given concrete structure. In other cases, the test method specification documents do not provide sufficient details about the procedures to follow, which gives a great degree of freedom to adjust the testing methods, and consequently the results obtained might not be comparable among different studies, or applicable in predicting the long-term performance of concrete. This is of particular concern as novel cement and concrete formulations are being developed to decarbonise the construction sector, and there is a growing acceptance of the need to adopt performance-based standards to enable their use [1]. Often, performance of blended or alternative cementitious materials is tested by following prescriptive standards that have been developed for Portland cement concrete, that do not account for the differences in reaction kinetics, chemistry, and/or microstructure in concrete with supplementary cementitious materials (SCMs). This necessitates evaluation of the testing methodologies that are currently used to evaluate chemical durability of modern concretes, to assess their applicability for determining whether a particular concrete will meet the demands of a specified application.
Carbonation is one of the major deterioration mechanisms of concrete structures, and as discussed by von Greve-Dierfeld et al. [2], it can lead to significant changes in the microstructure and performance of concrete containing different SCMs. It is expected that carbonation will occur slowly over several decades during the service life of concrete structures; however, increasing CO2 concentrations in the air, changes in climate, as well as changes in cementitious binder formulation chemistry affect how rapidly carbonation might occur [3, 4]. Accelerated testing methods are often used to evaluate the resistance of concrete to carbonation during accessible time frames (months rather than years or decades) under controlled exposure conditions. It is well known that the exposure conditions adopted in standards to determine chemical durability of concrete are not representative of all natural environments or climates, but it is in many cases intended that the testing conditions simulate worst-case scenarios. It is thus important to ensure that the accelerated test methods produce results that are relevant in understanding, and hopefully also predicting, how concrete might perform during its service life, and beyond this time if concrete re-carbonation after demolition is considered to be relevant.
The RILEM Technical Committee (TC) 281-CCC conducted two extensive interlaboratory tests with the goal of determining the reproducibility of concrete carbonation rate results when evaluated under different accelerated [5] or natural [5] exposure conditions. For concrete with SCMs tested under accelerated carbonation, it was concluded [5] that conventionally used standardised testing methods or recommendations provide similar carbonation resistance rankings, although the carbonation rate values were significantly different across different exposure environments. Of significance for service life prediction, no correlations between the fresh or hardened properties of concretes and their accelerated carbonation performance were identified. When evaluating similar mortar and/or concrete mixes under natural carbonation exposure conditions [5] similar rankings were also recorded, and the differences associated with climatic variations tested in this study (e.g. relative humidity/ temperature, with shelter/ unsheltered) were deemed statistically insignificant. By using the data collected in these interlaboratory tests, it was investigated whether a direct correlation between accelerated and natural carbonation rates could be drawn for the concrete mixes evaluated. The results revealed that the best correlation is achieved when materials were tested according to existing EN standards (EN 12390-10 and EN 12390-12). The results from this extensive testing campaign further demonstrate the significant impact of the testing methods adopted in the response of concrete when exposed to carbonation.
As early as 1907, in an effort to simulate and predict how concretes could perform during their service life, natural carbonation exposure studies were conducted to investigate the relationship between carbonation and reinforcement corrosion, and those observations underpinned the evolution of accelerated testing exposure conditions that were accepted by industry and development for over a century [7]. Carbonation resistance testing standards have been developed for many decades, as it became evident that carbonation posed a major threat to the concrete-based built environment. Historically, standardisation organisations across the world have developed such standards in close collaboration with different stakeholders with expertise in their subject matter and who know the needs of the organisations or sectors they represent, with the aim to reach a consensus and agree on a way to conduct such tests. One of the earlier recommendations developed as a guideline for carbonation testing of concrete is RILEM CPC 18 ‘Measurement of hardened concrete carbonation depth’ [8] from 1988, which underpinned the development of later European standards to determine carbonation resistance of concrete. This recommendation created awareness of the different parameters that needed to be recorded and understood when reporting carbonation results, as they influence the performance of concrete.
1.1 Case study—History of carbonation standards in Japan
In different regions, the development of standards for evaluating carbonation resistance of concrete has taken place over the past decades. As an example, the evolution of standards in Japan is briefly discussed. Nakamura et al. [7] surveyed and summarised testing conditions and methods adopted in Japan before and after a prescriptive standard was established, comparing such methods with the accelerated carbonation tests from Europe followed at the time (see Fig. 1). For creating the standards, the Japanese Concrete Institute (JCI) organised a Japanese Industrial Standards (JIS) drafting committee in 2001, which promoted the creation of the Japanese Standards Association (JSA) JIS A 1152 ‘Method for measuring carbonation depth of concrete’ [9] in 2002 (revised in 2011 and 2018) for evaluating concrete produced in the laboratory or core specimens from existing structures.
Evolution of concrete accelerated carbonation tests and other related testing methods in Japan. Some columns indicate the type of material (concrete, polymer, coatings) for which different tests were developed (including accelerated carbonation), as well as the key properties (e.g. method for determining carbonation, length changes) determined with such tests. Adapted from [7]
In 2003, JIS A 1153 ‘Method of accelerated carbonation test for concrete’ [10] was published. This standard was based on ISO 1920-12 ‘Determination of the carbonation resistance of concrete — Accelerated carbonation method’. Both test standards were based on the guidelines prescribed by the Architectural Institute of Japan [1]. In addition to those standards, NDIS 3419 ‘Method of test for neutralization depth of concrete in structures with drilling powder’ [11] was approved in 1999 and revised in 2011. Before JIS A 1152 and NDIS 3419 were established, various testing methods were proposed and used to determine carbonation resistance of concrete in Japan.
As illustrated for the case of Japan, empirical evidence and extensive experimental trials motivate changes in prescriptive standards. A given standardised testing methodology that is widely accepted by the construction sector today might have been derived from extensive validation and experimental work for a given set of materials available at the time (e.g. plain Portland cement), which might not be similar to those that will be used in the years to come [12] (e.g. the blended cements currently in widespread use). Therefore, it is imperative to critically assess how durability of concrete is tested, and the limitations of the results obtained from those tests, so that the results can be adequately interpreted in context.
2 Standardised testing methods for determining carbonation resistance of concrete
Several studies have highlighted different existing standardised testing procedures to determine carbonation resistance of concrete, with significant differences in sample pre-conditioning [13], exposure conditions (CO2 concentrations, relative humidity), and the method of measurement of carbonation extent (e.g., changes in alkalinity) [14, 15]. A compilation of the existing carbonation standards was created in consultation with the members of RILEM TC 281-CCC and is shown in Table 1.
2.1 Approaches for determining carbonation in regions where no testing methods or standards are defined
Despite the large number of dedicated standards for evaluation of carbonation resistance of cementitious materials (Table 1), there is no ASTM standard for determining carbonation resistance. ASTM standards are some of the most widely used globally for construction materials testing and are often translated or adapted in different countries for implementation as national standards. This is of particular interest considering that regions such as the American and African continents, which often adopt ASTM standards, are some of the major producers and consumers of concrete, and have a variety of climates in which carbonation-related degradation of concrete may be expected to be significant. Experts based in the United States and Canada have indicated that in the case of North America carbonation, or its potential effect on steel reinforcement corrosion, is not considered a major threat for concrete, as empirical experience from practitioners, up to now, shows that the durability of reinforced concrete structures in that region is mainly compromised by chloride exposure (particularly in coastal areas, or due to use of de-icing salts) before carbonation is identifiable as a notable durability issue. The American Concrete Institute code ACI 318-19 (22) [32] also does not explicitly include provisions related to carbonation. In North America, if carbonation of concrete needs to be tested, the European standards may be followed.
In Africa, there is no consensus across different countries for determining carbonation resistance of concrete, and standards have not been developed for it. When carbonation tests are conducted, they are conducted according to the European or the British Standards. In South Africa, which has a highly developed set of performance-based design protocols for concrete, the oxygen permeability index test may be used to provide information for carbonation rate prediction (among other concrete properties) rather than testing carbonation directly [33]. Africa is rapidly urbanising with increased consumption of concrete, and has regions of high temperature and varying humidity, making the durability challenges associated with carbonation inevitable, in at least some parts of that continent. Thus, there is need to develop an approach to measure carbonation under conditions that are representative of these environments. The RILEM CPC-18 method [8] is cited in some research papers from institutes in Africa, as well as the EN 14630 protocol [20] for determining carbonation depths using the phenolphthalein indicator, but these do not specify the protocols to follow to induce carbonation prior to that analysis.
Australia also does not have a national standard for testing carbonation resistance of concrete. It follows a similar approach to North America where European standards are adopted when this property needs to be tested. In this country the areas of high population density, and consequently concrete structures, are mainly located in coastal areas (Fig. 2), where chloride-induced corrosion is the main threat to the integrity of steel reinforced concrete. Wang et al. [3] simulated the potential effects of climate change on the carbonation of concrete in Australia, and the consequent likelihood of corrosion of steel reinforcement (Fig. 3). With and without considering changes in climate, the structures in regions with highest population density (Fig. 2) will have the lowest probability of carbonation-induced corrosion (Fig. 3); therefore, chloride-induced corrosion, rather than carbonation-induced corrosion, is considered the major practical threat to longevity for the majority of structural concretes in Australia. This explains why perhaps the development of a dedicated standard has not taken place.
Population density in Australia. Reproduced from [34], copyright Newebcreations (https://australiamap360.com), used by permission
Maps showing the probability of carbonation induced corrosion of concrete structures in Australia by 2100. a Probability of corrosion damage without consideration of climate change, and b change in probability of corrosion damage for IPCC A1FI emission scenario. Reproduced from [3], copyright Springer Science + Business Media B.V.
