Abstract
Developing a method to predict the behavior of cementitious materials against natural carbonation, which causes degradation in reinforced concrete structures, has been an area of interest for researchers. Although the natural process is gradual and takes years to manifest, accelerated carbonation tests have been devised to simulate this process in a shorter duration. Nevertheless, various factors have an influence on the accuracy of these tests, in predicting natural carbonation, including CO2 concentration, humidity, temperature, type of cement and aggregate, curing time, and duration of exposure to CO2. This review emphasizes the significance of considering these factors when using accelerated carbonation tests to evaluate the durability of concrete and to prevent corrosion. Taking these factors into account can help ensure that designers estimate the service life of concrete structures accurately and that appropriate measures are taken to mitigate any potential damage caused by natural carbonation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The process of natural concrete carbonation occurs slowly, and its effects become noticeable only after several years of exposure to CO2. During the initial stage of CO2 penetration into concrete, the alkaline content of Portland cements has a significant impact on natural carbonation. This chemical process reduces porosity in concrete by causing precipitation of calcium carbonate (CaCO3) resulting from the reaction with hydrated cement products [1]. The reaction involves portlandite (Ca (OH)2), a product obtained from cement hydration, reacting with atmospheric CO2 to form calcium carbonate (CaCO3) while releasing water. This chemical reaction is described by the Eq. (1):
Reinforced concrete structures have a shortened lifespan due to the process of carbonating concrete. The occurrence of this is attributed to the existence of carbon dioxide (CO2) in the atmosphere, with concentrations ranging from 0.03% to 1%. Carbon dioxide present in the air enters by means of porous covering of the concrete and reacts primarily with the hydrated cement product, known as portlandite (Ca (OH)2), Consequently, calcium carbonate (CaCO3) is formed as a result. The outcome of this chemical reaction is the reduction of portlandite, which plays a vital role in safeguarding the steel reinforcements from corrosion by maintaining a basic environment with a pH value of 13[2].
The carbonation of concrete causes the concrete cover to completely carbonate, resulting in an acidic pH level of approximately 9, leaving the steel reinforcement without protection and initiating corrosion. This corrosion is the primary degradation observed in reinforced concrete structures, posing a risk to their stability and reducing their service life. Repairing such structures incurs additional costs in addition to the initial construction costs. Assessing the impact of this phenomenon on the durability of reinforced concrete structures is a time-consuming process that spans several years. To expedite the process, researchers have developed an accelerated test known as the “accelerated carbonation test.” This test involves introducing concrete samples into a chamber rich in CO2 with varying concentrations, allowing for the rapid acceleration of the phenomenon in the laboratory. The primary objective of this test is to carbonate concrete samples within a short timeframe to predict their behavior, upon exposure to natural conditions. The outcomes of this test can assist in predicting the extent of carbonation in concrete when subjected to CO2 gas under natural conditions.
Several factors, including CO2 concentration, exposure time, temperature, relative humidity, and the type of cement, can influence the results of this test [3]. These factors also influence the derived relationships from this test, which are employed to establish a correlation between the accelerated carbonation test and natural carbonation. Utilizing these relationships, it becomes possible to estimate the depth of carbonation in natural conditions by considering the depth of accelerated carbonation obtained from the accelerated test, In order to estimate the service life of reinforced concrete structures against this phenomenon, designers use this relationship. The objective of this review is to provide a comprehensive summary of prior research concerning the correlation between the outcomes of accelerated testing and natural carbonation in concrete. The statement presents an evaluation of the primary findings and proposes suggestions for future research.
2 Methods for accelerating the carbonation of concrete
Accelerated carbonation testing methods aim to simulate the long-term effects of carbonation under controlled, accelerated conditions. These methods are essential for predicting the durability and service life of concrete structures in a shorter time frame. Techniques such as elevated CO2 concentrations, elevated temperatures and controlled humidity levels are commonly used. For example, the work of [4]provides a comprehensive review of these accelerated methods and their effectiveness in simulating real-world carbonation processes.
In this section, the used methods to accelerate the concrete carbonation were described.
2.1 Method 1
The first accelerated carbonation chamber was designed by [5].The time required to the carbonation of concrete samples is difficult to estimate since the microstructure and initial moisture are influenced by the cure time and method of pouring the concrete.
The first one, is performed, in two steps, before the test of accelerated carbonation, samples concrete must be dried.
The relative humidity of the samples before the accelerated carbonation test must be below 70%. To achieve this object, samples should be kept in a laboratory in ambient air with 40% relative humidity and temperature of 25 °C for 8 weeks.
Application of CO2 at high pressure (15 bar), this increases the CO2 solubility in the water of the concrete pores and the total carbonation of the samples will take place after two weeks [6, 7].
2.2 Method 2
This method was also used to characterize the behavior of concretes, mortars and cement pastes. It was developed by (French Association of Competitive Clusters,French association for research and testing on materials and construction) AFPC-AFREM [7]. This method consists to follow the development of the carbonation depth of concrete kept in a carbon dioxide-enriched atmosphere (50% CO2 + 50% air, 20° ± 2 C). The test protocol is composed of two steps, the period of samples preconditioning (the samples are stored for 28 days in an atmosphere of 95% relative humidity), the period of exposure at the environment rich in CO2 during 28 days. During this period, the samples are extracted from the carbonation chamber after 7, 14, and 28 days and then the samples must be cut in half. Phenolphthalein is sprayed on the cut faces of samples and the carbonation depth measurements are taken. This protocol recommends that the dimensions of samples have three times the diameter of the largest used aggregate [8]
2.3 Method 3
In another way, a new method to measure the carbonation depth of concrete under accelerated condition was developed by Lijuan Kong et al. [9], it is a novel non-destructive technique called nonlinear ultrasonics evaluation with the aim to characterize the impact of carbonation on concrete. The experimental findings demonstrate a notable reduction in the measured non-linearity parameter, the most probable outcome is the deposition of CaCO3, the carbonation product, in pre-existing voids and microcracks. [9, 10].
2.4 Method 4
Following a bibliographic review, F. G. Da Silva [11] proposes a method for carrying out the accelerated carbonation test. This method recommends the dimensions of 40 × 40x160mm for concrete and mortar samples with aggregate dimensions not exceeding 6.3 mm, and 40 × 40x400mm for aggregate dimensions exceeding 9.5 mm. After preparation, the samples must be kept covered with plastic for 24 h at a temperature of 23 ± 2 °C, then they will be taken out from the mold and kept in an environment with higher humidity than 95% for 28 days. A period of preconditioning of samples is necessary to ensure a relative humidity of 75 ± 2% and a temperature of 23 ± 2 °C. During this period (varying from hours to weeks), samples should be dried at temperature of 50 °C until having a constant mass. After preparation and preconditioning, the samples should be introduced into the carbonation chamber at an atmosphere containing 5% CO2 at a temperature of 23 ± 2 °C and a relative humidity of 75 ± 2%. To allow CO2 diffusion between samples, they should be spaced at least 20 mm apart [11]. The carbonation depth must be determined along the longitudinal axis of the samples of 50 mm length, as close as possible and perpendicular to the exterior surface of the concrete. The surfaces of the cut samples should be cleaned of dust using only the phenolphthalein indicator solution. The portion of the concrete surface exhibiting a purple coloration should be promptly measured and recorded within a 10-min interval after spraying. The carbonation depth values should be determined in accordance with Fig. 1, representing the average carbonation depth on each side denoted as "e." Results for the top and bottom of the samples should not be used. Subsequently, the measurements should be replicated for duplicate samples. The carbonation depth should exhibit a maximum deviation of 20%. If the difference is superior than 20%, both values must be re-measured. If the difference is inferior than 20%, take the average value [11].
