Abstract
Background
The gut microbiota (GM) and their metabolites have garnered significant attention for their roles in metabolic syndrome (MetS) and associated conditions. MetS, characterized by a cluster of metabolic abnormalities, significantly increases the risk of cardiovascular disease (CVD), obesity, insulin resistance, and type 2 diabetes mellitus (T2DM). The dysbiosis of gut microbiota, marked by changes in microbial composition and function, has been implicated in the pathogenesis of MetS.
Main body
This review synthesizes recent findings elucidating the influence of GM composition and microbiota-derived metabolites on MetS pathogenesis and progression. Notably, alterations in GM composition and dysregulation of metabolites such as short-chain fatty acids (SCFAs), trimethylamine N-oxide (TMAO), polyamines, amino acids, and indole derivatives have been implicated in MetS development. These metabolites play crucial roles in metabolic processes, and their imbalance can trigger or exacerbate metabolic disturbances associated with MetS. Various therapeutic approaches, including dietary interventions, probiotics, prebiotics, and precision medicine targeting specific metabolites, offer promising strategies for managing MetS. These interventions aim to restore a healthy GM balance and regulate the production of beneficial metabolites.
Conclusion
The complexity of GM interactions and their systemic effects necessitate more standardized research methodologies. Future investigations focusing on personalized therapeutic interventions and non-invasive diagnostic tools are warranted to address the complexities of MetS management. Advancing our understanding of the GM-metabolite-MetS axis will be crucial for developing effective, targeted treatments and improving patient outcomes in MetS.
Similar content being viewed by others
Introduction
Metabolic syndrome (MetS) comprises a cluster of metabolic disorders that significantly increase the risk of cardiovascular disease (CVD), obesity, insulin resistance, and type 2 diabetes mellitus (T2DM). The prevalence of MetS demonstrates considerable variability worldwide, influenced by factors such as geographical location, diagnostic criteria, age, gender, and associated health conditions [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15]. For instance, while Africa reports an overall prevalence of 32.4%, Pakistan exhibits a prevalence of 28.8%, with noteworthy regional variations [1, 4]. In contrast, the USA witnesses an escalating trend in MetS prevalence, particularly among individuals with lower educational attainment [9]. Gender disparities are evident, with higher prevalence observed among females in various regions such as Pakistan and India [1, 8]. Similarly, studies in China, Brazil, Finland, Mexico, Iran, and Zahedan highlight the global burden of MetS [2, 6, 7, 10,11,12, 15].
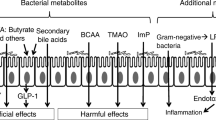
Recent research has underscored the intricate interplay between gut microbiota (GM), microbiota-derived metabolites, and host physiology in MetS pathogenesis [16]. Understanding these relationships is vital for developing effective therapeutic strategies for MetS management. The gut microbiota, comprising trillions of microorganisms, plays a crucial role in host metabolism by fermenting dietary substrates and producing various metabolites that can impact systemic health [17]. Among these metabolites, short-chain fatty acids (SCFAs), such as acetate, propionate, and butyrate, have garnered significant attention due to their pleiotropic effects on metabolic processes, inflammation, and gut barrier function [18,19,20]. Alterations in the composition and function of the gut microbiota, known as dysbiosis, have been linked to the development of MetS, partly through perturbations in SCFA production and signaling [21,22,23]. Additionally, trimethylamine N-oxide (TMAO), a metabolite derived from gut microbial metabolism of dietary nutrients like choline and carnitine, has emerged as a biomarker and mediator in MetS, influencing various metabolic pathways and contributing to cardiovascular risk [24,25,26].
Furthermore, polyamines, tryptophan and indole derivatives derived from gut microbial metabolism exhibit intricate relationships with MetS, affecting polyamine synthesis, amino acid utilization, and signaling pathways implicated in metabolic regulation [27,28,29]. Additionally, dysregulated bile acid metabolism plays a significant role in the development of metabolic syndrome [30]. Alterations in gut microbiota composition and function can perturb the balance of these metabolites, contributing to metabolic dysregulation and MetS pathophysiology [31,32,33].
This review aims to synthesize recent findings on the relevance of GM composition and microbiota-derived metabolites in MetS and seeks to outline clear directions for future research, suggesting that a deeper exploration into the causative links between microbiota-derived metabolites and metabolic dysfunctions could lead to more targeted therapeutic interventions.
Gut microbiota metabolites in metabolic syndrome
The pivotal role of the gut microbiota (GM) in metabolic syndrome (MetS) and related disorders has been extensively studied in recent years as highlighted in Table 1. Gradisteanu et al. examined microbiome patterns in MetS patients, revealing significant alterations correlated with metabolic abnormalities [16]. Several studies highlight the crucial role of gut microbiota in the development and progression of metabolic syndrome (MetS). The gut microbiome composition can be influenced by various factors such as diet, antibiotics, and maternal microbiota, which in turn affects disease susceptibility and metabolic health [21,22,23]. Alterations in the gut microbial community have been associated with obesity-related metabolic syndrome, insulin resistance, dyslipidemia, and inflammation by gut microbial metabolites [34,35,36].