In the following sections, the specificities of the standardised testing methods that can influence carbonation results are discussed in detail.
3 Sample specifications, pre-conditioning, and curing age when testing
3.1 Sample geometry and extraction methods
The geometry of the concrete samples used for carbonation experiments varies significantly among different standards (see Table 2). Some recommend the use of cylinders, while others recommend prisms or cubes of different sizes, depending on the number of measurements to be conducted. The shape of concrete samples is also influenced by the source of the concrete specimens to be tested: whether laboratory produced or extracted from real concrete structures. Laboratory concrete samples are often cast in prismatic and/or cubic forms, the latter often being used when a single measurement is being conducted per sample, as the necessary moulds are widely available and used for casting compressive strength testing specimens. Concrete prisms are also recommended in standardised procedures as they allow multiple measurements of the carbonation depth after different periods of carbonation exposure.
In the cast surface of the specimen, accumulation of portlandite is often observed [35], which is expected to influence the measured carbonation depth. The RILEM TC 281-CCC interlaboratory test results showed [5] that beside the trowelled surface (top side), the bottom side also presented a significantly different carbonation depth compared to the other sides (trowelled surface or lateral sides) of mortar specimens, particularly when CEM II and CEM III were used as binders. However, a corresponding trend was not observed in concrete specimens. In the case of inducing accelerated carbonation in loaded concrete specimens, Yao et al. [36] reported that the carbonation of the bottom surface was only 1/3 of that of the top trowelled surface and the side surfaces. A similar effect has also been observed in durability tests evaluating the sulfate resistance of mortars [37]. Therefore, it is preferable to measure the carbonation depth along the side surfaces, excluding the corners. The top and bottom surfaces should be excluded to minimise the effects of varying surface qualities and sample inhomogeneity resulting from the casting process.
For obtaining drilled samples from concrete structures, the standard EN 12504-1 [38] is the procedure that is typically followed, and is cited in the CEN carbonation standards (e.g. EN 12390-12). Detailed procedures for obtaining drilled samples are not provided in the other carbonation standards evaluated here, with the exception of SIA 262/1 [29] where drilling of samples with a minimum size of 50 (diameter) × 100 (length) mm is described. ASTM C42 [39] also describes drilling of core samples, but with a specific focus on obtaining specimens for strength testing.
In addition to the various shapes of concrete specimens, the shortest dimension of the concrete/ mortar specimen specified in norms, and used in different studies, varies from 40 up to 120 mm depending on the maximum size of the used coarse aggregate as shown in Fig. 4.
Sample dimensions used for carbonation experiments on concrete samples. The lines indicate the relationship of the smallest dimension of the specimen to the maximum aggregate size specified in standards ISO 1920-12, EN 12390-10:2018 and RILEM CPC-18. Data obtained from Neves et al. [40], Younsi et al. [41]; Fattuhi [42]; Lo et al. [43]; Liu et al. [44]; Shi et al. [45]; Khunthingkeaw et al. [46]; Chang et al. [47]; Sanjuán et al. [15]; Cui et al. [48]; Khan et al. [49]; Neves et al. [50]; Villain et al. [51, 52]; Bucher et al. [53]; Turcry et al. [13]; Vogler et al. [54]
Some standards do not provide specific dimensions of the concrete specimen, but instead indicate that the shortest dimension of the specimens must be at least three or four times the maximum nominal aggregate size [8, 17, 22]. However, such low ratios between specimen and maximum aggregate size are less conservative than common norms such as the ACI 318-19 [55] for producing concrete, where the maximum size of the coarse aggregate is limited to one-fifth of the narrowest dimension of the samples. Most published studies on carbonation used sample dimensions that met both the requirement of the carbonation standards, and the requirements of the norms for concrete production. While the ratio of sample dimension to maximum aggregate size is expected to have a minor influence on carbonation depth itself, a very low ratio will increase the variability of the results.
3.2 Specified curing age and conditions prior to pre-conditioning and testing
Prior to exposing specimens to CO2, most of the standards recommend specific conditions and durations of curing and/or pre-conditioning. This is done with the aim of allowing the binder or matrix in the concrete to achieve a microstructural maturity that will be roughly representative of the material during service, and to ensure that the degree of internal saturation (relative humidity) of the material is homogenised, so carbonation can actually proceed (and is not affected by zones of different internal RH). The intention is that different samples can be compared with each other in an equitable manner—although this is not always possible when the materials being tested are of very different chemistry or microstructure. From a CO2 transport perspective, it is well known that the relative humidity or degree of saturation of the concrete plays a key role in the carbonation progress. When the pore structure is saturated with water this prevents CO2 from entering the material, and conversely, when the pore structure is completely dried, although the diffusivity of CO2 increases, there is not sufficient pore water for CO2 to dissolve to form carbonic acid and trigger the carbonation reaction [56]. This will be discussed in more detail in Sect. 3.3.
Curing and pre-conditioning of concrete specimens will affect the degree of Portland cement hydration and latent hydraulic and pozzolanic reactions, and thus the amount of hydrates formed and the resulting pore structure. This will be more significant in concrete with some SCMs, as it is well-known that they generally show later strength development compared with plain Portland cement concrete [57, 58]. Any differences in curing or pre-conditioning methods and durations will impact the measured carbonation resistance of the material. Although very different types of curing methods or sample pre-conditioning are specified in various carbonation standards (Table 3) or in published studies, including water curing, moist curing, air curing, sealed curing, saturated lime bath curing, heat curing and even using curing compounds, very little research on the influence of curing types on carbonation resistance is available in the literature, as reviewed in [59, 60].
Ekolu et al. [61] concluded that the use of different common curing methods (with similar duration) led to similar carbonation behaviour in real structures. A similar conclusion was reached by RILEM TC 281-CCC as part of the round robin test evaluating carbonation performance of concrete under different standardised test methods [5]. Similar concrete rankings (i.e. placing the samples with different cement types in order from more to less resistant to carbonation) were achieved when adopting different curing methods. However, predefined sealed curing for 28 days at 20 °C resulted in higher carbonation depths compared to those measured in specimens cured for 28 days under water, especially for CEM I samples (e.g. the results indicate 60% higher carbonation depths for CEM I concrete samples vs. 19% higher for CEM III concrete). On the other hand, samples water-cured for 3 or 7 days rather than 28 days exhibited larger carbonation than those sealed cured, with the magnitude of the difference depending on the cement type [5]. This highlights that investigation of the potential effects of curing and pre-conditioning is required for adoption of more complex concrete mix designs with increased use of SCMs.
The curing duration is a well-known parameter that has a significant impact on carbonation resistance, particularly for blended cements with SCMs, and this is closely linked to the matrix maturity achieved as the material ages. A detailed review of 287 papers on carbonation of concretes containing blast furnace slag [62] identified significant variations in the curing duration prior to testing. 50% of the papers used a curing duration between 1–14 days, 29% between 15–28 days, 6% between 56–91 days, 3% more than 91 days, and for the remaining 12% no curing duration was mentioned. Only a few studies have compared the effect of different curing durations on carbonation resistance of the comparable concrete [42, 43, 63,64,65]. An example of the significant influence of curing duration on carbonation is shown in Fig. 5 [64], where it can be seen that in the case of slag-containing concretes, at contents of 50 wt.% slag, significant changes in carbonation depth are observed with differences in curing duration from 1 to 18 months.
Effect of curing duration (1, 3, 6 or 18 months at ∼20 °C and > 95% RH) on the carbonation depth of concrete with 0, 50, 70 and 85 wt.% replacement of Portland cements by BFS (denoted as S in the legend), exposed to 10 vol.% CO2. Reproduced with permission from [64], copyright Elsevier B.V.
Generally, the minimum duration of curing required for realistic evaluation of the carbonation performance of a concrete is dependent on the cement replacement level, the type of SCM used, and the exposure CO2 concentration. Currently, insufficient systematic investigations are available to define the minimum acceptable curing duration for concrete containing SCMs and/or cured under different exposure conditions. The ingress of CO2 depends on the degree of hydration and the consequent pore structure formed at the time of exposure. Considering that blended Portland cement concretes have a different microstructure and phase assemblage, and are generally less mature compared to Portland cement concrete at a specified early curing time (e.g. 28 days), they are almost inevitably more prone to carbonation when tested at such ages. Since construction practice recommends longer curing for concrete with SCMs [66], durability testing standards need to be modified accordingly. It must also be considered that under natural carbonation conditions, the carbonation takes place over a longer time span, when the SCM-containing concrete is already more mature. For long term prediction of natural carbonation from accelerated carbonation results, fib Bulletin 34 [67] recommends accounting for curing duration using a curing factor (Kc), which is not well quantified or reported for new SCMs.
3.3 Influence of pre-conditioning period
At the end of a recommended curing period, additional pre-conditioning prior to carbonation exposure is required by some standards (for example XP P18 458 [21], BSI 1881-210 [31], CUR-Aanbeveling 48 [30], EN 12390-10 [17], EN 12390-12 [18] and others). This step generally includes storage of the samples at natural CO2 concentrations at similar temperature and relative humidity (RH) to that of the accelerated carbonation testing period (Table 3). The aim of such pre-treatment is to homogenise the internal water saturation of concrete to facilitate the ingress of CO2 into the material. This might only partially be guaranteed as concrete density and pore structure will impact transport properties. For example, Shi [68] reported that drying of 20 mm thick specimens containing 31.9 wt.% metakaolin at 50 °C to constant weight took roughly 65 days, much longer than drying of Portland cement specimens which took only 10 days. For natural carbonation conditions, pre-conditioning is generally not considered to be necessary, since (i) carbonation kinetics are slower than the evaporation of water from wet concrete; (ii) preconditioning under a CO2-free environment is not always feasible for concrete samples; and (iii) real concrete infrastructure elements are usually not preconditioned, except possibly for some precast concrete elements.