2.5 Other methods
The choice of the carbonation depth measurement method to be used depends on the objectives sought and the technical and economic constraints. Methods such as the optical microscope, analysis of isotope ratios and X-Ray diffraction give precise information on the microstructure of carbonated concrete, however, they are poorly suited to the expected needs of the accelerated carbonation test which is intended for provide information on the behavior of concrete with respect to carbonation within an acceptable timeframe [12].
3 Methods of highlighting the concrete carbonation depth
Furthermore, the most widely used method for measuring the carbonation depth is the spraying of phenolphthalein on the surface of the concrete. Several pH indicators have been used to highlight the carbonation depth of concrete such as, thymolphthalein, Alizarin yellow GG, Alizarin yellow R [11]. In addition to the method of spraying phenolphthalein on the concrete surface for the determination of the concrete carbonation depth, there are other methods such as, the optical microscope, isotope ratio analyses and X-ray diffraction [12]. The application of phenolphthalein on the concrete surface as a method of detection is unable to differentiate between the loss of alkalinity due to carbonation and that caused by other acid gases [12]. In cases where alternative factors leading to carbonation are suspected, their impacts can be assessed through laboratory analysis [11]. In the same way, two methods of measuring the carbonation rate of concrete can also be cited, such as, gamma densimeter and thermogravimetric analysis (TGA) associated with chemical analysis (CA). The gamma densimeter makes it possible to measure the rate of CO2 which has penetrated into the concrete and to control the carbonation process during the accelerated carbonation test, the TGA-CA makes it possible to measure the CaCO3 resulting from the CSH carbonation and calculate the portlandite consumed by the carbonation reaction [4]. Another method of measuring the carbonation rate of concrete based on the measurement of pH value. This method was tested on three types of concrete, concrete containing 30% fly ash, concrete containing 50% granulated blast furnace slag crushed, concrete containing 10% meta kaolin and 10% micro silica and concrete with Portland cement pure, these four mixtures were subjected to accelerated carbonation (5% CO2) for six weeks and pH measurements were measured as the carbonation progressed. Measurements of carbonation depths, air permeability, Additionally, measurements of resistivity and calcium hydroxide content were conducted. The results obtained show that the pH profiles depend on the type of cement (without or with additions) and the time of exposure to CO2. Moreover, thermogravimetric analysis (TGA) indicates a correlation between the calcium hydroxide content and the apparent pH of carbonated concrete. From what we have cited above, it can be concluded that the pH profiles can be used to measure the carbonation resistance of concretes containing cements with additions [13].
From this review, the main finding was the existence of several measuring methods of the accelerated carbonation depth with different preconditioning methods which can influence the correlation relations between the accelerated conditions and the natural exposure conditions obtained by each method.
4 Factors influencing the accelerated carbonation test
4.1 Physical parameters
The main physical parameters are: carbon dioxide diffusion, microstructure, water content, and distribution within the porosity of concrete [6]. In addition, the accelerated carbonation is influenced also by the relative humidity of the sample. A relative humidity between 52 and 75% is recommended to ensure complete carbonation reaction [11, 14,15,16].
In another hand, the relative humidity HR can be affected in the case of the use of the sodium nitrite saline solution, to control relative humidity in the case of accelerated carbonation in an atmosphere concentrated in carbon dioxide, this can cause the evolution of nitrogen oxide gas, which can contaminate the interstitial solution using nitrite and the nitrate salt, it is preferable to use other alternatives to control relative humidity in the case of accelerated carbonation in order to avoid any risk of corrosion of the concrete reinforcements [17]. In addition, the present water in the pores of the cement paste plays a key role in carbonation. It acts as a barrier against the diffusion of CO2 into the pores, and it provides a favorable environment for the reaction of CO2 with portlandite (Ca (OH) 2). This can be explained by the fact that the depth of carbonation increases with increasing relative humidity (up to an optimum of 50%). Carbonation decreases the porosity of the cement paste [18,19,20,21]. The pores are covered with a thickness “e” of water given by relation (2)
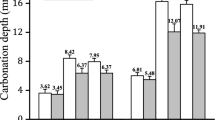
Moreover, the carbonation depth decreases with increasing cure time in water [7, 22, 23], this decrease is given in Table 1 [7].
Furthermore, concrete samples that underwent a 28 day curing period before accelerated carbonation exhibited higher resistance to carbonation compared to those cured for 7 and 14 days. [22].
The permeability of concrete is influenced by carbonation, as it affects the porosity. The rate of carbonation is dependent on both the porosity and the humidity of the concrete. [24]. Moreover, the calculated coefficient of concrete permeability (utilizing a heat of hydration model) is directly proportional to the square root of the porosity. Additionally, the permeability coefficient of the concrete can be determined through the application of Darcy's law.[25,26,27,28].
Conversely, the porosity tests did not reveal any correlation with the carbonation depths or the absorption coefficient. [29].
In addition, the depth of carbonation decreases with increasing concrete class and decreasing W / C ratio [14, 15, 30, 31]. The dimensions of the pores have also an influence on the concrete carbonation [15, 32]. In a similar context, other studies indicate that raising the relative humidity from 57 to 80% (with a 4% concentration of CO2) affects the concrete's resistance to carbonation. [33]. Other research concludes that for to get a maximum carbonation, the relative humidity should be between 60 and 65% [34].
In the same context, the temperature, the relative humidity and the CO2 concentration have significant effects on the carbonation depth [35]. Furthermore, in the accelerated carbonation test, the temperature range between 20° to 40° has no effect on the accelerated carbonation of concrete [4, 26]. As the relative humidity increases, the carbonation depth of concrete also increases, reaching its maximum value at a relative humidity of 70%.[36]. The compressive strength of concrete and the W / C ratio can be used to predict the carbonation depth [37]. In the same main, a simple test method has been established to predict the service life of concrete against carbonation based on four parameters, namely compressive strength, carbonation depth, capillary absorption and porosity. The results showed the existence of a direct relationship between the carbonation depth and the capillary absorption coefficient. Furthermore, carbonation-hardened concrete exhibited a decrease in water absorption, porosity, chloride permeability, and carbonation depth, indicating a reduction in the surface permeability of the concrete [3]. [10, 29]. The accelerated carbonation of concrete is also influenced by premature drying during the carbonation reaction, the temperature during the accelerated carbonation test and the reduction in pore volume by the precipitation of calcite [38]. Moreover, accelerated carbonation reduces the permeability and porosity of concrete, especially for poor quality concrete [39,40,41]. Research demonstrates that materials with low relative humidity (RH = 25%) exhibit the highest carbonation depths prior to commencing the accelerated carbonation test. These findings underscore the significance of accounting for moisture transfer when predicting the carbonation kinetics of cementitious materials. [24]. Furthermore, the carbonation depth of samples equilibrated at 65% relative humidity (RH) reveals that the carbonation rate is not at its maximum. The rates are higher when the material is pre-conditioned to 25% RH. In fact, apart from CO2 transport, water vapor sorption takes place at 25% RH, expediting the advancement of the CO2-RH interaction towards the inner regions of the material. [24]. In a separate line of research, investigations have demonstrated that the carbonation of concrete exerts an impact on the structural performance of reinforced concrete structures under load. [42, 43]. Recent research has revealed a positive correlation between the carbonation depth of concrete and both the initial stress levels and duration of carbonation [44].