The pathogenetic mechanisms through which these metabolites contribute to the development of metabolic syndrome (MetS) are multifaceted. Short-chain fatty acids (SCFAs), such as acetate and butyrate, exert regulatory effects on glucose and lipid metabolism, inflammation, and intestinal barrier function, all critical factors in MetS pathology [18,19,20, 37,38,39,40]. Trimethylamine N-oxide (TMAO) serves as both a biomarker and a mediator in MetS, exacerbating metabolic dysfunction by triggering inflammatory responses and oxidative stress pathways [24,25,26, 41,42,43,44,45,46]. Bile acids, by activating receptors like FXR and GPBAR1, influence glucose homeostasis and modulate gut microbial composition and energy metabolism, contributing significantly to MetS progression [47,48,49,50].
Additionally, polyamines, including spermine, spermidine, and putrescine, synthesized by gut microbiota such as Lactobacillus and Clostridium, are integral to cellular processes like growth and proliferation. Dysregulated levels of polyamines contribute to oxidative stress and inflammation, impacting gut barrier integrity and immune function, thereby exacerbating insulin resistance and dyslipidemia observed in metabolic syndrome (MetS) [29, 33, 51,52,53,54]. Tryptophan derivatives, such as indole-3-aldehyde (3-IAld) and indole-3-acetic acid (IAA), produced by bacterial metabolism of dietary tryptophan, activate pathways like the aryl hydrocarbon receptor (AhR), influencing immune responses and adipose tissue metabolism. Disrupted tryptophan metabolism by gut microbiota may thus promote systemic inflammation, insulin resistance, and dyslipidemia, pivotal aspects of MetS [27, 55,56,57,58].
Xavier-Santos et al. highlighted the impact of GM on metabolic processes and proposed interventions like probiotics and prebiotics for managing MetS [59]. Therapeutic interventions targeting GM and microbiota-derived metabolites have shown promise in managing MetS. These include probiotics, prebiotics, synbiotics, fecal microbiota transplantation (FMT], dietary interventions, and precision medicine approaches [37, 39, 60, 61]. For example, dietary adjustments and supplementation with specific nutrients have been proposed to modulate GM composition and metabolite production [62, 63].
Short-chain fatty acids in metabolic syndrome
Short-chain fatty acids (SCFAs), particularly acetate, propionate, and butyrate, are metabolites produced by gut microbiota fermentation of dietary fiber. SCFAs play a vital role in regulating metabolic processes, including glucose and lipid metabolism, inflammation, and gut barrier integrity [18,19,20]. Dysregulation of SCFA levels has been implicated in the pathogenesis of metabolic syndrome. Ganesan et al. and Zhang et al. both emphasize the dysregulation of SCFAs in metabolic disorders [64, 65]. Ganesan et al. found decreased SCFA levels, particularly acetate and butyrate, in NAFLD patients, while Zhang et al. observed decreased propionic acid levels in Cushing's syndrome patients. These findings suggest a potential link between SCFAs and metabolic syndrome severity.
Nogal et al. examined the relationships between circulating acetate levels, gut microbiome composition, and visceral fat in a large population-based cohort [66]. They found that acetate levels were positively correlated with gut microbiota diversity and negatively associated with visceral fat. This study suggests that gut microbiota composition and SCFA production could influence cardiometabolic health by modulating visceral fat accumulation. Various dietary components, including fiber, dairy products, anthocyanins, and betaine, have been studied for their effects on gut microbiota composition and metabolic syndrome.
Impact of dietary intervention on metabolic syndrome targeting SCFA
Consumption of fiber-rich diets promotes the growth of beneficial bacteria and SCFA production, thereby attenuating metabolic syndrome risk [39, 67, 68]. Dairy product consumption, particularly low-fat options, has been associated with beneficial alterations in plasma metabolome profiles and mitigation of metabolic syndrome [34]. Additionally, dietary supplements like betaine have shown promise in improving gut microbiota dysbiosis and metabolic syndrome parameters via the gut microbiota-derived miR-378a/YY1 regulatory axis [69].
Li et al. explored the effects of different types of dietary fiber (DF) on the growth and development of Magang geese [70]. They observed that low-, medium-, and high-viscosity DFs reduced lipid levels in geese by promoting the production of SCFAs and activating the AMPK pathway-related genes. This study emphasizes the beneficial effects of DFs on lipid metabolism through SCFA production, providing insights into dietary interventions for metabolic syndrome. He et al. and Maurer et al. investigated dietary interventions with mulberry leaf extract (MLE) and citrus extract, respectively [71, 72]. Both studies found that these extracts positively influenced SCFA production, contributing to improved metabolic profiles in MetS subjects. He et al. demonstrated that MLE modulated SCFA production through the AMPK signaling pathway, while Maurer et al. observed increased butyrate production with citrus extract supplementation.
Wang et al. and Li et al. focused on the effects of dietary interventions on SCFA production by gut microbiota modulation [73, 74]. Wang et al. found that Lactobacillus administration increased SCFA production, particularly acetic acid, contributing to improved metabolic parameters. Li et al. demonstrated that dietary butyrate reduced weight gain and improved insulin resistance in mice, with the effects mediated by gut microbiota, particularly Lachnospiraceae bacterium 28–4.