Pre-conditioning is crucial for accelerated carbonation testing, to avoid any differences in the performance due to exposure of concrete at levels of internal RH dissimilar to those applied during the accelerated carbonation testing. The influence of pre-conditioning before carbonation in accelerated carbonation experiments has been less explored than the effect of curing duration, and its necessity remains debatable. Lye et al. [62] evaluated 198 papers on carbonation of slag concretes, identifying that only 1/3 of the papers mentioned that preconditioning of the concrete samples was applied. This highlights that differences in carbonation rates reported in different studies cannot always be explained simply on the basis of the chemistry of the binder or concrete strength class, as these can also be influenced by differences in sample curing and pre-conditioning. In particular, the internal relative humidity influences the microstructure development at a young age as well as the carbonation kinetics. The water saturation degree of concrete will also play a key role in the diffusivity of CO2, which will also be influenced by the pre-conditioning step. Artificially creating a homogeneous moisture distribution might not be representative of the actual performances of materials; however, accelerated tests aim to simulate a worst-case scenario. There is still need for research to understand and quantify the effect of pre-conditioning and RH on (accelerated) carbonation for evolving SCMs.
RILEM TC 116-PCD developed a recommendation for moisture redistribution pre-conditioning [69] for the measurement of the gas permeability and capillary absorption. This procedure consists of pre-drying of the concrete test specimens to an intermediate/average moisture concentration, which is in equilibrium and results in a uniform distribution of the evaporable water in the test specimens. This is achieved by storing the specimens at 50 °C either sealed or in ‘small sample containers’, for a minimum of 14 days. A revision of this recommendation was made, and an improved procedure was then proposed which also included a redistribution/homogenisation step at 50 °C [70]. This proposed approach could be considered for sample pre-conditioning prior to carbonation testing, as this methodology has been proven to be more effective to ensure homogeneous moisture distributions in cementitious materials.
4 Specified accelerated carbonation exposure conditions
The accelerated carbonation exposure conditions are the main variables across different studies evaluating carbonation performance of concrete, and therefore their effect on the results obtained from such tests needs to be critically evaluated. As mentioned earlier, a round robin test conducted by RILEM TC 281-CCC [5], assessing carbonation performance of concrete produced with Portland, Portland-fly ash and blast-furnace slag blended cements, and adopting different standardised testing protocols, demonstrated how different exposure conditions enabled similar rankings of the concrete evaluated but led to significant differences in the carbonation rates recorded. In the following sections the variability of exposure conditions recommended by different standards and their effect on the results obtained will be briefly described.
4.1 CO2 concentration
As indicated in Table 4, the exposure conditions used during carbonation testing vary significantly from test to test, particularly the CO2 concentrations adopted. CO2 concentrations in accelerated tests range from 1 to 20%, but most of the standards prescribe CO2 levels between 1 and 5%. There were only two standards recommending testing above such CO2 concentrations: (i) the previous French standard recommended 50% CO2 (XP P18 458:2008), which changed in 2022 to 3% (XP P18 458:2022-12) in agreement with the European standard, and (ii) the Chinese standard (GB T50082-2009), which recommends testing at 20% CO2.
It is anticipated that at a higher CO2 exposure concentration, the carbonation front will progress faster (Fig. 6) and assessment of the carbonation performance can take place in a shorter timeframe. However, the mechanism of carbonation and consequently the phase assemblage of the hydrated cement matrix can change significantly at a higher CO2 exposure concentration, which will not represent the carbonation mechanism occurring in a natural environment. The effects of different exposure conditions on phase assemblage have been discussed in detail in the literature review by von Greve-Dierfeld et al. [2] and will only be briefly mentioned here.
Effect of CO2 concentration on carbonation depth of a Portland cement concrete with a 28d compressive strength of 30 MPa, as a function of curing time. Reproduced with permission from [48], copyright Elsevier B.V.
There is no consensus neither in standards nor in the literature regarding maximum CO2 concentrations to be applied in accelerated tests so that similar carbonation reaction products to those identified in pastes exposed to natural carbonation are forming. For example, Liu et al. [71] identified in carbonated Portland cement specimens that the preferential calcium carbonate polymorph forming was strongly dependent on the degree of calcium silicate hydrate (C–S–H) carbonation and the carbonation duration, which were not strongly affected by the CO2 concentration (being 0.03%, 3%, 10%, 20% 50% and 100% in their research), although C–S–H decalcification is more pronounced in accelerated conditions. However, it should be noted that besides temperature and CO2 concentration, the internal RH as well as the composition of the pore solution significantly influence changes in phase assemblage and microstructure during carbonation. Based on the finding that the ratio of decalcified to remaining C–S–H was similar for tests at 3% and 20% CO2, it was then concluded that a CO2 level up to 20% can be applied in accelerated tests. Figure 6 shows the effect of the CO2 concentration on carbonation depth of Portland cement concrete, measured at different curing and exposure times, as obtained by Cui et al. [48]. It was identified that the carbonation depth increases linearly at CO2 exposure concentrations up to 20%, and does not change significantly beyond 20%, which was attributed to the dense surface layer formed upon carbonation, lowering the CO2 diffusion. These observations have been used to limit the CO2 concentration up to 20% for prediction of natural carbonation based on accelerated tests. This CO2 concentration is the one recommended by the Chinese standard (GB T50082-2009, [16]).
Castellotte et al. [72] conducted a study using 0.03%, 3%, 10% and 100% CO2 concentrations on pastes with Portland cement with low alumina content, and 5% addition of limestone. They showed that up to 3% CO2 exposure no significant changes occurred in the type of reaction products formed upon carbonation, with comparable Ca/Si ratios to those identified in naturally carbonated conditions, while ettringite and anhydrous cement remained present in both cases. For CO2 levels of 10% or higher, significant changes were detected. Comparable results were also identified by Auroy et al. [73] when evaluating Portland cement pastes, concluding that using 3% CO2 made it possible to reach a carbonation state that was representative of long-term carbonation in natural conditions. Another aspect that should be taken into account when selecting a suitable CO2 level in accelerated tests is the release of water upon carbonation [74, 75] which can block the capillary pores and hinder CO2 diffusion. This effect was investigated by Van den Heede et al. [76] in fly ash blended concrete, who identified that carbonation-induced water formation at different CO2 exposure concentrations (0.04%, 1% or 10% CO2) affects carbonation progress, and using a concentration of 10% CO2 was considered too high to simulate natural carbonation. Carbonation shrinkage is also likely to increase at higher CO2 exposure concentrations [77].
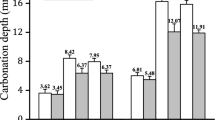
Results from the RILEM TC 281-CCC round robin test on the effect of carbonation depths as a function of the CO2 carbonation exposure conditions are shown in Fig. 7 for CEM II- and CEM III-containing concretes. It can be clearly seen that independent of the type of concrete evaluated, the differences in CO2 concentration during carbonation testing will lead to different carbonation depth values. From this study it was not possible to draw a clear conclusion regarding the effect of the CO2 concentration in isolation, as each of the different standardised testing methods adopted also presented variabilities regarding sample pre-conditioning period and conditions. Nevertheless, there seems to be a clearer trend in the results of the CEM III concrete where a reduced carbonation depth variability is observed, and higher CO2 concentrations lead to higher carbonation depths.
Box-and-whisker plot and the individual mean per laboratory of the carbonation depths of concrete measured after 91 days of accelerated carbonation for A CEM II and B CEM III, grouped by the applied accelerated carbonation method. Reproduced from [5], copyright RILEM. It is noted that the LNCE E391 recommend exposing specimens at 5% CO2 (Table 4) rather than 4% CO2
Several standards recommend an exposure concentration of 4% CO2 or above. However, awareness of the impact of CO2 concentration in carbonation testing is increasing. The European standard for accelerated carbonation testing (EN 12390-12) had a reduction in the CO2 concentration from 4 to 3% in its recent revision in 2020, and the French standard (XP P18-458) reduced the CO2 concentration of exposure from 50 to 3% in 2022. Further studies need to be conducted to understand the influence of this parameter specifically for concretes containing SCMs, and the suitability of existing accelerated carbonation testing methods in determining their carbonation potential under service conditions.
4.2 Relative humidity (RH) and temperature
Table 4 shows that the specified RH during accelerated carbonation testing can vary from 55 to 70%. It is generally accepted for Portland cement based materials that carbonation will take place at humidity ranges between 50% as a lower limit, and 70 [78] or 80% [79] as an upper limit. A low humidity does not allow sufficient dissolution of CO2 to form carbonic acid, and high humidity hinders the diffusion of CO2. The RH ranges adopted in standards are broad, which means that adopting either the lower or upper limiting values of a particular standard can have significant effects on the carbonation results [79]. A detailed discussion of the effect of RH and temperature on the phase assemblages of cementitious materials with SCMs under carbonation is reported in [2]. One of the main outcomes of this analysis is that for materials containing SCMs, the RH range at which carbonation proceeds the fastest may differ from the values determined for Portland cement.