The value of CO2 concentration in the carbonation chamber is a very important parameter. The CO2 concentration in the carbonation chamber between 7 to 18% does not have a visible effect on carbonation for concretes having a compressive strength that exceeds 40 MPa. In addition, there is a relationship between the compressive strength of concrete and the absorption and sorption of water [14]. This constatation show that the value of the compressive strength and the CO2 concentration rate into the carbonation chamber have a high effect on the accelerated carbonation rate.
Other studies also show that the rate of carbonation decreases with the improve of the concrete quality [23]. Other research specifies that CO2 concentrations varying from 0.045 to 4% with a relative humidity of 57% during an accelerated carbonation test on concrete samples have no effect on the variation in the depth of accelerated carbonation [33]. For concrete of low strength and high permeability, the carbonation rate increases with the CO2 concentration [14]. Accelerated carbonation has the same effect on the microstructure of concrete as natural carbonation [6]. The accelerated carbonation test changes the strength class of concrete [45]. In addition, the accelerated carbonation is not always fasted by the increase in the concentration of CO2 inside the carbonation chamber, but it depends also on the type of cement used. This effect can only be demonstrated under conditions of natural exposure as the accelerated carbonation effects a change on the concrete class [27, 45].
4.2 Chemical parameters.Concrete carbonation is a process in which carbon dioxide (CO2) from the air reacts with calcium hydroxide in the concrete to form calcium carbonate. This process can affect the durability of concrete structures.
The chemical parameters concern the reserve of reactive constituents of the cement. The maintenance of the reserve of these reactants is mainly affected by characteristics linked to the material such as the type and dosage of cement and the concrete curing conditions [6]. The type of cement has an effect on the accelerated carbonation, the alkaline content of Portland cement has a weak effect on the kinetics of carbonation [1].
Several chemical parameters influence the rate and extent of carbonation in concrete, these parameters are cited below:
-
Calcium Hydroxide Content: Higher levels of calcium hydroxide (Ca(OH)2) facilitate the carbonation process as this compound readily reacts with carbon dioxide to form calcium carbonate [46].
-
Alkalinity: The initial pH of concrete is high due to the presence of alkaline compounds like NaOH and KOH. A higher pH slows down the carbonation process since CO2 must neutralize these alkalis before reacting with Ca(OH)2
-
. Water-Cement Ratio (w/c ratio):A higher water-cement ratio increases concrete's porosity, facilitating CO2 penetration and accelerating carbonation [47].
-
Pozzolanic Materials: pozzolanic materials like fly ash, silica fume, or slag consume Ca(OH)2 in pozzolanic reactions, reducing the amount available for carbonation but also refining pore structure, which may hinder CO2 diffusion [48].
-
Cement Type: Different types of cement contain varying amounts of Ca (OH)2 and other compounds affecting carbonation. Portland limestone cement may carbonate faster than ordinary Portland cement [49].
-
Curing Conditions: Proper curing leads to a denser microstructure, reducing permeability and slowing carbonation. Inadequate curing increases porosity and accelerates carbonation [50].
4.3 Effect of additions and cement dosage on carbonation rate
The carbonation resistance increases with the decrease in the water / cement ratio and with the decrease in the rate of fly ash (less than 30%). Increasing the CaO content in fly ash improves the carbonation concrete resistance [51,52,53,54].
In addition, concrete containing the fly ash with a rate between of 50% to 60% decreases the resistance to carbonation. The effect of fly ash become neglected if the rate does not exceed 10% in the mixture [51, 55, 56].
The carbonation process of ternary hydrated Portland cement (TPC), which incorporates thermally activated paper sludge and fly ash, occurs 2.2 times faster in comparison to Portland cement [57]. Moreover, the concrete mixtures with fly ash have a significantly higher carbonation coefficient in both accelerated and natural exposures compared to concretes with ordinary Portland cement (OPC) [34, 58, 59].
Slag concretes formulated with a water / cement ratio between 0.42 and 0.48 exhibit the same carbonation depths in natural exposure, while these concretes have different permeability’s after 28 days of hardening. In addition, increasing the dosage of cement to 500 kg / m3 considerably reduces the penetration of CO2, which becomes almost negligible (of the order of 2 mm) [60]. In addition, fly ash concrete modified with nanoparticles decrease the depth of carbonation. Among the different types of fly ash concrete, concrete with fly ash and TiO2 with an equal ratio of nanoparticles, CaCO3 and TiO2 shows a higher resistance to the carbonation in all environments [61].
5 CO2 concentration of the accelerated carbonation test and the equivalence time with the natural carbonation of concrete
The concentration of CO2 in the carbonation chamber significantly impacts the carbonation of concrete. [62]. The temperature and CO2 concentration respectively affect the crystal form and size of carbonate products [35]. A CO2 concentration of 100% accelerates the carbonation of concrete of 100 times, a CO2 concentration of 5% accelerates the carbonation process of 5 times [45]. Another study shows that concrete samples exposed to accelerated carbonation under a CO2 concentration of 100% and under a relative humidity of 65% for one hour is equivalent to 36 days in outdoor natural exposure and 54 days in indoor natural exposure [63]. The used concentration of CO2 in the accelerated carbonation test must ensure a correlation between accelerated carbonation tests and the results of natural carbonation [11]. EN 13295: 2004 recommends using a CO2 concentration of 1% and a temperature of 21 ± 2° C to ensure a correlation with natural exposure conditions (0.03% CO2) [11]. Other research recommends a 10-day exposure of the concrete at a concentration of 5% CO2 to have acceptable results [5, 45, 62, 64]. On the other hand, the carbonation of concrete at low CO2 concentration may very well reflect the process of the natural carbonation of concrete [65, 66]. When CO2 concentrations deviate slightly from 5% in accelerated carbonation resistance tests, it is recommended to adjust the acceleration factor by taking the square root of the ratio between the accelerated test concentration and 5%. [67]. In the same context, a study has shown that with a concentration of 4% of CO2 and a relative humidity of 57%, the resistance to accelerated carbonation of concrete can be used to predict the carbonation depth in the natural environment [33]. Moreover, during an accelerated carbonation test on concrete samples, the concentration of CO2 has an influence on the carbonation depths, the concentration of 5% is the most recommended [27, 41, 68]. Other research recommends 2% CO2 concentration to have carbonation depths similar to natural carbonation depths. Furthermore, the consumption of Ca (OH) 2 in the case of a CO2 concentration of 20% is 1.5 to 2 times compared to the consumption of Ca (OH) 2 in the case of a CO2 concentration of 2% [69]. In addition, during an accelerated carbonation test on concrete samples having the same ages, CO2 concentrations varying from 2 to 20% lead to high carbonation depths. On the other hand, CO2 concentrations varying from 50 to 100%, lead to low carbonation depths. This is due to the fact that carbonation with CO2 concentrations varying from 50 to 100% accelerates the phenomenon of carbonation which reduces the porosity of the coating and consequently reduces the diffusion of CO2 in the concrete [70, 71]. Other study shows that the mineralogy and pore structure of the solid phases of carbonated samples are a function of the CO2 concentration [17].