Trimethylamine N-oxide (TMAO) in metabolic syndrome (MetS)
Emerging research suggests that gut microbiome-derived metabolites, particularly trimethylamine N-oxide (TMAO), may play a pivotal role in the pathogenesis of MetS and its associated complications. Several studies investigate the relationship between TMAO and metabolic syndrome (MetS), emphasizing its potential as a biomarker and its role in the pathogenesis of MetS. Kuo et al. found a positive association between serum TMAO levels and MetS in patients with coronary artery disease (CAD) [24]. Similarly, Mirzababaei et al. reported a significant correlation between elevated TMAO levels and MetS, indicating its potential as a predictive marker for MetS [25]. Moreover, Sun et al. highlighted the association between TMAO levels and the severity of psoriasis, suggesting its role as an indicator of disease severity in psoriatic patients with MetS [26].
Metabolomics studies reveal a distinctive urinary metabolite signature associated with metabolic syndrome progression. Bruzzone et al. identified TMAO and sixteen other metabolites that evolve with MetS progression, providing insights into the molecular basis of the syndrome and offering potential biomarkers for early detection and monitoring [60]. Nurwanti and Bai found that both TMAO and BMI were independently associated with an increased risk of metabolic syndrome [44]. TMAO exhibited a higher odds ratio for metabolic syndrome compared to BMI, suggesting its potential as a more sensitive biomarker for metabolic syndrome risk in middle-aged and elderly adults.
While TMAO is associated with MetS, its role in atherosclerotic cardiovascular disease (ASCVD) remains under discussion. Ringel et al. found no independent association between TMAO plasma levels and stable ASCVD but identified associations with obesity and diabetes mellitus (DM), indicating a potential functional role in metabolic syndrome [45]. Additionally, Hoyles et al. demonstrated the direct interaction of microbiome-associated methylamines, including TMAO, with the blood–brain barrier (BBB), influencing cerebrovascular and cognitive function [75]. This highlights the intricate relationship between gut microbiota-derived metabolites and brain health, with implications for neurological disorders associated with metabolic syndrome.
Effects of dietary intervention on TMAO levels
The gut microbiota plays a crucial role in metabolic syndrome, influencing TMAO production and other metabolic pathways. Gao et al. demonstrated that L-carnitine supplementation modulated gut microbiota composition and attenuated high-fat diet-induced metabolic syndrome in mice, providing evidence for the therapeutic potential of L-carnitine in metabolic syndrome [76].
Thomas et al. compared the effects of egg intake and choline supplementation on TMAO formation and gut microbiota in MetS individuals, observing an increase in plasma carotenoids with no significant effect on TMAO levels but noting correlations between microbiota diversity and metabolic parameters [61]. Additionally, Franck et al. investigated the effects of red raspberry consumption on metabolic parameters and trimethylamine N-oxide (TMAO) levels [77]. Despite changes in gene expression and metabolomic profiles, raspberry supplementation did not lead to significant metabolic improvements, emphasizing the complexity of dietary interventions. Collectively, these studies underscore the role of dietary interventions and gut microbiota modulation in influencing MetS and associated complications, with TMAO emerging as a potential mediator whose precise mechanisms warrant further investigation for developing targeted therapeutic strategies.
Role of bile acids in metabolic syndrome
Bile acids, synthesized from cholesterol in the liver, undergo further modification by the gut microbiota, leading to the generation of a diverse pool of bile acid species. Dysregulated bile acid metabolism has been implicated in the pathogenesis of metabolic syndrome. Children with MetS exhibit elevated levels of total, secondary, and 12α-hydroxylated bile acids, along with deoxycholic acid, which correlates with dyslipidemia and insulin resistance markers [30]. Moreover, dysregulated bile acid profiles negatively correlate with gut bacterial diversity, potentially contributing to gut microbial dysbiosis in MetS [30].
Studies have elucidated the role of bile acid-activated receptors (BARs), such as the farnesoid-x-receptor (FXR) and G protein Bile Acid Receptor (GPBAR1), in metabolic regulation. Activation of these receptors modulates inflammatory responses and metabolic pathways, suggesting therapeutic potential in addressing metabolic disorders [78].
Interventions on bile acids for treating metabolic syndrome
Various interventions targeting bile acid metabolism have shown promise in ameliorating metabolic syndrome. For instance, dietary supplementation with xanthohumol derivatives leads to improvements in obesity and MetS parameters, partly mediated by alterations in gut microbiota composition and bile acid metabolism [79]. Similarly, caffeine treatment improves metabolic syndrome in high-fat diet-induced obese mice by alleviating insulin resistance and serum lipid disorders [80]. The underlying mechanisms involve alterations in gut microbiota composition and bile acid metabolism. Additionally, synbiotic interventions, combining beneficial bacteria like Akkermansia muciniphila with antioxidants, demonstrate efficacy in modulating gut microbiota and bile acid profiles, thereby attenuating obesity and NAFLD progression [81].
Polyamines in metabolic syndrome
Polyamines, such as putrescine, spermidine, and spermine, are organic compounds that occur naturally in many foods and are also synthesized within the human body. Although not directly produced by the gut microbiota, these compounds are influenced by microbial activities within the gut. For instance, a study demonstrated that polyamines like putrescine, derived from the gut microbiota, help promote colonic epithelial proliferation and regulate macrophage differentiation [53]. This suggests a vital role for microbial polyamines in maintaining mucosal homeostasis. Similarly, Ma et al. found that spermidine supplementation led to weight loss and improved insulin resistance in diet-induced obese mice, with these effects linked to enhanced intestinal barrier function and significant changes in the gut microbiota composition, including an increase in the SCFA-producing bacterium Lachnospiraceae NK4A136 group [51].