It is also worth noting that the RH of the exposure environment does not necessarily correspond to the internal relative humidity in the concrete. Upon drying, a gradient in the internal humidity exists between the inner and the outer layers. This is dependent on the moisture transport, which in turn is influenced by the microstructure of the concrete [80]. Also, the effect of temperature and carbonation-induced water formation will influence internal gradients in the moisture distribution. Pre-conditioning of specimens before accelerated carbonation tests (as discussed in Sect. 3.3) is typically conducted to minimise such effects. The fact that measuring internal RH gradients in specimens prior or during carbonation is not a conventional practice means that there is uncertainty about the effectiveness of the pre-conditioning or RH exposure conditions in ensuring that a certain degree of saturation of the specimens is achieved upon testing [70].
There is more consistency in the recommended testing temperature across the different standardised testing methods (Table 4). The majority of carbonation standards specify a temperature of ~ 20 °C. Although this temperature is far from being representative of all micro-climates in which carbonation can occur, this is a conventional value adopted for the testing of construction materials in controlled laboratory conditions. For example, IS 516-2 recommends 27 ± 2 °C, which is the standard laboratory temperature in Indian standards, and also relevant to the temperature conditions in other hot or tropical countries (see Table 4). It is important to consider that the temperature during CO2 exposure will have an impact on the carbonation rate [2]. Figure 8 illustrates how carbonation depth can change as a function of the temperature of exposure, for concretes of different strength classes.
Curves of carbonation depth of fly ash blended concretes of different strength classes (indicated as ‘C’ in the legend) exposed to 20% CO2 and 70% relative humidity for 28d or 56d of exposure, versus temperature. The days in the legend represent the carbonation exposure time. Adapted from [44]
Currently, only the ISO standard [22] and IS 516 [24] provide a specification for adopting 27 °C for simulating tropical climate conditions. The significant differences between the exposure temperatures under natural environmental conditions and accelerated carbonation standardised tests will create significant deviations in the carbonation kinetics and results derived from those tests [81]. This has implications for predicting natural carbonation potential from accelerated carbonation results, which is often overlooked but will be discussed in more detail in Sect. 6.
5 Determining changes in alkalinity (pH indicators)
All the existing testing standards evaluating carbonation performance of concrete recommend the use of a pH indicator, mostly phenolphthalein, to determine the changes in pH associated with the carbonation reaction, particularly the carbonation of portlandite. Such measurements are accepted as representative of the carbonation depth. The phenolphthalein indicator shows colour changes at pH between 8.2–10.0 in solution. The main difference across standardised testing methods is in the preparation of the phenolphthalein solution as well as how and when the readings need to be performed. Most standards (e.g. BSI 1881-210, CUR-Aanbeveling 48, EN 12390-10, EN 12390-12, EN 13295, ISO 1920-12, SIA 262/1, UNE83993-1, RILEM CPC-18, LNEC E391) specify dissolving 1 g of phenolphthalein powder in 100 mL of a mixed solvent (70 mL isopropanol or ethanol + 30 mL distilled or deionised water). The Spanish standards (UNI 9944) recommend 0.1% of phenolphthalein in isopropanol, while the Chinese standard (GB T50082) specifies a 1% phenolphthalein solution prepared in water diluted ethanol (ethanol to water ratio 4:1). The Norwegian test (NT Build 357) recommends dissolving 1 g phenolphthalein in 500 mL of distilled/ion exchanged water and 500 mL ethanol.
In concrete with SCMs it has been identified that there are issues with clearly identifying colour change boundaries, and therefore the carbonation depth, when using phenolphthalein solutions produced as per recommended standards (mixed solvents), e.g. Figure 9A. This has been attributed to the potential leaching of alkalis (Na+/K+) in the carbonated region [5]. This is not the case when using an isopropanol or ethanol solution without addition of water, where well defined areas of discoloration are detected (Fig. 9B). The application of indicators to concrete produced from blast furnace slag-containing cements may also be problematic due to the very dark colour of the hydrate regions in some slag cement concretes [82], as the indicator colour changes are difficult to see against the background of a dark green material, and the carbonation front may or may not correspond with other colour changes that take place in the specimens due to redox processes in the cement itself.
Photographs of partially carbonated A Portland cement concrete, and B concrete containing calcined clays, after spraying with a 0.1% phenolphthalein solution produced with A 70% isopropanol + 30% distilled water and B 100% isopropanol. The photographs were taken immediately after spraying the concrete with the indicator solution. Courtesy of Y. Dhandapani
An overview of the recommendations included in different standards for collecting the carbonation measurements is reported in Table 5. The times when the readings need to be performed, in terms of carbonation duration or for revealing the pH changes, vary depending on the standard (or in some cases are not clearly specified), which will introduce significant variability between different studies and measurements. A clear example of such variability was identified during the RILEM TC 281-CCC round robin testing programme [5], where it was observed that, when using a phenolphthalein solution in 70% isopropanol/30% water, the time when the carbonation depths are measured (24 h, as in the RILEM CPC-18 method, instead of 1 h as recommended in EN 12390-12, see Table 5) was crucial for clearly distinguishing pH changes, particularly in CEM I and CEM II mortars or concretes. Perhaps of all the variables discussed here, the timing of when the carbonation depth needs to be measured is the most relevant, because if no clear boundary is observed, the measurements will not be reliable. Further investigation into the impact of when and how carbonation measurements are collected is required, so standards can be modified accordingly.
Thiel et al. [83] evaluated the effectiveness of using other pH indicators (0.5% curcumin, 0.1% thymolphthalein, rainbow indicator) and identified that the colour of the thymolphthalein indicator faded after 24 h, and consequently recommended shorter times after spraying for collecting carbonation depth data. They also estimated that phenolphthalein underestimated carbonation (associated with consumption of portlandite) by about 20%, compared with the carbonation depths determined by analytical techniques such as thermogravimetry or laser ablation inductively coupled plasma mass spectroscopy. It is well-known that all calcium-bearing hydrated and anhydrous phases present in the concrete matrix will eventually carbonate [2], but the extent of carbonation of each phase is not captured by pH indicators, as shown in Fig. 13.
It is worth noting that although all standards include information about how the carbonation front can be determined using a pH indicator, there is only one dedicated standardised testing method for measuring the carbonation depth, EN 14630 [20], whose procedure is similar to that described in EN 12390-12 [18], but provides further details for the carbonation depth determination in large fragments or core samples that may be removed from real structures. RILEM CPC-18 [8] discusses the importance of exposure conditions for inducing carbonation of concrete specimens, but does not provide a specific guideline for exposure of the material, therefore it is often used as a guideline for carbonation depth data collection, and even cited in the BSI 1881-210 and CUR-Aanbeveling 48 standards (Table 5).
Another variation in the standards is the precision level specified for the carbonation depth measurements (Table 5), which is mentioned in very few standards. While most standards establish a precision of 0.5 mm, EN 14630 specifies reporting carbonation depth to the closest millimetre, and ISO 1920-12 requires reporting to a precision of the closest 0.2 mm. These differences will further increase variability in the carbonation depth readings for a given material. The statistical power of the measurements will also differ between standards, with as many as 24 readings (EN 12390-10 with two prisms) collected per time interval for some standards, while others require only 4–5 measurements (Table 5).
Given that changes in the solvent used for preparing the pH indicating solution, as well as the time when the readings are performed, can introduce significant changes in the thickness of the layer experiencing pH changes, there are increased concerns about identifying whether changes in pH can be truly correlated to carbonation of the phase assemblage of a given cementitious system. Consequently, a variety of methods and approaches have been explored over the past decade. Villain et al. [52] described a methodology to determine carbonation via thermogravimetry, chemical analysis and gamma densimetry. Quantification of portlandite using thermogravimetry has particularly been reported in numerous studies [47, 51, 84, 85]. Van den Heede & De Belie [86] found that the colorimetric carbonation depth obtained through phenolphthalein spraying usually slightly underestimated the microscopically assessed carbonation depth measured on thin sections under crossed polars, both for Portland cement based and for fly ash containing concrete. However, the discussion here of what is truly relevant when measuring carbonation depth depends on whether the main impact of carbonation is considered to be the alteration of the binder mineralogy, or the pH decrease and its consequent effects on corrosion susceptibility. This discussion will depend to a significant extent on the intended engineering usage of the materials and does not appear to have a single definitive answer.
A pH indicator is still the most widely used method for determining the carbonation front, despite the clear evidence that it is uncertain what is being captured by alterations of the pH. Attention has recently been drawn to the fact that phenolphthalein has been identified as carcinogenic [87], and consequently it should be removed from the standards. The standard EN 12390-10 [18] is the only one that specifies the use of an alternative indicator such as thymolphthalein for carbonation assessment. Alternatives such as thymolphthalein, alizarin yellow and rainbow indicators have been successfully used to identify changes in alkalinity in carbonated concrete [54], and so are being more widely adopted in laboratory studies. An added advantage of using such activators is that their colour transitions occur at different pH values from those of phenolphthalein (Table 6), and therefore the colourless regions could be more clearly associated with specific reactions happening in the binders upon carbonation. A natural pH indicator, curcumin, extracted from commercial turmeric powder and from the rhizomes of the turmeric plant, has proven to be effective in detecting changes in pH in cementitious materials, yielding a red colour in areas where there is an alkaline reserve (pH ~ 12) and yellow in carbonated zones (pH ≤ 9) [88]. Also, natural pH indicators based on anthocyanin extracted from petals of the Chinese violet cress, Orychophragmus violaceus [89], grape, red cabbage or black carrot [90] have proven to be effective to identify the pH changes in carbonated concrete. Despite the promising results obtained when using these synthetic and natural pH indicators, as long as the standards continue requiring the use of phenolphthalein, the use of such indicators in practice will continue to be limited.