Furthermore, numerous studies have investigated the relationship between natural carbonation and accelerated carbonation. The results of these research are summarized in the following:
The short-term exposure of concrete to an environment rich in CO2 (10% by volume) with a relative humidity ranging from 40 to 90% with a temperature of 20° is comparable to an exposure to atmospheric CO2 for a period of two years [19],
The study of [45], show that the exposure of concrete to the accelerated carbonation test for a period of 7 to 15 days with a CO2 concentration of 4 to 5% is equivalent to one year of exposure under the conditions normal (RH = 50–60%).
The optimal compressive strength of concrete under accelerated carbonation occurs when the relative humidity ranges between 40 and 50%. This phenomenon can be attributed to the reaction between portlandite (Ca (OH) 2) and CO2, resulting in the formation of calcium carbonate (CaCO3). This chemical reaction leads to an increase in volume molar from 33.15 cm3 to 36.76 cm3, representing a significant 11% expansion. [19, 72].
The water absorption of samples subjected to accelerated carbonation is lower than that of samples exposed to atmospheric CO2. This is because relative humidity does not have a big effect on samples under normal exposure,
Water absorption and porosity are proportional to the depth of carbonation induced by the accelerated carbonation process [19],
In conclusion, the change created by accelerated carbonation may influence the comparison with normal carbonation [19].
In the accelerated carbonation test, the coefficient of carbonation of concrete is higher than the coefficient observed under ambient conditions. This disparity can be attributed to the variation in CO2 concentration between the accelerated and normal conditions.[15, 59].
Carbonation at a rate of 90% of portlandite (transformed into calcite) is reached after 8 days [38].
Other researchers concluded, that it is difficult to extrapolate between the results of accelerated carbonation to natural carbonation [45, 73]. A comparison between the accelerated carbonation and the natural carbonation of a mortar without and with pozzolan was performed, the results show that the mass gain of concrete samples increases by a rate of 6% during the accelerated carbonation process (8 days), on the other hand during natural carbonation, the mass of samples only reaches 0.5% for an exposure time of 20 days. After 4 months, this rate reaches 1.75%. From this result, it takes one year of exposure of the mortar samples under normal carbonation conditions to achieve the 6% mass increase rate recorded for the accelerated carbonation test [38]. In addition, the carbonation depth of concrete samples increases with the duration of exposure in the chamber rich of CO2 [20]. The mass gain in the case of accelerated carbonation is 6%. This rate corresponds to a carbonation of 75.85% of the initial mass of portlandite. For natural carbonation, the mass gain is 1.5%, which corresponds to carbonation of 20.23% of the initial mass of portlandite [38]. A comparison between the natural carbonation (98% relative humidity + ambient CO2 rate) and the accelerated carbonation (CO2 rate of 5 to 20% and a relative humidity of 65 to 98%) of concretes based on Portland cement with reagent MgO and pulverized combustible ash was carried out. Regarding the accelerated carbonation, the results obtained show that the hydrated MgO becomes burcite and gives nesquehonite, while the Portland cement gives calcite. The accelerated carbonation damaged mixtures without MgO, although it did not change the characteristics of concrete containing MgO and Portland cement in equal proportions. Portland cement is carbonated at intermediate relative humidity levels (65% to 98%) releasing water, whereas Portland cement with MgO is carbonated at high humidity levels (98%) by consumption of water during the carbonation of burcite [74]. In the same context, MgO as an addition (0–40%) to Portland cement reduces the porosity of the cement paste and increases the resistance to carbonation compared to the Portland cement paste [75, 76]. The accelerated carbonation depth (3% CO2 concentration) for cement-based concrete with additions (fly ash, blast furnace slag) is 10 times greater than the natural carbonation depth (0.03%) [77]. In another study, C. T. Tam et al. [37] shows that the carbonation rate is 15 times greater in accelerated carbonation compared to the rate recorded in the event of natural carbonation. Exposure of concrete for 3 days in accelerated carbonation (7% CO2 concentration) is equivalent to 15 days of exposure to natural environment [37]. The results obtained by [37, 77] show that the prediction of the carbonation depth of concrete using the accelerated carbonation test presents uncertainties. Moreover, the carbonation rate of concrete samples exposed to an environment rich in CO2 for two months (98% CO2) corresponds to 312 days in a natural environment (0.03% CO2) [78]. On the other hand, the pH values and phase components of the carbonation layer in concrete exhibited similar variations under accelerated climatic environments with high CO2 concentrations as they did under natural conditions. [79]. Furthermore, the age of the concrete, which refers to the duration of exposure to natural conditions, does not affect the ratio between the coefficients of accelerated and natural carbonation. Instead, it is the environmental conditions that have an impact on this ratio. [67]. Additionally, changes in relative humidity impact the compactness of the microstructure [33]. Conversely, it has been observed that the pH value of carbonated concrete follows an exponential function in relation to the depth of carbonation. This relationship can be regarded as an indicator showcasing the degree of carbonation variation. [80].In addition, carbonation of concrete was already produced during curing, which has effects for extrapolating the results of carbonation tests to longer service life periods [81].
6 Effect of CO2 pressure on accelerated carbonation
During an accelerated carbonation test, concrete samples were subjected to four different levels of CO2 pressure: 0.03%, 10%, 25%, and 50% of atmospheric pressure (Patm). The aim was to investigate the influence of CO2 pressure on the rate of carbonation and porosity. XRD analyses revealed significant carbonation of portlandite, ettringite, and aluminates under low CO2 pressures. However, mercury intrusion analyses indicated a decrease in porosity within the carbonate zone as the CO2 pressure increased. DTA/TGA analyses highlighted that the reduction in porosity was attributed to the higher carbonation rate induced by higher CO2 pressures compared to lower pressures. Furthermore, the carbonation rate of C-S–H was found to be dependent on the partial pressure of CO2. [82]. Therefore, an elementary model was proposed, rationally regrouping all these observations, in which the amount of carbonated calcium had two sources:
-
Calcium result from the portlandite, ettringite and aluminates.
-
This calcium is totally carbonated whatever the partial pressure of CO2;
The calcium of CSH. The carbonation rate is given by a function (PCO2 / Patm) n resulting from three types of cement and four pressure levels [82].
In the scenario of accelerated carbonation, where a significant CO2 pressure gradient is applied, gas phase advection plays a crucial role in the carbonation process. A model has been developed to forecast the evolution of the microstructure and transport properties of cement pastes under accelerated conditions, where a pressure gradient of pure CO2 is employed. This model relies on a macroscopic mass balance of carbon dioxide in the gas and water phases. In addition to predicting changes in transport properties such as diffusivity and permeability, the model is also capable of forecasting alterations in the microstructure. [83].
Additionally, the pressure in an accelerated carbonation test controls the rate of change in compressive strength as well as mass gain [84]. The recommended optimum pressure is around 60 psi applied for 10 h [85].