Further exploring the impact of gut microbiota on polyamine metabolism, Sheng et al. investigated men with metabolic syndrome and discovered alterations in gut microbial species, coupled with increased polyamine metabolism pathways. This activity was associated with elevated levels of gamma-glutamyl transpeptidase (GGT), a marker often linked with metabolic dysfunction. By modulating glutathione levels and maintaining cellular oxidative balance, GGT indirectly supports the enzymatic processes involved in polyamine synthesis and degradation in the gut [33]. Additionally, Tari Selcuk et al. conducted a study that found correlations between dietary polyamine intake and various metabolic risk parameters in postmenopausal women [54]. The intake of spermidine was found to be positively associated with increased waist circumference, systolic and diastolic blood pressure, body mass index (BMI), and waist-to-height ratio (WHtR). In contrast, the intake of spermine was negatively associated with waist circumference, systolic blood pressure, BMI, and WHtR, underscoring the significant impact of dietary sources of polyamines. These findings collectively highlight the complex interplay between dietary polyamines, gut microbiota, and the host's metabolic health, emphasizing the indirect but profound influence that gut microbiota have on polyamine levels and their broader systemic effects.
Effect of bariatric surgery on polyamine levels and metabolic syndrome improvement
Ocaña-Wilhelmi et al. investigated the impact of bariatric surgery on serum polyamine levels in morbidly obese patients with metabolic syndrome [29]. They observed a significant increase in polyamine metabolites after surgery, particularly putrescine and acetyl derivatives of spermidine and spermine. Moreover, changes in putrescine and acetyl putrescine levels were associated with the resolution of metabolic syndrome post-surgery.
Understanding the complex mechanisms involving polyamines and gut microbiota could pave the way for novel interventions targeting metabolic disorders. However, further research is warranted to elucidate underlying mechanisms and optimize therapeutic strategies.
Impact of dietary intervention on metabolic syndrome targeting polyamines
Dietary intake of polyamines, which are abundant in foods such as soy, legumes, and mushrooms, has been shown to enhance gut microbiota composition and function. A study by Vasquez et al. suggests that dietary intervention can modulate the gut microbiome and subsequently influence polyamine levels, which could improve gut health and reduce inflammation [82]. Similarly, Xiao et al. demonstrated that dietary fibers and polyamines could enrich short-chain fatty acid (SCFA)-producing bacteria and genes involved in tryptophan metabolism, further supporting the notion that diet significantly affects gut microbiota and metabolite production [83].
Tryptophan and indole derivatives in metabolic syndrome
Indole and its derivatives are metabolites produced by the gut microbiota from the amino acid tryptophan. Tryptophan metabolism produces several bioactive compounds, including indole-3-aldehyde (3-IAId), indole-3-acetic acid, indole-3-aldehyde (IAA), indole-3-lactic acid, and indole-3-propionic acid (IPA), which have shown significant cardiometabolic effects. Several studies underscore the therapeutic potential of indole derivatives.
The interplay between tryptophan metabolism and cardiovascular disease (CVD) is notable. Melhem and Taleb discussed the intricate relationship between tryptophan metabolites, particularly kynurenine pathway intermediates, and CVD pathogenesis, suggesting avenues for tailored therapeutic interventions [84]. Furthermore, indole derivatives, particularly those from gut microbiota metabolism, play crucial signaling roles. Shatova and Shestopalov elucidated the signaling functions of indole derivatives, emphasizing their role in gut-microbiota crosstalk and metabolic syndrome [85]. Su et al. highlighted the immunomodulatory effects of gut microbiota-derived tryptophan metabolites, offering insights into potential immunotherapeutic strategies [86].
Indole derivatives, such as Indole-3-aldehyde (3-IAId) and indole-3-propionic acid (IPA), play significant roles in the context of metabolic syndrome, particularly in relation to metabolic dysfunction-associated steatotic liver disease (MASLD). Studies have indicated a close relationship between MASLD and gut microbiota-derived metabolites, including indole derivatives. A study by Min et al. demonstrated that patients with hepatic steatosis exhibited decreased levels of IPA and indole-3-acetic acid (IAA) in their feces compared to healthy controls [57]. This finding is significant as it indicates a potential link between indole derivatives and the development of hepatic steatosis.
Moreover, research has shown that the administration of IPA and IAA can ameliorate hepatic steatosis and inflammation in animal models of MASLD induced by a Western diet (WD). This effect is achieved through the suppression of the NF-κB signaling pathway, which is associated with a reduction in endotoxin levels and the inactivation of macrophages [57]. Specifically, Bifidobacterium bifidum, a gut bacterium, metabolizes tryptophan to produce IAA, which effectively prevents hepatic steatosis and inflammation. Also, Alam et al. investigated the efficacy of Lysimachia candida Lindl. extract in mitigating metabolic syndrome phenotypes in rats, highlighting its ability to restore metabolic pathways crucial for glucose homeostasis and insulin sensitivity [27]. This suggests a potential therapeutic role for indole derivatives derived from the gut microbiota in the treatment of MASLD.