In recent years, Grengg et al. [91] developed a chemical sensor for high-resolution optical pH imaging of concrete (Fig. 10), providing for the first time 2D visualisation of the pH in a concrete cross section. This is a reliable and highly accurate method that offers promising advantages for diagnosis of concrete carbonation.
pH image of a concrete specimen previously exposed to accelerated carbonation. A pH image of the sample, B image of the concrete surface used for pH imaging. C Combined image of A and B including reference pH measurements using a flat surface electrode. Reproduced with permission from [91], copyright Elsevier B.V.
Sakoparnig et al. [92] utilised such optical sensors and compared the changes in pH in carbonated materials with those obtained using a phenolphthalein solution and quantification of portlandite/calcium carbonates (determined by thermogravimetry analysis). Their results demonstrate that ‘carbonation depths’ determined by the phenolphthalein method do not capture the full conversion of portlandite to calcium carbonate, while the optical sensor does (Fig. 11).
Comparative results of pH measurements conducted by the phenolphthalein indicator methods vs. an optical sensor, along with quantified contents of portlandite and calcium carbonates determined by thermogravimetric analysis of a Portland cement mortar. The pink region is indicating the carbonation depth measured by the phenolphthalein method, not the range of pH values detected by this method. Courtesy of M. Sakoparnig
Discrepancies between portlandite consumption, carbonate formation, and pH changes identified by the phenolphthalein method have also been highlighted in other studies [47]. This has been attributed to the gradient of carbonation that will be present in the material (Fig. 12). The formation of a partially carbonated front is known to be controlled by the micro-climate (such as RH level) in the cementitious materials [93] and is often not captured by the phenolphthalein method. This further highlight that the progression of the microstructural alterations occurring due to the carbonation reaction itself is not fully captured by using a pH indicator.
Schematic of the pH gradient expected in carbonated concrete specimens. Adapted from [93]
With the increased use of SCMs in concrete production, the use of a pH indicator for determining carbonation has been extensively questioned, as it is well known that concrete with blended cements will have a lower content of portlandite due to a reduced clinker factor and pozzolanic reaction [94]. In Fig. 13 it is illustrated how the changes in pH are linked to changes in phase assemblage upon carbonation, in blended Portland cements containing calcined clays. A change in pH identified by the phenolphthalein method does not capture the decalcification of Ca-bearing phases (e.g., portlandite/calcium silicate hydrates) or changes in porosity.
pH profiles related to the changes in phase assemblages predicted by thermodynamic modelling in blended Portland cement containing calcined clays. The right bar indicates the gradual colour change of phenolphthalein from fuchsia to colourless upon pH changes from 10 to 8.2. P corresponds to 100 wt% white Portland cement; L—68.1 wt% white Portland cement + 31.9 wt% limestone; ML to 68.1 wt% white Portland cement + 25.5 wt% metakaolin + 6.4 wt% limestone; and M – 68.1 wt% white Portland cement + 31.9 wt% metakaolin. Reproduced with permission from [35], copyright Elsevier B.V
In plain Portland cement systems, carbonation is not considered a major durability threat for the concrete per se, as it is known that this phenomenon can lead to densification of the material [95]. Historically carbonation of cement and concrete is of concern when considering its potential impact in the longevity of reinforced concrete structures, linked to the corrosion performance of the steel rebars. It is believed that the reduction in alkalinity of the concrete cover layer due to the consumption of portlandite and decalcification of C–S–H during the carbonation process, can lead to the destabilisation/depassivation of the steel reinforcement, and a consequent increase in the risk of failure of concrete structures. A critical analysis of the impact of carbonation on potential corrosion of steel reinforcement, published by Angst et al. [96] within the context of RILEM TC 281-CCC, demonstrated that a reduced pH of the concrete cover layer alone is not sufficient to lead to significant corrosion of steel rebars in concrete. It is important to stress that assuming that concrete carbonation will automatically lead to corrosion of steel in reinforced concrete is a simplification of the mechanism of corrosion of the steel reinforcement, as there is no consistent evidence in concrete structures that carbonation of concrete will lead to corrosion. Other factors such as pore structure, moisture content, and composition of the pore solution at the steel–concrete interface, will influence how and to what extent carbonation-induced corrosion of the steel reinforcement will proceed [97].
6 Natural versus accelerated carbonation
6.1 Natural carbonation exposure
Several standards (e.g. CUR-Aanbeveling 48:2010, NT Build 357, RILEM CPC-18, UNE 83993 – 1: 2013) mention how and/or how often the reading of carbonation depths in concrete specimens can be performed using the phenolphthalein indicator and how results can be reported, when specimens are exposed to natural carbonation conditions. The environmental conditions (RH, T and CO2 concentration) must be collected periodically and reported along with the carbonation rates.
EN 12390-10 is the only available standard that provides detailed information about how to care for samples exposed to natural carbonation in different climatic conditions (e.g. indoor natural environment, indoor controlled environment, outdoor sheltered or outdoor unsheltered). Additionally, it prescribes the conditions to be applied in a climate-controlled chamber if it is used to simulate an indoor environment. In such cases, relative humidity (RH) and CO2 concentration must be recorded at least once every two hours. The average for the CO2 concentration over the test duration should be (0.040 ± 0.001)% by volume with no variation outside (0.040 ± 0.005)%, and the RH should be (65 ± 2)%. Temperature must be measured at least once per day at a constant time, and remain in the range (20 ± 2) °C. Results from the RILEM TC 281-CCC interlaboratory test show that although temperature and RH are usually well controlled, none of the climatic chambers used by the different laboratories were able to comply with the specific CO2 value ranges required by the standard [5]. This demonstrates that even under controlled environments, variations in the exposure conditions will occur.
6.2 Natural versus accelerated carbonation
General information about service life prediction models that account for carbonation of concrete can be found in Alexander et al. [98], where a critical state of the art review about durability, service life prediction and modelling for reinforced concrete structures is reported. It is not the objective of the present analysis to revisit such studies. Instead, we intend to create awareness about the current approaches in using accelerated carbonation results from standardised testing methods, attempting to correlate natural and accelerated carbonation not just with the intention of predicting concrete durability, but also attempting to quantify the CO2 uptake capacity of concrete.
Many studies and reviews have been published correlating accelerated and natural carbonation results of concrete including [14, 15, 40, 99], just to mention a few. Also within the framework of RILEM TC 281-CCC an extensive interlaboratory test campaign was performed to compare natural carbonation of mortar and concrete with CEM I, CEM II/B-V and CEM III/B, and to investigate its relationship to accelerated carbonation [5]. Often, accelerated carbonation results are used to develop models which are included in codes and standards such as the fib Model Codes (e.g. fib MC 2010, Bulletin 34 Model code for service life design [100]) and ISO 13823:2008 [101] with the aim of predicting service life performance, and/or to aid the design of concretes accounting for their potential durability [102].
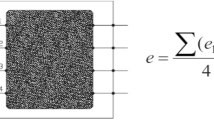
Despite the extensive scientific evidence that carbonation of cementitious materials is both a chemical reaction and a diffusion-controlled mechanism, particularly for concrete with SCMs [2], a direct correlation between natural and accelerated carbonation results is generally made according to Eq. (1). This equation is derived from the assumption that carbonation is only a diffusion-controlled mechanism, and therefore can be described by Fick’s first law of diffusion. According to Eq. (1), there is a direct correlation between the carbonation coefficient (kc) and the concentration of CO2 under natural (cCO2, nat) or accelerated (cCO2, acc) carbonation exposure conditions [103].
Such a relationship is well accepted, and it has been extensively used for correlating natural and accelerated carbonation results. However, this relationship is only valid if the degrees of saturation of the concrete and the maturity of the binding phase (concrete matrix) are comparable at the moment of testing, so the mechanism of carbonation under different exposure conditions is comparable. It is worth noting that the fib model code as well as in the SIA standard include empirically determined correcting factors or equations to account for the differences in carbonation kinetics and material age influencing the calculated carbonation rates when using natural or accelerated carbonation data.
This important aspect is often overlooked, as it is impossible to simulate, under accelerated carbonation conditions, all the variety of climates at which natural carbonation assessment is conducted. Consequently, Eq. (1) is often directly applied to draw conclusions about natural or accelerated carbonation performance. It is well documented in literature that this equation is not valid for converting carbonation rates obtained at a certain CO2 concentration to another one, even for situations where the temperature and RH are virtually the same. Figure 14 shows data of Van den Heede [104] where the carbonation coefficients corresponding with exposure to 10% CO2 were implemented in this equation to estimate a carbonation coefficient for a 1% CO2 environment.
Effect of the applied CO2 concentration on the carbonation coefficient of mixtures (a) with 50% fly ash in the binder and (c) a PC reference with w/c ratio of 0.55; measured values are shown by markers, calculated values for 1% CO2 based on Eq. (1) by the bold line. Courtesy of P. Van den Heede
If Eq. (1) held, then the estimated carbonation coefficient for 1% CO2 should not differ too much from the measured value. This was clearly not the case, as for both mixtures F(1)50 with 50% fly ash in the binder and for the PC reference T(0.55), the estimated values (bold line) were only 55% and 38% of the measured values. One possible explanation for this underestimation of carbonation rates when testing at high CO2 concentrations and using Eq. (1) for conversion, is the excessive production of water during carbonation at 10% CO2 which induces pore blocking. Van den Heede et al. [76] further investigated this phenomenon for high-volume fly ash (HVFA) mortars with 50% PC and 50% fly ash via carbonation tests at ± 0.04% CO2 (natural carbonation), 1% CO2 and 10% CO2. Internal humidity sensor monitoring and 1H NMR relaxometry revealed the highest water vapour and liquid water contents after carbonation at 10% CO2 (Fig. 15) [76].