7 Effect of the nature of the cement and the size of the aggregates on the accelerated and natural carbonation of concrete
The effects of accelerated carbonation on the physicochemical properties (evolution of the main crystal phases) of ordinary Portland cement paste was analysed by x-ray diffraction and thermogravimetric analysis. The pore structure was explored by adsorption–desorption of N2 at low temperature and by mercury intrusion, the microstructure was observed by Scanning Electron Microscope. The results of these investigations show:
The chemical control of the formation of calcium ions from solid portlandite or CSH gel governed the rate of accelerated carbonation and calcium carbonate precipitation. Carbonation led to a reduction in the total volume of pores in the studied cement pastes, attributed to the deposition of formed CaCO3. Samples subjected to accelerated carbonation conditions exhibited a greater volume of gel pores compared to those obtained through natural carbonation [86]. There are compaction pores, air pores, capillary pores and gel pores.
Compaction pores (vibration compaction) have a significant influence on the sportiness and carbonation of concrete. Vibration compaction improves the resistance to carbonation of concrete [87]. Calcite from the carbonation of the cement paste also comes from other solid phases other than portlandite, it represents 50% in addition to the carbonation of portlandite [88].
In addition, concretes based on pozzolanic cements (fly ash, blast furnace slag) carbonate (accelerated carbonation) more quickly than concretes based on Portland cements [23, 53, 55, 77, 89]. To protect the steels in these concretes (in the case of up to 50% replacement in fly ash) against corrosion for a duration of 50 years, the concrete cover must be between 35 and 40 mm [77]. Portland cement-based concretes are less sensitive to carbonation compared to the cement-based concrete with additions. For these concretes, the carbonation depth is less than 15 mm for all curing durations [4, 77, 90]. Additionally, the average depths of carbonation were minimally affected by the aspect ratio and angularity of the aggregates [91]. Recycled aggregates influence the depth of carbonation compared to normal aggregates [34]. In another study, it was found that carbonation of recycled concrete aggregates under optimal conditions densified the outer surface of hydrated cement of recycled concrete aggregates by gradual deposition of carbonates on the outside of the recycled concrete aggregates [92].
8 Effect of accelerated and natural carbonation on the microstructure of cement paste
Carbonation of concrete leads to a gradual polymerization of CSH and causes the formation of silica modified Ca gel and calcium carbonate. Carbonation of CSH and portlandite occur simultaneously. The polymerization of CSH increases with increasing CO2 concentration. For carbonation with CO2 concentrations of 10% and 100%, the CSH gel disappears completely. Accelerated carbonation with 3% CO2 concentration leads to a microstructure very similar to natural carbonation with a 0.03% CO2 concentration [40, 88, 93]. In addition, the rate of corrosion of the reinforcements of reinforced concrete structures caused by accelerated carbonation is higher than the rate of corrosion caused by natural carbonation [94]. The accelerated carbonation modifies the microstructure of concrete, and leads to its densification by precipitation of CaCO3 in the voids [10, 21, 35, 84].
9 Effect of geometry, cure, precondition, relative humidity, CO2 concentration, temperature and method of measuring the carbonation depth of concrete samples
Curing of pozzolanic cement-based concrete is necessary to extend their lifespan [77]. Furthermore, the curing time plays an important role in the formation of the microstructure of the pores, the curing conditions have an effect on the properties of the concrete cover which has a determining role for the protection of steels against aggressive agents [11, 30, 55, 90, 95]. In addition, the curing conditions (curing time and curing methods) are a most factors that governing the formation of the pore structure which influences the carbonation process. Normally, a longer cure results in a higher degree of hydration and a denser microstructure [34].
The geometry of the samples is a parameter that influences the results in a carbonation test. The diffusion of CO2 in a cylindrical shaped sample is not the same as that in a prismatic shaped sample, this can lead to misleading situations. Diffusion occurs towards the core of the cylindrical sample; this situation leads to higher carbonation depths than those prismatic samples. Thus, to compare the results of the carbonation depth for the two geometries of the samples (cylindrical and prismatic), the same test conditions must be had [11]. With this in mind, RILEM and EN 13295: 2004 recommend using prismatic samples of concrete, mortar or grout with the sizes (40 × 40x160 mm) for accelerated carbonation tests and 100 × 100x400 mm for concretes with dimensions of aggregates greater than 10 mm [11]. Moreover, the preconditioning conditions correspond to the duration of the hardening time and the exposure of the concrete to CO2. The degree of preconditioning plays a crucial role in an accelerated carbonation test as it alters the pore saturation level of the concrete. When samples are introduced into a carbonation chamber with low saturation, they will absorb water until they match the chamber's moisture level. On the other hand, if the initial saturation is high, it will take a longer time for the samples to lose moisture and reach the chamber's conditions. This variation in preconditioning can lead to significant differences in the test results. [11, 16, 96]. In addition, the high temperature has a noticeable effect on the degradation of concrete, and the life of the concrete decreases as the temperature increases, which significantly reduces the resistance to carbonation of the concrete [44].
10 connection between accelerated carbonation and natural carbonation
Recent studies highlight several key aspects of the relationship between accelerated carbonation and natural carbonation:
-
Rate comparison: accelerated carbonation achieves in days or weeks what natural carbonation takes years to accomplish. It involves controlled conditions (high CO2 concentration, pressure, and humidity) to expedite the process [97].
-
Structural changes: both processes alter the microstructure and porosity of materials like concrete, but accelerated carbonation tends to produce more pronounced and uniform changes in a shorter time [97]
-
Predictive models: models have been developed to relate accelerated carbonation results to natural carbonation outcomes. For instance, it is estimated that 7–15 days of accelerated carbonation can simulate approximately one year of natural exposure [97]
-
Material testing: studies have shown that accelerated carbonation tests are effective in predicting long-term performance and durability of materials, providing a quicker assessment method compared to natural carbonation [97]
These insights underline the practical applications of accelerated carbonation in material testing and carbon sequestration efforts, offering a valuable tool for researchers and engineers to predict and enhance the long-term performance of construction materials.
11 Conclusions
In this paper, a review concerning the natural and accelerated carbonation of cementitious materials is presented. The paper summarizes the main results recorded for improving the accelerated carbonation test to reflect the reality of natural carbonation. This work also presents also the main models for predicting natural carbonation from the results of the accelerated carbonation test. However, the results of the accelerated carbonation test used to predict natural carbonation are influenced by several parameters, such as, the concentration of CO2 in the carbonation chamber, temperature, relative humidity, type of cement, cure time, accelerated carbonation test method, time of exposure to CO2, geometry of the sample as well as the CO2 pressure. From this review, the main conclusions can be written:
-
1.
The curing time in water of the concrete samples before the accelerated carbonation test considerably reduces the carbonation rate (of the order of 17% of the total volume of the carbonate sample for a curing time in the water. 28-day water). AFPC-AFREM] recommends a cure time in water of 28 days before starting the accelerated carbonation test.
-
2.
In the literature, several concentrations of carbon dioxide are proposed to conduct an accelerated carbonation test. The concentrations suggested by various studies are summarized in Table 2.
From Table 2, it can be seen that the concentrations of 2 to 5% CO2 with a temperature of 21 ± 2 °C give acceptable results. This leads us to conclude that small concentrations of accelerated carbonation CO2 are recommended to be able to predict acceptable carbonation depths in natural exposure.
Relative humidity (RH) during the accelerated carbonation test is a key parameter in the carbonation process. Table 3 gives some values of relative humidity recommended by researchers for performing the accelerated carbonation test.