Effects of dietary intervention on tryptophan and indole derivatives
Dietary tryptophan, found in foods like turkey, eggs, and cheese, can be modulated through specific dietary interventions. Selective nourishment of gut microbiota with amino acids, including tryptophan, could serve as a novel prebiotic approach to improve gut health [87]. Dietary interventions with specific lipids, such as phosphatidylcholine and sphingomyelin, can modulate endogenous tryptophan metabolism and gut microbiota composition, with phosphatidylcholine showing more promise in reducing colitis symptoms and inflammation in preclinical models [88].
A polyphenol-rich diet can influence GM and enhance the production of bioactive metabolites, specifically indole derivatives from dietary tryptophan, which are linked to maintaining intestinal barrier integrity. In a randomized controlled trial with older adults, a polyphenol-rich diet significantly increased serum levels of IPA in participants with normal renal function, but not in those with impaired RF. The study found that IPA variations were associated with changes in C-reactive protein and shifts in GM composition, particularly within the Clostridiales and Enterobacteriales orders [89]. These results suggest that a polyphenol-rich diet may be beneficial for older adults with normal renal function, highlighting the importance of considering renal function when defining dietary interventions aimed at improving gut health and metabolic outcomes.
Dietary interventions and gut microbiota in metabolic syndrome
Wang et al. and Zeng et al. explore dietary interventions and their effects on metabolism and gut microbiota. Wang et al. focus on kelp-resistant starch (KRS) and its impact on intestinal morphology and function, highlighting changes in amino acid metabolism among other pathways [90, 91]. Zeng et al. investigate a citrus polymethoxyflavone-rich extract (PMFE) and its ability to alleviate metabolic syndrome through modulation of gut microbiota and regulation of branched-chain amino acid (BCAA) metabolism. Both studies underscore the intricate relationship between dietary interventions, gut microbiota, and metabolic pathways. Li and Song utilized Mendelian randomization to investigate the causal relationship between antioxidants, minerals, and vitamins with MetS traits [92]. They found associations between specific vitamins and minerals with components of MetS, providing insights into potential causal relationships.
Association between vitamins and metabolic syndrome
Several studies have investigated the association between various vitamins and metabolic syndrome (MetS) as highlighted in Table 2. Nguyen et al. found that lower intakes of vitamins B1, B2, B3, C, and A were associated with MetS, while higher serum levels of heavy metals (Pb, Hg, Cd), vitamin A, E, and hs-CRP were observed in subjects with MetS [101]. Pei et al. explored water-soluble vitamins (VC, VB9, VB12) and found negative associations with MetS, with higher quartiles of VC associated with lower MetS risk [103]. Nguyen and Kim found that vitamin B2 intake and high curry consumption were associated with reduced MetS risk in postmenopausal women [102].
Zhang et al. investigated the effects of diet-induced gut microbiota dysbiosis on spermatogenesis in a MetS sheep model. They found a notable reduction in bile acid levels, affecting vitamin A absorption, which contributed to abnormal spermatogenesis [105]. This study highlights the intricate relationship between gut microbiota, vitamin absorption, and MetS-related outcomes. Boughanem et al. explored the modulation of gut microbiota and serum vitamin D levels by a Mediterranean diet (MedDiet) intervention in obese patients with MetS [97]. They observed differences in gut microbiota composition and functionality between groups with optimal and low vitamin D levels. The study suggests a potential link between gut microbiota, vitamin D status, and metabolic pathways, emphasizing the role of dietary interventions in managing MetS. Zhang et al. investigated the effects of vitamin D supplementation on high-fat diet-induced NAFLD in rats [100]. They found that vitamin D treatment improved NAFLD by modulating gut microbiota composition and metabolites. This study suggests a potential therapeutic role for vitamin D in NAFLD through gut microbiota regulation.
Zhu et al. conducted a prospective study in a US cohort and found inverse associations between folate, vitamin B6, and vitamin B12 intakes or serum concentrations with incident MetS [96]. This study underscores the importance of B vitamin status in MetS risk reduction. Soto-Martin et al. investigated the growth requirements of butyrate-producing gut bacteria, emphasizing their dependence on dietary vitamins [106]. The study highlights the role of vitamins, particularly thiamine and folate, in supporting the growth and function of beneficial gut bacteria associated with metabolic health. Villatoro-Santos et al. explored the associations between vitamins B6, B12, and folate with MetS prevalence in Mesoamerican children and adults [107]. Their findings suggest differential associations between vitamins and MetS across age groups.
HighliKim and Kang found a dose-dependent association between high serum retinol (vitamin A) and α-tocopherol (vitamin E) levels with increased MetS risk among Korean adults [95]. Barzegar-Amini et al. reported lower serum vitamin E levels in individuals with MetS, suggesting an inverse association between vitamin E status and MetS presence [94]. Overall, these studies demonstrate the complex and varied relationships between vitamin intake, serum levels, gut microbiota, and metabolic syndrome (MetS).
Role of dietary supplements
Interventions targeting the gut microbiota and circulating metabolites offer promising avenues for metabolic syndrome management. Gholami et al. investigated the association between dietary supplement intake and MetS, while initial findings indicated significant differences in supplement consumption among those with MetS, the relationship was not significant after adjusting for covariates in the multivariate regression model [108]. Cano-Ibáñez et al. found that despite being overweight, participants with MetS exhibited suboptimal nutrient intake, particularly among men, with nutrient density positively associated with female sex, higher education, better Mediterranean diet adherence, non-smoking, and active lifestyles [109]. Qiu et al. and Zhang et al. demonstrated the therapeutic potential of traditional Chinese herbal medicine and prebiotic supplementation in ameliorating metabolic disorders [110, 111]. Additionally, Alhamoud et al. highlighted the anti-obesity effects of 6-gingerol through microbiota modulation and lipid metabolism alterations [112].