Evolution of the internal humidity measured with humidity sensors at a depth of 15 mm during carbonation at 1% CO2, 20 °C, 60% RH and 10% CO2, 20 °C, 60% RH in high volume fly ash (HVFA) mortars, with indication of when the carbonation depth reached 15 mm. Reproduced with permission from [76], copyright Elsevier B.V
7 Beyond durability assessment
Carbonation coefficients determined via extensive experimentation are included in some standards such as EN 16757:2022 ‘Sustainability of construction works. Environmental Products Declarations’ [105], and the related document PD CEN/TR 17310:2019 ‘Carbonation and CO2 uptake in concrete’ [106]. Tables reporting carbonation coefficient values are explicitly given as a function of the concrete strength class, along with a table for correcting such carbonation coefficients for concrete containing SCMs (limited to limestone, silica fume, fly ash and blast furnace slag). As the carbonation coefficient varies as a function of the time and environmental exposure conditions, using a single or average value to describe the performance of concrete accounting solely for their compressive strength is not an accurate representation of the processes that take place under real-world conditions, but is a simplification that is easy to use in practical engineering applications.
The same simplified approach to approximate the carbonation rates of different concrete has been used in previous estimates of the CO2 uptake (re-carbonation) of concrete in Northern Europe [107] and globally [108]. The carbonation rates used in these studies and the above-mentioned standards derive from early, seminal reports, which provided a first collation of recommended values to be used for kc to estimate the CO2 uptake by cementitious materials during lifetime and after demolition [109, 110]. However, these reports were prepared about two decades ago, and significant new developments have occurred in cement technology, yielding to extensive new data and insights into the carbonation process. The reports themselves advised that the values will be revised when sufficient new data is available.
In this context, it is imperative to harmonise standards for determining carbonation performance of concrete, to ensure that the different carbonation coefficient values reported in the literature are comparable and can have some validity in service life prediction models or re-carbonation calculations. As highlighted in Sect. 3, the limited accuracy of the pH indicator method for revealing carbonation progress opens the question of how reliable it is as a method to establish (up to a certain extent) the sustainability credentials of cement, concrete or structures using carbonation coefficients determined via this method. As discussed throughout this critical overview, there are other testing parameters that introduce significant variability in the results, when adopting different standardised testing methods. Therefore, it is uncertain which carbonation results can be deemed correct for CO2 uptake potential calculations, because in a practical sense, as long as the data are obtained according to a standardised testing methodology, it will be accepted as representative of a given material.
8 Concluding remarks
Carbonation is one of the major durability threats of modern blended Portland cement systems. Despite the extensive knowledge gained over the past decades about this phenomenon, there is a lack of consensus about how carbonation of concrete needs to be evaluated, as evidenced by the fact that many standardised testing methodologies can be applied for this purpose. There are notable differences among existing standardised testing methods that will introduce variability of the results. Therefore, accelerated carbonation testing methods should be considered only to distinguish carbonation performance among concretes tested under comparable conditions.
The sample pre-conditioning and curing will influence the maturity of the binding phase, and consequently its response to CO2 exposure. The incorporation of a maturity index within methodologies for evaluating durability of concrete with SCMs is urgently needed, and should be prioritised in future iterations of standards, so that concrete longevity can be determined more accurately. Accelerated carbonation exposure conditions influence the carbonation mechanism and the extent of changes the material will experience. A consensus needs to be reached about exposure conditions to be adopted across different standards, particularly defining the CO2 concentration and relative humidity for testing. For Portland cement and blended Portland cements it seems that 1 to 3% CO2 concentration is adequate for inducing comparable phase assemblage changes to those identified during natural carbonation exposures. Therefore, testing accelerated carbonation of concrete at CO2 exposure concentrations beyond this value is not recommended. It is important to promote the harmonisation of this parameter across existing standards to enable more direct comparisons between results from different studies, to increase the understanding of how the intrinsic properties of individual concrete can be linked to their carbonation resistance.
There is still need for research to translate carbonation rates obtained via accelerated tests accurately to carbonation rates under natural conditions for different types of concrete. Concrete with SCMs will undergo different microstructural changes due to carbonation than ordinary Portland cement concrete (often coarsening instead of refinement of the pore structure). Exposing these concretes to high concentrations of CO2 will lead to rapid carbonation, which in turn can lead to higher production of water internally (as a reactant of the carbonation reaction). The differences in water release upon carbonation might also influence the RH conditions at which the carbonation rate will be higher.
Carbonation of concrete has been historically linked to a reduction of the pH, which is determined using a phenolphthalein solution. The analysis performed in this paper has led to two conclusions related to this premise: (i) the use of this carcinogenic substance could be removed from existing standards, and alternative synthetic or natural pH indicators need to be recommended instead; (ii) the use of a pH indicator does not fully capture the carbonation reactions taking place in concrete. For Portland cement concrete, it can be argued that changes in alkalinity induced by carbonation are of relevance in the context of steel corrosion. For blended Portland cement concrete, carbonation can lead to a reduced loading capacity and increased porosity of the material, so independent of the changes in alkalinity that can be associated with carbonation, it is of relevance to determine the extent of microstructural changes carbonation has induced, which the pH indicator method is failing to reveal. The limited accuracy of the methodologies used for determining carbonation depths calls for selection of suitable approaches to answer the questions raised when planning such experiments. Carbonation coefficients determined from carbonation depth readings using the pH indicator method might not be truly representative of the actual carbonation the material is experiencing. This is concerning for service life prediction models, particularly for concrete with SCMs.
The oversimplified recommendations that are in place to calculate CO2 uptake by concrete using standards originally developed for accelerated carbonation rate determination, and consequently define the sustainability credentials of cements, concrete or structures assuming constant carbonation coefficient values, are potentially misleading at a technical level. The carbonation coefficients estimated from carbonation depth results determined according to the different standardised testing methodologies are imprecise, and unique to individual concrete mix designs and environmental exposure conditions. Often no direct correlation between carbonation coefficient and compressive strength is identified in concrete with SCMs, particularly when performance is determined under accelerated testing conditions. Therefore, such recommendations based on using carbonation coefficients tabulated in such standards need to be questioned.
References
Beushausen H, Torrent R, Alexander MG (2019) Performance-based approaches for concrete durability: state of the art and future research needs. Cem Concr Res 119:11–20
von Greve-Dierfeld S et al (2020) Understanding the carbonation of concrete with supplementary cementitious materials: a critical review by RILEM TC 281-CCC. Mater Struct 53(6):136
Wang X, Stewart MG, Nguyen M (2012) Impact of climate change on corrosion and damage to concrete infrastructure in Australia. Clim Change 110(3–4):941–957
Yoon I-S, Çopuroğlu O, Park K-B (2007) Effect of global climatic change on carbonation progress of concrete. Atmos Environ 41(34):7274–7285
Vanoutrive H et al (2022) Report of RILEM TC 281-CCC: outcomes of a round robin on the resistance to accelerated carbonation of Portland, Portland-fly ash and blast-furnace blended cements. Mater Struct 55(3):99 https://doi.org/10.1617/s11527-022-01927-7
Vanoutrive H et al (2024) Report of RILEM TC 281-CCC: Outcomes of a round robin on the resistance to natural carbonation of Portland, Portland-fly ash and blast-furnace blended cements and its relation to accelerated carbonation. Mater Struct (under review)
Nakamura N, Michihiko A (2014) Investigation on change of test methods in accelerated carbonation of concrete. AIJ J Technol Design 20(46):845–850
RILEM (1988) CPC-18 Measurement of hardened concrete carbonation depth. Mater Struct 21(6):453–455