From Table 3, it can be seen that there is a variety of recommended relative humidity (RH) values varying from 25 to 90%. However, most recommend relative humidity’s between 40 and 70%.
The temperature to be maintained during the accelerated carbonation test is also an important parameter, Table 4 summarizes the recommendations of various researchers for the temperature in the accelerated carbonation chamber.
The optimum recommended temperature for the accelerated carbonation test is an average of 20° C.
-
1.
The CO2 pressure inside the optimal accelerated carbonation chamber recommended is in the order of 60 psi and applied for 10 h.
-
2.
The accelerated carbonation modifies the microstructure of concrete, and leads to its densification by precipitation of CaCO3 in the voids.
-
The chemical control of the accelerated carbonation rate involved the formation of calcium ions from either the solid portlandite or the CSH gel, leading to the precipitation of calcium carbonate.
-
As carbonation occurred, the cement pastes under study exhibited a reduction in the overall volume of their pores, attributed to the deposition of the formed CaCO3.
-
The cement samples subjected to accelerated carbonation conditions demonstrated a greater formation of gel pores compared to those achieved through natural carbonation
-
The average depths of carbonation were minimally affected by the aspect ratio and angularity of the aggregates, demonstrating little influence.
-
12 3. There are various methods for determining the carbonation depth, these methods are:
-
The spraying of phenolphthalein on the concrete surface is the most commonly employed and straightforward method for measuring carbonation depth. In this method, the carbonated concrete retains its colourlessness while the unaffected concrete appears purple.
-
Other pH indicators such as thymolphtalein, Alizarin yellow GG, Alizarin yellow R can also be used to measure the rate of carbonation
-
There are other methods for the characterization of carbonated concrete, such as the optical microscope, analysis of isotope ratios, X-ray diffraction, however, they are poorly suited to the expected needs of the accelerated carbonation test which is intended to provide information on the behaviour of concrete vis-à-vis carbonation within an acceptable time gamma densimeter and thermogravimetric analysis (TGA) associated with chemical analysis (CA)
4. From the obtained results from the accelerated carbonation, several mathematical models have been proposed in order to predict the depth of carbonation of concrete in the long term (under natural conditions). These models are based on the parameters that influence the carbonation of concrete namely relative humidity (RH), CO2 concentration, exposure time, temperature, pH and type of cement.
Each of these models has been developed for a specific environment, in terms of materials (cement, aggregates), accelerated carbonation test methods adopted, CO2 concentrations and pressure, relative humidity and temperature.
5. The shape of the samples used in an accelerated carbonation test affects the prediction of the long-term carbonation depth.
In conclusion, and from the above, the prediction of natural carbonation from the results of accelerated carbonation is influenced by the above parameters. Therefore, it is difficult to standardize a prediction model for determining the long-term carbonation rate of concrete structures, but it is recommended to adapt the developed models to local environmental conditions.
Finally, the accelerated carbonation is a test which undoubtedly allows us to predict the behaviour of cementitious materials against the natural phenomenon of carbonation. Nevertheless, this test is influenced by the parameters mentioned above. therefore, and in order to obtain acceptable results from this test, we recommend to create an international working group which brings together all the experts in the field in order to establish the standard to conduct this test, this work must take into account the following recommendations:
-
The concentration of CO2 used during the test depends on the exposure time,
-
The temperature is fixed for most research at 20° C,
-
The CO2 pressure inside the carbonation chamber is 15 bars,
-
The Type of cement is an important parameter in the evaluation of the depth of accelerated carbonation,
-
The most used method to demonstrate the depth of accelerated carbonation is spraying phenolphthalein because it is the easiest to use,
Data availability
No datasets were generated or analysed during the current study.
References
Goñi S, Gaztañaga MT, Guerrero A. Role of cement type on carbonation attack. J Mater Res. 2002;17:1834–42.
AL-Ameeri A, Rafiq M I and Tsioulou O effect of cracks on alkalinity level of concrete structures exposed to carbon dioxide environment condition
AL-Ameeri A S, Nahhab A H and AL-Baghdadi H M 2021 The effects of carbonation on the chloride resistance of concretes with supplementary cementitious materials (SCMs) Young Researchers’ Forum V 5
Villain G, Thiery M, Platret G. Measurement methods of carbonation profiles in concrete: Thermogravimetry, chemical analysis and gammadensimetry. Cement Concr Res. 2007;37:1182–92.
Elgalhud AA, Dhir RK, Ghataora GS. Carbonation resistance of concrete: limestone addition effect. Mag Concr Res. 2017;69:84–106.
Al-Kadhimi TKH, Banfill PFG, Millard SG, Bungey JH. An accelerated carbonation procedure for studies on concrete. Adv Cem Res. 1996;8:47–59.
Fattuhi NI. Concrete carbonation as influenced by curing regime. Cem Concr Res. 1988;18:426–30.
Ollivier J P 1997 Essai de carbonatation accéléré, mesure de l’épaisseur de béton carbonate (Laboratoire des Matériaux et Durabilité des Constructions Toulouse, France)
Kim G, Kim J-Y, Kurtis KE, Jacobs LJ, Le Pape Y, Guimaraes M. Quantitative evaluation of carbonation in concrete using nonlinear ultrasound. Mater Struct. 2016;49:399–409.
Sharma D, Goyal S. Effect of accelerated carbonation curing on near surface properties of concrete. Eur J Environ Civil Eng. 2020;1:22.
Da Silva FG, Helene P, Castro-Borges P, Liborio JB. Sources of variations when comparing concrete carbonation results. J Mater Civil Eng. 2009;21:333–42.
Turcry P 2019 Carbonatation des matériaux cimentaires
McPolin DO, Basheer PA, Long AE, Grattan KT, Sun T. New test method to obtain pH profiles due to carbonation of concretes containing supplementary cementitious materials. J Mater Civ Eng. 2007;19:936–46.
Loo YH, Chin MS, Tam CT, Ong KCG. A carbonation prediction model for accelerated carbonation testing of concrete. Mag Concr Res. 1994;46:191–200.
Roy SK, Poh KB, Northwood DO. Durability of concrete—accelerated carbonation and weathering studies. Build Environ. 1999;34:597–606.
Turcry P, Younsi A, Jacquemot F, Aït-Mokhtar A, Rougeau P. Influence of in situ concrete variability on accelerated carbonation test. Eur J Environ Civil Eng. 2012;16:288–97.
Anstice DJ, Page CL, Page MM. The pore solution phase of carbonated cement pastes. Cem Concr Res. 2005;35:377–83.
Papadakis VG, Vayenas CG, Fardis MN. A reaction engineering approach to the problem of concrete carbonation. AIChE J. 1989;35:1639–50.
De Ceukelaire L, Van Nieuwenburg D. Accelerated carbonation of a blast-furnace cement concrete. Cem Concr Res. 1993;23:442–52.
Hussain S, Bhunia D, Singh SB. Comparative study of accelerated carbonation of plain cement and fly-ash concrete. J Build Eng. 2017;10:26–31.
Zhang H, Rodriguez CR, Dong H, Gan Y, Schlangen E, Šavija B. Elucidating the effect of accelerated carbonation on porosity and mechanical properties of hydrated Portland cement paste using X-ray tomography and advanced micromechanical testing. Micromachines. 2020;11:471.