These studies collectively underscore the complex interplay between dietary nutrients, serum vitamin levels, and metabolic health outcomes like MetS and obesity. While some nutrients demonstrate protective effects against MetS, others may increase risk factors. Understanding these associations can inform dietary recommendations and intervention strategies for improving metabolic health.
Metformin's role in gut health and metabolic syndrome management
Metformin plays a pivotal role in managing metabolic syndrome, demonstrating its crucial importance in improving not only glucose metabolism but also broader aspects of metabolic health. Metformin positively impacts gut microbiota, enhancing its therapeutic effects in treating metabolic syndrome (MetS). It promotes the growth of short-chain fatty acid (SCFA) producing bacteria, including Blautia, Bacteroides, Butyricoccus, Bifidobacterium, Prevotella, Megasphaera, and Butyrivibrio, leading to increased fecal concentrations of lactate and succinate [113, 114]. Metformin also boosts mucin-degrading bacteria like Akkermansia muciniphila, which improves glucose metabolism by regulating gut permeability, reducing lipopolysaccharides (LPS), and enhancing postprandial insulin secretion through GLP-1 interactions [114, 115].
Further studies highlight metformin’s influence on the gut microbiome and its role in improving metabolic health. Metformin administration impacts metabolic syndrome by modulating the gut microbiota, leading to changes in microbial communities such as Prevotellaceae, Rikenellaceae, and Clostridiales, which may influence glucose metabolism, lipid profiles, and overall gut health, helping to distinguish MetS from type 2 diabetes mellitus (T2DM) and highlighting the unique microbiome signatures associated with each condition [116]. In accordance with the findings of Lee et al., metformin regulates metabolic syndrome by modulating the gut microbiome, which enhances glucose metabolism, increases short-chain fatty acids, strengthens intestinal permeability against lipopolysaccharides, modulates the immune response, and interacts with bile acids [117].
Additionally, metformin may offer cardiovascular benefits by reducing trimethylamine N-oxide (TMAO) concentrations and its precursor metabolites, which are linked to cardiovascular diseases [118]. Overall, metformin enhances gut and cardiovascular health by modulating the gut microbiota, increasing beneficial bacterial species, and reducing harmful metabolites. Furthermore, Ahmadi et al. provide evidence that metformin reduces aging-related microbiome dysbiosis and improves cognitive function by modulating the gut microbiome/goblet cell/mucin axis [119]. This multifaceted influence underscores metformin’s potential beyond glucose regulation, suggesting it can also play a significant role in overall metabolic health, inflammation reduction, and the maintenance of gut integrity. These findings highlight the importance of understanding the gut microbiome’s role in therapeutic interventions and open avenues for future research on metformin's broader health benefits.
Therapeutic potential of probiotics, prebiotics, and synbiotics in metabolic syndrome and associated metabolic disorders
The complex interplay between the composition of gut microbiota, the metabolites they produce, and the body’s physiological processes significantly influences the development of metabolic syndrome (MetS). Among the array of therapeutic strategies, probiotics, prebiotics, and synbiotics have emerged as promising interventions for modulating gut microbiota and improving metabolic health.
Probiotics, live microorganisms with health benefits when consumed adequately, play a pivotal role in managing MetS. They foster the growth of beneficial bacteria to restore microbial balance. This restoration of microbial balance has been linked to reductions in obesity-related MetS, insulin resistance, dyslipidemia, and inflammation. Notably, probiotics exert their effects by influencing various metabolic pathways beyond SCFA production. They modulate bile acid composition, polyamine metabolism, amino acid metabolism, and indole derivative production, thereby impacting metabolic function [53, 120, 121].
Recent studies have expanded on these findings, exploring the potential of fecal microbiota transplantation (FMT) as an innovative approach to treating metabolic syndrome and associated metabolic disorders. For instance, a randomized controlled trial demonstrated that FMT combined with low-fermentable fiber supplementation significantly improved insulin sensitivity in patients with severe obesity and metabolic syndrome [122]. Similarly, another study found that FMT capsules derived from lean donors led to sustained changes in the gut microbiome of obese patients, although it did not result in significant weight loss [123]. Moreover, in adolescents with obesity, FMT did not affect BMI but showed promising reductions in abdominal adiposity [124]. Further emphasizing the potential of FMT, a trial in obese subjects with type 2 diabetes demonstrated enhanced microbiota engraftment and improved metabolic parameters following repeated FMT treatments [125].
Prebiotics, non-digestible fibers that selectively nourish beneficial gut bacteria, offer another avenue for MetS management. By serving as substrates for SCFA production, prebiotics regulate glucose and lipid metabolism while modulating gut microbiota composition [19]. Dietary interventions enriched with prebiotics have demonstrated efficacy in improving metabolic parameters associated with MetS [37]. Furthermore, prebiotics contribute to metabolic health by promoting bile acid homeostasis, polyamine balance, amino acid metabolism, and indole production through their influence on gut microbiota [51, 58, 126, 127].