JIS A 1152 (2018) Method for measuring carbonation depth of concrete. Japanese Standards Association (JSA)
JIS A 1153 (2003) Method of accelerated carbonation test for concrete. Japanese Standards Association (JSA)
NDIS 3419 (2011) Method for neutralization depth of concrete in structures in drilling powder. http://www.jsndi.jp/jis-ndis/pdf/NDIS3419.pdf
Provis JL, Bernal SA, Zhang Z (2024) The decarbonization of construction—How can alkali-activated materials contribute? Engineering 32(6):17–20
Turcry P et al (2014) Analysis of an accelerated carbonation test with severe preconditioning. Cem Concr Res 57:70–78
Leemann A, Moro F (2017) Carbonation of concrete: the role of CO2 concentration, relative humidity and CO2 buffer capacity. Mater Struct 50:30
Sanjuán M, Andrade C, Cheyrezy M (2003) Concrete carbonation tests in natural and accelerated conditions. Adv Cem Res 15(4):171–180
GB/T 50082-2009 (2009) Standard for test methods of long-term performance and durability of ordinary concrete. China Academy of Building Research
EN 12390-10:2018 (2018) Testing hardened concrete. Part 10. Determination of the carbonation resistance of concrete at atmospheric levels of carbon dioxide. European Committee for Standardization (CEN)
EN 12390-12:2020 (2020) Testing hardened concrete. Part 12. Determination of the carbonation resistance of concrete. Accelerated carbonation method. European Committee for Standardization (CEN)
EN 13295:2004 (2004) Testing hardened concrete. Determination of the carbonation resistance of concrete. Accelerated carbonation method. European Committee for Standardization (CEN)
EN 14630:2006 (2006) Products and systems for the protection and repair of concrete structures. Test methods. Determination of resistance to carbonation, in European Committee for Standardization (CEN)
XP P18 458:2022 (2022) Tests for hardened concrete. Accelerated carbonation test - measurement of the thickness of carbonated concrete. Association Française de Normalisation (AFNOR)
ISO/DIS 1920-15 (2015) Testing of concrete—Part 12: Determination of the carbonation resistance of concrete—Accelerated carbonation method. International Organization for Standardization (ISO)
UNI 9944:1992 (1992) Corrosion and protection of reinforcing steel in concrete. Determination of the carbonation depth and of the chlorides penetration profile in concrete. Ente Nazionale Italiano di Unificazione (UNI)
IS 516 (2021) Hardened concrete—Methods of test Part 2: Properties of hardened concrete other than strength. Section 4: Determination of the carbonation resistance by accelerated carbonation method. Bureau of Indian Standards
NT Build 357 (1989) Concrete, repairing materials and protective coating: Carbonation resistance, Nordic Innovation Centre (NORDTEST)
LNEC E391:1993 (1993) Specification. Concrete. Determination of accelerated carbonation. Laboratório Nacional de Engenharia Civil (LNEC)
UNE 83993-1: 2013 (2013) Concrete durability. Testing method. Determining the carbonation penetration rate in hardened concrete. Part 1: Natural method. Organismo de Normalización en España (UNE)
UNE 83993-2:2013 (2013) Durability of concrete. Test method. Measurement of carbonation penetration rate in hardened concrete. Part 2: Accelerated method. Organismo de Normalización en España (UNE)
prSIA 262/1: 2017-11 (2017) Concrete construction—Additional specifications. Swiss Society of Engineers and Architects (SIA)
CUR-Aanbeveling 48:2010 (2010) Procedures, criteria and test methods for testing the suitability of novel cements for application in concrete and for the equivalent performance of concrete with fillers. CROW-CUR
BSI (2013) BSI 1881-210:2013 Testing hardened concrete. Determination of the potential carbonation resistance of concrete. Accelerated carbonation method. The British Standards Institute
American Concrete Institute (2022) ACI 318–19(22): Building code requirements for structural concrete/commentary on building code requirements for structural concrete (ACI 318R–19). ACI, Farmington Hills
Salvoldi B, Beushausen H, Alexander M (2015) Oxygen permeability of concrete and its relation to carbonation. Constr Build Mater 85:30–37
https://australiamap360.com/pdf/australia-population-density-map-pdf.pdf
Shi Z et al (2016) Experimental studies and thermodynamic modeling of the carbonation of Portland cement, metakaolin and limestone mortars. Cem Concr Res 88:60–72
Yao Y et al (2023) Report of RILEM TC 281-CCC: effect of loading on the carbonation performance of concrete with supplementary cementitious materials—An interlaboratory comparison of different test methods and related observations. Mater Struct 56(6):110
Shi Z et al (2019) Sulfate resistance of calcined clay—Limestone—Portland cements. Cem Concr Res 116:238–251
EN 12504-1 (2019) Testing concrete in structures—Cored specimens. Taking, examining and testing in compression. European Committee for Standardization
ASTM International (2020) ASTM C42/C42M-20—Standard test method for obtaining and testing drilled cores and sawed beams of concrete
Neves R, Branco F, De Brito J (2013) Field assessment of the relationship between natural and accelerated concrete carbonation resistance. Cement Concr Compos 41:9–15
Younsi A et al (2011) Performance-based design and carbonation of concrete with high fly ash content. Cement Concr Compos 33(10):993–1000
Fattuhi N (1988) Concrete carbonation as influenced by curing regime. Cem Concr Res 18(3):426–430
Lo Y, Lee H (2002) Curing effects on carbonation of concrete using a phenolphthalein indicator and fourier-transform infrared spectroscopy. Build Environ 37(5):507–514
Liu P, Yu Z, Chen Y (2020) Carbonation depth model and carbonated acceleration rate of concrete under different environment. Cement Concr Compos 114:103736
Shi H-S, Xu B-W, Zhou X-C (2009) Influence of mineral admixtures on compressive strength, gas permeability and carbonation of high performance concrete. Constr Build Mater 23(5):1980–1985
Khunthongkeaw J, Tangtermsirikul S, Leelawat T (2006) A study on carbonation depth prediction for fly ash concrete. Constr Build Mater 20(9):744–753
Chang C-F, Chen J-W (2006) The experimental investigation of concrete carbonation depth. Cem Concr Res 36(9):1760–1767
Cui H et al (2015) Experimental study on effects of CO2 concentrations on concrete carbonation and diffusion mechanisms. Constr Build Mater 93:522–527
Khan MI, Lynsdale C (2002) Strength, permeability, and carbonation of high-performance concrete. Cem Concr Res 32(1):123–131
Neves R et al (2015) Assessing concrete carbonation resistance through air permeability measurements. Constr Build Mater 82:304–309
Villain G, Platret G (2006) Two experimental methods to determine carbonation profiles in concrete. ACI Mater J 103(4):265
Villain G, Thiery M, Platret G (2007) Measurement methods of carbonation profiles in concrete: thermogravimetry, chemical analysis and gammadensimetry. Cem Concr Res 37(8):1182–1192
Bucher R et al (2017) Service life of metakaolin-based concrete exposed to carbonation: Comparison with blended cement containing fly ash, blast furnace slag and limestone filler. Cem Concr Res 99:18–29
Vogler N et al (2020) Alternative pH-indicators for determination of carbonation depth on cement-based concretes. Cement Concr Compos 109:103565
ACI CODE-318-19 (22) (2022) Building code requirements for structural concrete and commentary. American Concrete Institute, Farmington Hills
Papadakis VG, Vayenas CG, Fardis MN (1991) Experimental investigation and mathematical modeling of the concrete carbonation problem. Chem Eng Sci 46(5–6):1333–1338
De Belie N, Soutsos M, Gruyaert E (eds) (2018) Properties of fresh and hardened concrete containing supplementary cementitious materials. State-of-the-Art Report of the RILEM Technical Committee 238-SCM, Working Group 4. Springer, Cham
Vollpracht A, Soutsos M, Kanavaris F (2018) Strength development of GGBS and fly ash concretes and applicability of fib model code’s maturity function—A critical review. Constr Build Mater 162:830–846
Ekolu SO (2016) A review on effects of curing, sheltering, and CO2 concentration upon natural carbonation of concrete. Constr Build Mater 127:306–320
Pacheco-Torgal F et al (2012) An overview on concrete carbonation in the context of eco-efficient construction: evaluation, use of SCMs and/or RAC. Constr Build Mater 36:141–150
Ekolu SO (2018) Model for practical prediction of natural carbonation in reinforced concrete: Part 1-formulation. Cement Concr Compos 86:40–56
Lye C-Q, Dhir RK, Ghataora GS (2016) Carbonation resistance of GGBS concrete. Mag Concr Res 68(18):936–969
Ho DWS, Lewis RK (1987) Carbonation of concrete and its prediction. Cem Concr Res 17(3):489–504
Gruyaert E, Van den Heede P, De Belie N (2013) Carbonation of slag concrete: effect of the cement replacement level and curing on the carbonation coefficient—Effect of carbonation on the pore structure. Cement Concr Compos 35(1):39–48
Balayssac JP, Détriché CH, Grandet J (1995) Effects of curing upon carbonation of concrete. Constr Build Mater 9(2):91–95
Bouzoubaâ N, Fournier B (2005) Current situation with the production and use of supplementary cementitious materials (SCMs) in concrete construction in Canada. Can J Civ Eng 32(1):129–143
Schießl P, Gehlen C (2006) fib 34: Model code for service-life design. FIB: Lausanne, Switzerland, 126 pp
Shi Z (2016) Durability of Portland cement-calcined clay-limestone blends. PhD thesis. Aarhus University, Denmark
RILEM TC 116-PCD (1999) Tests for gas permeability of concrete: a preconditioning of concrete test specimens for the measurement of gas permeability and capillary absorption of water. Mater Struct 32:174–179