Hussain S, Bhunia D, Singh SB. Influence of curing duration on accelerated carbonation of concrete and the uncertainties in its measurement Mater. Sci Eng. 2018;431: 052013.
Otieno M, Ikotun J, Ballim Y. Experimental investigations on the effect of concrete quality, exposure conditions and duration of initial moist curing on carbonation rate in concretes exposed to urban, inland environment. Construct Build Mater. 2020;246:118443.
Metalssi OO, Aït-Mokhtar A, Turcry P. A proposed modelling of coupling carbonation-porosity-moisture transfer in concrete based on mass balance equilibrium. Construct Build Mater. 2020;230:116997.
Song H-W, Kwon S-J. Permeability characteristics of carbonated concrete considering capillary pore structure. Cem Concr Res. 2007;37:909–15.
Chávez-Ulloa E, Camacho-Chab R, Sosa-Baz M, Castro-Borges P, Pérez-López T. Corrosion process of reinforced concrete by carbonation in a natural environment and an accelerated test chamber Int. J Electrochem Sci. 2013;8:9015–29.
Ekolu SO. A review on effects of curing, sheltering, and CO2 concentration upon natural carbonation of concrete. Construct Build Mater. 2016;127:306–20.
Šavija B, Luković M. Carbonation of cement paste: Understanding, challenges, and opportunities. Construct Build Mater. 2016;117:285–301.
Carvajal AM, Maturana P, Pino C, Poblete J. Analysis of the relation between accelerated carbonation, porosity, compressive strength and capillary absortion in concrete, in the search of a new control method by durability. Revista de la Construcción. 2009;8:129–35.
Jiang L, Lin B, Cai Y. A model for predicting carbonation of high-volume fly ash concrete. Cem Concr Res. 2000;30:699–702.
Ribeiro AB, Santos T, Gonçalves A. Performance of concrete exposed to natural carbonation: Use of the k-value concept. Constr Build Mater. 2018;175:360–70.
De Weerdt K, Plusquellec G, Revert AB, Geiker MR, Lothenbach B. Effect of carbonation on the pore solution of mortar. Cem Concr Res. 2019;118:38–56.
Leemann A, Moro F. Carbonation of concrete: the role of CO 2 concentration, relative humidity and CO2 buffer capacity. Mater Struct. 2017;50:30.
Qiu Q. A state-of-the-art review on the carbonation process in cementitious materials: Fundamentals and characterization techniques. Constr Build Mater. 2020;247: 118503.
Liu P, Yu Z, Chen Y. Carbonation depth model and carbonated acceleration rate of concrete under different environment. Cement Concr Compos. 2020;114: 103736.
Chen Y, Liu P, Yu Z. Effects of environmental factors on concrete carbonation depth and compressive strength. Materials. 2018;11:2167.
Tam CT, Lim HB, Sisomphon K. Carbonation of concrete in the tropical environment of Singapore. IES J Part A. 2008;1:146–53.
Cultrone G, Sebastian E, Huertas MO. Forced and natural carbonation of lime-based mortars with and without additives: Mineralogical and textural changes. Cem Concr Res. 2005;35:2278–89.
Claisse PA, El-Sayad H, Shaaban IG. Permeability and pore volume of carbonated concrete. ACI Mater J. 1999;96:378–81.
Fang Y, Chang J. Microstructure changes of waste hydrated cement paste induced by accelerated carbonation. Constr Build Mater. 2015;76:360–5.
Hussain S, Bhunia D, Singh SB. An experimental investigation of accelerated carbonation on properties of concrete. Eng J. 2016;20:29–38.
Wang X-H, Val DV, Zheng L, Jones MR. Carbonation of loaded RC elements made of different concrete types: accelerated testing and future predictions. Constr Build Mater. 2020;243: 118259.
Mi R, Pan G, Liew KM. Predicting carbonation service life of reinforced concrete beams reflecting distribution of carbonation zones. Constr Build Mater. 2020;255: 119367.
Zhang D, Yang Q, Mao M, Li J. Carbonation performance of concrete with fly ash as fine aggregate after stress damage and high temperature exposure. Constr Build Mater. 2020;242: 118125.
Sanjuan MA, Andrade C, Cheyrezy M. Concrete carbonation tests in natural and accelerated conditions. Adv Cem Res. 2003;15:171–80.
Papadakis VG, Fardis MN, Vayenas CG. Effect of composition, environmental factors and cement-lime mortar coating on concrete carbonation. Mater Struct. 1992;25:293–304.
Parrott L J 1987 A review of carbonation in reinforced concrete
Roy DM. Hydration, structure, and properties of blast furnace slag cements, mortars, and concrete. J Proceed. 1982;79:444–57.
Monteiro PJ, Kurtis KE. Time to failure for concrete exposed to severe sulfate attack. Cement Concrete Res. 2003;33:987–93.
Bentur A, Berke N, Diamond S. Steel corrosion in concrete: fundamentals and civil engineering practice. Boca Raton: CRC Press; 1997.
Khunthongkeaw J, Tangtermsirikul S, Leelawat T. A study on carbonation depth prediction for fly ash concrete. Construct Build Mater. 2006;20:744–53.
Helene PRDL, Castro-Borges P. A novel method to predict concrete carbonation Concreto y cemento. Investigación y desarrollo. 2009;1:25–35.
Czarnecki L, Woyciechowski P. Concrete carbonation as a limited process and its relevance to concrete cover thickness. ACI Mater J. 2012;109:275.
Leemann A, Nygaard P, Kaufmann J, Loser R. Relation between carbonation resistance, mix design and exposure of mortar and concrete. Cement Concrete Compos. 2015;62:33–43.
Bouzoubaâ N, Bilodeau A, Tamtsia B, Foo S. Carbonation of fly ash concrete: laboratory and field data. Can J Civ Eng. 2010;37:1535–49.
Morandeau A, Thiéry M, Dangla P. Impact of accelerated carbonation on OPC cement paste blended with fly ash. Cem Concr Res. 2015;67:226–36.
Frías M, Goñi S. Accelerated carbonation effect on behaviour of ternary Portland cements. Compos B Eng. 2013;48:122–8.
Ashraf W. Carbonation of cement-based materials: challenges and opportunities. Constr Build Mater. 2016;120:558–70.
Duran-Herrera A, Mendoza-Rangel JM, De-Los-Santos EU, Vazquez F, Valdez P, Bentz DP. Accelerated and natural carbonation of concretes with internal curing and shrinkage/viscosity modifiers. Mater Struct. 2015;48:1207–14.
Bernal SA, San Nicolas R, Provis JL, De Gutiérrez RM, van Deventer JS. Natural carbonation of aged alkali-activated slag concretes. Mater struct. 2014;47:693–707.
Ramachandran D, Uthaman S, Vishwakarma V. Studies of carbonation process in nanoparticles modified fly ash concrete. Constr Build Mater. 2020;252: 119127.
Revert AB, De Weerdt K, Jakobsen UH, Geiker MR. Impact of accelerated carbonation on microstructure and phase assemblage. Nordic Concrete Res. 2018;59:111–26.
Galan I, Andrade C, Castellote M. Natural and accelerated CO2 binding kinetics in cement paste at different relative humidities. Cement Concrete Res. 2013;49:21–8.