Synbiotics, which combine probiotics and prebiotics, offer an integrated approach to enhancing gut microbiota and metabolic health. By providing a conducive environment for probiotic proliferation, prebiotics augment the efficacy of probiotic supplementation in MetS management [39]. This synergistic effect addresses dysbiosis-induced metabolic dysfunction comprehensively. For instance, research by Ouyang et al. underscores the therapeutic potential of synbiotics in alleviating small intestinal bacterial overgrowth and improving lipid metabolism, particularly in pregnant women with subclinical hypothyroidism [128].
Probiotics, prebiotics, and synbiotics represent promising strategies for managing MetS by modulating gut microbiota composition and influencing various metabolic pathways. Their multifaceted effects underscore their potential as holistic approaches to metabolic health.
Conclusion and future directions
In conclusion, the gut microbiota and microbiota-derived metabolites represent key players in the pathogenesis of metabolic syndrome and associated disorders. Understanding the complex interplay between GM composition, microbial metabolites, and host physiology holds promise for the development of personalized therapeutic interventions and non-invasive diagnostic tools for metabolic disorders. However, addressing challenges such as methodological heterogeneity and the need for translational research is essential for advancing our understanding and clinical management of MetS.
Probiotics, prebiotics, and synbiotics represent promising therapeutic approaches for managing metabolic syndrome by modulating gut microbiota composition and microbial metabolite production. These interventions target the underlying dysbiosis and metabolic dysfunction associated with MetS, offering potential avenues for personalized therapy. Future research should focus on elucidating the mechanistic links between gut microbiota, metabolites, and MetS pathogenesis to optimize therapeutic strategies and develop non-invasive diagnostic tools.
Future research efforts should focus on elucidating the specific mechanisms by which gut microbiota and microbiota-derived metabolites influence MetS development and progression. Additionally, well-designed, controlled trials are needed to evaluate the efficacy of therapeutic interventions targeting GM composition and metabolite profiles in MetS management. Translational research aiming to leverage stool metabolites for non-invasive diagnostics and personalized therapeutic interventions represents a promising avenue for future investigation. Moreover, integrating omics technologies and systems biology approaches may provide deeper insights into the complex interplay between diet, microbiome, and cardiometabolic diseases, paving the way for precision medicine in MetS management.
Availability of data and materials
All data in this study are included in this study.
Abbreviations
- MetS:
-
Metabolic syndrome
- CVD:
-
Cardiovascular disease
- T2DM:
-
Type 2 diabetes mellitus
- GM:
-
Gut microbiota
- SCFA:
-
Short-chain fatty acid
- TMAO:
-
Trimethylamine N-oxide
- FMT:
-
Fecal microbiota transplantation
- DF:
-
Dietary fiber
- MLE:
-
Mulberry leaf extract
- AMPK:
-
AMP-Activated protein kinase
- ASCVD:
-
Atherosclerotic cardiovascular disease
- DM:
-
Diabetes mellitus
- BBB:
-
Blood-brain barrier
- FXR:
-
Farnesoid X receptor
- GPBAR1:
-
G protein bile acid receptor 1
- TGR5:
-
Takeda G-protein-coupled receptor 5
- NAFLD:
-
Non-alcoholic fatty liver disease
- BMI:
-
Body mass index
- BCAA:
-
Branched-chain amino acid
- GGT:
-
Gamma-glutamyl transpeptidase
- IAA:
-
Indole-3-acetic acid
- IPA:
-
Indole-3-propionic acid
- KRS:
-
Kelp-resistant starch
- MASLD:
-
Metabolic dysfunction-associated steatotic liver disease
- MedDiet:
-
Mediterranean diet
- NF-κB:
-
Nuclear factor kappa-light-chain-enhancer of activated B cells
- PMFE:
-
Polymethoxyflavone-rich extract
- 3-IAId:
-
Indole-3-aldehyde
- WD:
-
Western diet
- WHtR:
-
Waist-to-height ratio
References
Adil SO, Islam MA, Musa KI, Shafique K (2023) Prevalence of metabolic syndrome among apparently healthy adult population in Pakistan: a systematic review and meta-analysis. In: Healthcare. MDPI; p. 531. Available from: https://www.mdpi.com/2227-9032/11/4/531 Cited 2024 Jun 17
Bahar A, Kashi Z, Kheradmand M, Hedayatizadeh-Omran A, Moradinazar M, Ramezani F, et al. Prevalence of metabolic syndrome using international diabetes federation, National Cholesterol Education Panel- Adult Treatment Panel III and Iranian criteria: results of Tabari cohort study. J Diabetes Metab Disord. 2020 Jun;19(1):205–11. Available from: http://link.springer.com/10.1007/s40200-020-00492-6. Cited 17 Jun 2024
Belete R, Ataro Z, Abdu A, Sheleme M. Global prevalence of metabolic syndrome among patients with type I diabetes mellitus: a systematic review and meta-analysis. Diabetol Metab Syndr. 2021 Dec;13(1):25. Available from: https://dmsjournal.biomedcentral.com/articles/10.1186/s13098-021-00641-8. Cited 17 Jun 2024