Antón C et al (2013) An improved procedure for obtaining and maintaining well characterized partial water saturation states on concrete samples to be used for mass transport tests. Mater Struct 46:1389–1400
Liu W et al (2019) XRD and 29Si MAS NMR study on carbonated cement paste under accelerated carbonation using different concentration of CO2. Mater Today Commun 19:464–470
Castellote M et al (2009) Chemical changes and phase analysis of OPC pastes carbonated at different CO2 concentrations. Mater Struct 42:515–525
Auroy M et al (2018) Comparison between natural and accelerated carbonation (3% CO2): Impact on mineralogy, microstructure, water retention and cracking. Cem Concr Res 109:64–80
Georget F, Prévost JH, Huet B (2018) Impact of the microstructure model on coupled simulation of drying and accelerated carbonation. Cem Concr Res 104:1–12
Saetta AV, Vitaliani RV (2004) Experimental investigation and numerical modeling of carbonation process in reinforced concrete structures: Part I: theoretical formulation. Cem Concr Res 34(4):571–579
Van den Heede P, Thiel C, De Belie N (2020) Natural and accelerated carbonation behaviour of high-volume fly ash (HVFA) mortar: Effects on internal moisture, microstructure and carbonated phase proportioning. Cement Concr Compos 113:103713
Swenson EG, Sereda PJ (1968) Mechanism of the carbonatation shrinkage of lime and hydrated cement. J Appl Chem 18(4):111–117
Papadakis VG, Vayenas CG, Fardis MN (1991) Fundamental modeling and experimental investigation of concrete carbonation. ACI Mater J 88(4):363–373
Da Silva FG et al (2009) Sources of variations when comparing concrete carbonation results. J Mater Civ Eng 21(7):333–342
Steiner S et al (2020) Effect of relative humidity on the carbonation rate of portlandite, calcium silicate hydrates and ettringite. Cem Concr Res 135:106116
Thiel C et al (2022) Effect of internal moisture and outer relative humidity on concrete carbonation. CivilEng 3(4):1039–1052
Le Cornec D et al (2017) Greening effect in slag cement materials. Cement Concr Compos 84:93–98
Thiel C, Gehlen C (2019) On the determination of carbonation in cementitious materials. In: Proceedings of the International Conference on Sustainable Materials Systems and Structures (SMSS2019) Durability, Monitoring and Repair of Structures, Rovinj, Croatia
Vogler N et al (2021) Description of the concrete carbonation process with adjusted depth-resolved thermogravimetric analysis. J Therm Anal Calorim 147:167–6180
Neves Junior A et al (2014) A study of the carbonation profile of cement pastes by thermogravimetry and its effect on the compressive strength. J Therm Anal Calorim 116:69–76
Van den Heede P, De Belie N (2014) A service life based global warming potential for high-volume fly ash concrete exposed to carbonation. Constr Build Mater 55:183–193
Dunnick JK, Hailey JR (1996) Phenolphthalein exposure causes multiple carcinogenic effects in experimental model systems. Cancer Res 56(21):4922–4926
Chinchón-Payá S, Andrade C, Chinchón S (2016) Indicator of carbonation front in concrete as substitute to phenolphthalein. Cem Concr Res 82:87–91
Cui D et al (2022) Use of a novel pH indicator extracted from petals to investigate the carbonation behavior in cementitious materials. Cement Concr Compos 134:104804
Chinchón-Payá S, Andrade C, Chinchón S (2020) Use of anthocyanin solutions in Portland cement concrete to identify carbonation depth. Mater Struct 53(4):101
Grengg C et al (2019) High-resolution optical pH imaging of concrete exposed to chemically corrosive environments. Cem Concr Res 116:231–237
Sakoparnig M, Galan I, Müller B, Zoegl I, Juhart J, Mittermayr F, Autischer M, Grengg C (2023) New insights into the carbonation process of mortars and concretes. In: Jędrzejewska A, Kanavaris F, Azenha M, Benboudjema F, Schlicke D (eds) International RILEM conference on synergising expertise towards sustainability and robustness of cement-based materials and concrete structures: synercrete’23 - volume 2. Springer Nature Switzerland, Cham, pp 1122–1132
Houst YF (1996) The role of moisture in the carbonation of cementitious materials. Int Z Bauinstandsetz 2(1):49–66
Massazza F (1993) Pozzolanic cements. Cement Concr Compos 15(4):185–214
Houst YF, Wittmann FH (1994) Influence of porosity and water content on the diffusivity of CO2 and O2 through hydrated cement paste. Cem Concr Res 24(6):1165–1176
Angst U et al (2020) Corrosion of steel in carbonated concrete: mechanisms, practical experience, and research priorities—A critical review by RILEM TC 281-CCC. RILEM Tech Lett 5:85–100
Angst UM (2023) Steel corrosion in concrete—Achilles’ heel for sustainable concrete? Cem Concr Res 172:107239
Alexander M, Beushausen H (2019) Durability, service life prediction, and modelling for reinforced concrete structures–review and critique. Cem Concr Res 122:17–29
Vu QH et al (2019) Impact of different climates on the resistance of concrete to natural carbonation. Constr Build Mater 216:450–467
Fédération internationale du béton (2006) fib Bulletin 34 - Model Code for Service Life Design. Lausanne: fib
ISO 13823:2008 (2008) General principles on the design of structures for durability. International Organization for Standardization
Engelsen CJ et al (2005) Carbon dioxide uptake in demolished and crushed concrete. Norwegian Building Research Institute, Oslo
Sisomphon K, Franke L (2007) Carbonation rates of concretes containing high volume of pozzolanic materials. Cem Concr Res 37(12):1647–1653
Van den Heede P (2014) Durability and sustainability of concrete with high volumes of fly ash. PhD Thesis. Universiteit Gent: Belgium. 386 pp
EN 16757:2022 (2022) Sustainability of construction works. Environmental product declarations, European Committee for Standardization (CEN)
PD CEN/TR 17310:2019 (2019) Carbonation and CO2 uptake in concrete. 2019, European Committee for Standardization (CEN)
Pade C, Guimaraes M (2007) The CO2 uptake of concrete in a 100 year perspective. Cem Concr Res 37(9):1348–1356
Xi F et al (2016) Substantial global carbon uptake by cement carbonation. Nat Geosci 9(12):880–883
Lagerblad B (2005) Carbon dioxide uptake during concrete life cycle: State of the art. Stockholm: Swedish Cement and Concrete Research Institute Stockholm
Kjellsen KO, Guimaraes M, Nilsson A (2005) The CO2 balance of concrete in a life cycle perspective. Danish Technological Institute-DTI
Acknowledgements
The authors would like to thank all members of RILEM TC 281-CCC, for the valuable discussions regarding carbonation of concrete with SCMs, and their insight and valuable discussions during the preparation and review of this paper. The valuable discussions and comments on the paper by A.T.M. Marsh are greatly acknowledged.
Funding
Participation of S.A. Bernal was sponsored by the UK Engineering and Physical Sciences Research Council (EPSRC) through the Early Career Fellowship EP/R001642/1. The participation of Y. Dhandapani, J.L. Provis and M.C.G. Juenger was sponsored by EPSRC through grant EP/T008407/1 and by the National Science Foundation (NSF) through award 1903457. P. Van den Heede and N. De Belie acknowledge the Research Foundation Flanders (FWO-Vlaanderen) for the financial support through grant G062720N.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article was prepared within the framework of RILEM TC 281-CCC. The article has been reviewed and approved by all members of the TC.
TC Membership
Chair: Nele De Belie
Deputy Chair: Susan A. Bernal
Members: Natalia Alderete, Carmen Andrade, Ueli Angst, Tushar Bansal, Véronique Baroghel-Bouny, Muhammed P.A. Basheer, Nele de Belie, Susan Bernal Lopez, Hans D. Beushausen, Leon Black, Aires Camoes, Servando Chinchon-Paya, Özlem Cizer, Gisela Paola Cordoba, Martin Cyr, Patrick Dangla, Yuvaraj Dhandapani, Katja Dombrowski-Daube, Vilma Ducman, Yogarajah Elakneswaran, Jan Elsen, Juan Manuel Etcheverry, Miren Etxeberria, Ana Maria Fernandez-Jimenez, Lander Frederickx, Cassandre Le Galliard, Inès Garcia Lodeiro, Daniel Geddes, Christoph Gehlen, Mette Geiker, Guoqing Geng, Bahman Ghiassi, Gregor Gluth, Cyrill Grengg, Elke Gruyaert, R. Doug Hooton, Bruno Huet, Yu Huang, Andres Idiart, Ivan Ignjatovic, Kei-Ichi Imamoto, Shiju Joseph, Zuquan Jin, Maria C.G. Juenger, Siham Kamali-Bernard, Antonis Kanellopoulos, Xinyuan Ke, Sylvia Kessler, Heejeong Kim, Sabine Kruschwitz, Namkon Lee, Bin Li, Juan Li, Ning Li, Tung Chai Ling, Zhiyuan Liu, Qingfeng Liu, Barbara Lothenbach, Jingzhou Lu, Isabel Martins, José Fernando Martirena-Hernandez, César Medina Martinez, Renjie Mi, Fabrizio Moro, Shishir Mundra, Yeakleang Muy, Marija Nedeljkovic, Kolawole A. Olonade, José Pacheco, Christian Paglia, Angel Palomo, Sol Moi Park, Ravi Patel, Janez Perko, Quoc Tri Phung, Elodie Piolet, John L. Provis, Francisca Puertas, Nuria Rebolledo, Marlene Sakoparnig, Javier Sanchez Montero, Francesco Santoro, Sriram Pradeep Saridhe, Karen Scrivener, Marijana Serdar, Xinyu Shi, Zhenguo Shi, Kosmas K. Sideris, Ruben Snelings, Matteo Stefanoni, Charlotte Thiel, Karl Christian Thienel, Ilda Tole, Luca Valentini, Philip Van Den Heede, Hanne Vanoutrive, Yury Andrés Villagran Zaccardi, Visalakshi Talakokula, Anya Vollpracht, Stefanie Von Greve-Dierfeld, Brant Walkley, Fazhou Wang, Ling Wang, Zhendi Wang, Jinxin Wei, Lia Weiler, Bei Wu, Chuansheng Xiong, Yan Yao, Guang Ye, Maciej Zajac, Cheng Zhang, Zengfeng Zhao, Semion Zhutovsky
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bernal, S.A., Dhandapani, Y., Elakneswaran, Y. et al. Report of RILEM TC 281-CCC: A critical review of the standardised testing methods to determine carbonation resistance of concrete. Mater Struct 57, 173 (2024). https://doi.org/10.1617/s11527-024-02424-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1617/s11527-024-02424-9