Rathinarajan S and Pillai R G 2017 Carbonation rate and service life of reinforced concrete systems with mineral admixtures and special cements (CORCON)
Zhiguon N, Ri Y. Experimental investigation of concrete carbonation under different conditions. Study Civil Eng Architecture (SCEA). 2013;2:114.
Visser JHM. Influence of the carbon dioxide concentration on the resistance to carbonation of concrete. Constr Build Mater. 2014;67:8–13.
Neves R, Branco F, De Brito J. Field assessment of the relationship between natural and accelerated concrete carbonation resistance. Cement Concr Compos. 2013;41:9–15.
Fedorov PA, Anvarov AR, Lutsyk EV, Latypov VM, Latypova TV. Kinetics of fine concrete carbonation in humid operational environment. Int J Appl Eng Res. 2016;11:7439–45.
Yan Y, Guanbao T, Ling W, Suping C, Yin C. Difference between natural and accelerated carbonation of concrete at 2% Co2 and 20% Co2. Revista Romana de Materiale. 2018;48:70–5.
Cui H, Tang W, Liu W, Dong Z, Xing F. Experimental study on effects of CO2 concentrations on concrete carbonation and diffusion mechanisms. Construct Build Mater. 2015;93:522–7.
Hussain S, Bhunia D, Singh SB. Assessment of carbonation depth under natural and accelerated carbonation conditions. Indian Concr J. 2016;57:64.
Mo L, Zhang F, Deng M, Jin F, Al-Tabbaa A, Wang A. Accelerated carbonation and performance of concrete made with steel slag as binding materials and aggregates. Cement Concr Compos. 2017;83:138–45.
Pouhet R, Cyr M. Studies of natural and accelerated carbonation in metakaolin-based geopolymer. Adv Sci Technol. 2014;92:38–43.
Vandeperre LJ, Al-Tabbaa A. Accelerated carbonation of reactive MgO cements. Adv Cem Res. 2007;19:67–79.
Mo L, Panesar DK. Effects of accelerated carbonation on the microstructure of Portland cement pastes containing reactive MgO. Cem Concr Res. 2012;42:769–77.
Bernal SA, San Nicolas R, Myers RJ, de Gutiérrez RM, Puertas F, van Deventer JS, Provis JL. MgO content of slag controls phase evolution and structural changes induced by accelerated carbonation in alkali-activated binders. Cem Concr Res. 2014;57:33–43.
Sisomphon K, Franke L. Carbonation rates of concretes containing high volume of pozzolanic materials. Cem Concr Res. 2007;37:1647–53.
Stehlík M. Accelerated carbonation depth test in an atmosphere of 98% CO2. Statybin? S Konstrukcijos ir Technologijos. 2011;3:51–5.
Ji Y, Yuan Y, Tan Z, Wang W and Xia Y 2008 Correlation of concrete carbonation process under natural condition and high CO2 concentration artificial accelerated climate environments International Conference on Microstructure Related Durability of Cementitious Composites (RILEM Publications) pp 495–505
Kong L, Han M, Yang X. Evaluation on relationship between accelerated carbonation and deterioration of concrete subjected to a high-concentrated sewage environment. Constr Build Mater. 2020;237: 117650.
Gluth GJ, Arbi K, Bernal SA, Bondar D, Castel A, Chithiraputhiran S, Dehghan A, Dombrowski-Daube K, Dubey A, Ducman V. RILEM TC 247-DTA round robin test: carbonation and chloride penetration testing of alkali-activated concretes. Mater Struct. 2020;53:1–17.
Hyvert N, Sellier A, Duprat F, Rougeau P, Francisco P. Dependency of C-S–H carbonation rate on CO2 pressure to explain transition from accelerated tests to natural carbonation. Cement Concrete Res. 2010;40:1582–9.
Phung QT, Maes N, Jacques D, De Schutter G, Ye G. Evolution of microstructure and transport properties of cement pastes due to carbonation under a co2 pressure gradient—a modeling approach. Concreep. 2015;10:1032–41.
Georget F, Prévost JH, Huet B. Impact of the microstructure model on coupled simulation of drying and accelerated carbonation. Cement Concrete Res. 2018;104:1–12.
Ahmad S, Assaggaf RA, Maslehuddin M, Al-Amoudi OSB, Adekunle SK, Ali SI. Effects of carbonation pressure and duration on strength evolution of concrete subjected to accelerated carbonation curing. Constr Build Mater. 2017;136:565–73.
García-González CA, Hidalgo A, Andrade C, Alonso MC, Fraile J, López-Periago AM, Domingo C. Modification of composition and microstructure of Portland cement pastes as a result of natural and supercritical carbonation procedures. Industrial Eng Chem Res. 2006;45:4985–92.
Gonen T, Yazicioglu S. The influence of compaction pores on sorptivity and carbonation of concrete. Constr Build Mater. 2007;21:1040–5.
Castellote M, Fernandez L, Andrade C, Alonso C. Chemical changes and phase analysis of OPC pastes carbonated at different CO2 concentrations. Mater Struct. 2009;42:515–25.
Hussain S, Bhunia D, Singh SB, Aggrawal M. Mechanical strength and durability of mineral admixture concrete subjected to accelerated carbonation. J Struct Integrity Mainten. 2018;3:44–51.
Bahador S D and Arezoo R 2009 Three years natural carbonation of PC and blended cement concrete in Singapore exposure condition Proceedings of 34th International Conference on Our World in Concrete and Structures, Singapore
Jiang Z-L, Gu X-L, Huang Q-H, Zhang W-P. Statistical analysis of concrete carbonation depths considering different coarse aggregate shapes. Constr Build Mater. 2019;229: 116856.
Gholizadeh-Vayghan A, Bellinkx A, Snellings R, Vandoren B, Quaghebeur M. The effects of carbonation conditions on the physical and microstructural properties of recycled concrete coarse aggregates. Constr Build Mater. 2020;257: 119486.
Bernal SA, Provis JL, Walkley B, San Nicolas R, Gehman JD, Brice DG, Kilcullen AR, Duxson P, van Deventer JS. Gel nanostructure in alkali-activated binders based on slag and fly ash, and effects of accelerated carbonation. Cement Concrete Res. 2013;53:127–44.
Varjonen S. Accelerated carbonated concrete as corrosion environment. Nordic Concrete Res-Publicat. 2004;31:1.
Jia Y, Aruhan B, Yan P. Natural and accelerated carbonation of concrete containing fly ash and GGBS after different initial curing period. Mag Concr Res. 2012;64:143–50.
Turcry P, Oksri-Nelfia L, Younsi A, Aît-Mokhtar A. Analysis of an accelerated carbonation test with severe preconditioning. Cem Concr Res. 2014;57:70–8.
Carević V, Radević A, Ignjatović I. Influence of Fly Ash as Cement Substitution on Accelerated and Natural Carbonation of Concrete. In: Jędrzejewska A, Kanavaris F, Azenha M, Benboudjema F, Schlicke D, editors. International RILEM conference on synergising expertise towards sustainability and robustness of cement-based materials and concrete structures RILEM Bookseries. Cham: Springer; 2023.
Author information
Authors and Affiliations
Contributions
Ahmed MERAH wrote the main manuscript text and prepared figures All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Merah, A. Methods of concrete accelerated carbonation test: a review. Discov Civ Eng 1, 53 (2024). https://doi.org/10.1007/s44290-024-00057-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44290-024-00057-z