Bowo-Ngandji A, Kenmoe S, Ebogo-Belobo JT, Kenfack-Momo R, Takuissu GR, Kengne-Ndé C, et al. Prevalence of the metabolic syndrome in African populations: A systematic review and meta-analysis. PLoS One. 2023;18(7):e0289155. Available from: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0289155. Cited 17 Jun 2024
do Vale Moreira NC, Hussain A, Bhowmik B, Mdala I, Siddiquee T, Fernandes VO, et al (2020) Prevalence of Metabolic Syndrome by different definitions, and its association with type 2 diabetes, pre-diabetes, and cardiovascular disease risk in Brazil. Diabetes Metab Syndr Clin Res Rev. 14(5):1217–24. Available from: https://www.sciencedirect.com/science/article/pii/S1871402120301612. Cited 17 Jun 2024
Farmanfarma KK, Kaykhaei MA, Mohammadi M, Adineh HA, Ansari-Moghaddam A (2020) The prevalence and trend of metabolic syndrome in the South-East of Iran. J Med Life. 13(4):587. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7803315/. Cited 17 Jun 2024
Haverinen E, Paalanen L, Palmieri L, Padron-Monedero A, Noguer-Zambrano I, Sarmiento Suárez R, et al. Comparison of metabolic syndrome prevalence using four different definitions – a population-based study in Finland. Arch Public Health. 2021 Dec;79(1):231. Available from: https://archpublichealth.biomedcentral.com/articles/10.1186/s13690-021-00749-3. Cited 17 Jun 2024
Krishnamoorthy Y, Rajaa S, Murali S, Rehman T, Sahoo J, Kar SS. Prevalence of metabolic syndrome among adult population in India: a systematic review and meta-analysis. PLoS One. 2020;15(10):e0240971. Available from: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0240971. Cited 17 Jun 2024
Liang X, Or B, Tsoi MF, Cheung CL, Cheung BM. Prevalence of metabolic syndrome in the United States National Health and Nutrition Examination Survey 2011–18. Postgrad Med J. 2023;99(1175):985–92. Available from: https://academic.oup.com/pmj/article-abstract/99/1175/985/7076129. Cited 17 Jun 2024
Noubiap JJ, Nansseu JR, Lontchi-Yimagou E, Nkeck JR, Nyaga UF, Ngouo AT, et al. Geographic distribution of metabolic syndrome and its components in the general adult population: A meta-analysis of global data from 28 million individuals. Diabetes Res Clin Pract. 2022;188:109924. Available from: https://www.sciencedirect.com/science/article/pii/S0168822722007380. Cited 17 Jun 2024
Oliveira LVA. dos, SBNS, Machado, ÍE, Malta, DC, Velasquez-Melendez, G, and Felisbino-Mendes, MS. Prevalence of the metabolic syndrome and its components in the Brazilian adult population. Ciênc Saúde Coletiva. 2020;25:4269–80.
Ortiz-Rodríguez MA, Bautista-Ortiz LF, Villa AR, Antúnez-Bautista PK, Aldaz-Rodríguez MV, Estrada-Luna D, et al. Prevalence of metabolic syndrome among Mexican adults. Metab Syndr Relat Disord. 2022;20(5):264–72. Available from: https://www.liebertpub.com/doi/abs/10.1089/met.2021.0115. Cited 17 Jun 2024
Reisinger C, Nkeh-Chungag BN, Fredriksen PM, Goswami N. The prevalence of pediatric metabolic syndrome—A critical look on the discrepancies between definitions and its clinical importance. Int J Obes. 2021;45(1):12–24. Available from: https://www.nature.com/articles/s41366-020-00713-1. Cited 17 Jun 2024
Sigit FS, Tahapary DL, Trompet S, Sartono E, Willems Van Dijk K, Rosendaal FR, et al. The prevalence of metabolic syndrome and its association with body fat distribution in middle-aged individuals from Indonesia and the Netherlands: a cross-sectional analysis of two population-based studies. Diabetol Metab Syndr. 2020 Dec;12(1):2. Available from: https://dmsjournal.biomedcentral.com/articles/10.1186/s13098-019-0503-1. Cited 17 Jun 2024
Yao F, Bo Y, Zhao L, Li Y, Ju L, Fang H, et al. Prevalence and influencing factors of metabolic syndrome among adults in China from 2015 to 2017. Nutrients. 2021;13(12):4475. Available from: https://www.mdpi.com/2072-6643/13/12/4475. Cited 17 Jun 2024
Gradisteanu Pircalabioru G, Ilie I, Oprea L, Picu A, Petcu LM, Burlibasa L, et al. Microbiome, mycobiome and related metabolites alterations in patients with metabolic syndrome—A pilot study. Metabolites. 2022;12(3):218. Available from: https://www.mdpi.com/2218-1989/12/3/218. Cited 17 Jun 2024
Ansari MHR, Saher S, Parveen R, Khan W, Khan IA, Ahmad S. Role of gut microbiota metabolism and biotransformation on dietary natural products to human health implications with special reference to biochemoinformatics approach. J Tradit Complement Med. 2023;13(2):150–60. Available from: https://www.sciencedirect.com/science/article/pii/S2225411022000323. Cited 17 Jun 2024
Aoki R, Onuki M, Hattori K, Ito M, Yamada T, Kamikado K, et al. Commensal microbe-derived acetate suppresses NAFLD/NASH development via hepatic FFAR2 signalling in mice. Microbiome. 2021 Dec ;9(1):188. Available from: https://microbiomejournal.biomedcentral.com/articles/10.1186/s40168-021-01125-7. Cited 17 Jun 2024
Du L, Lü H, Chen Y, Yu X, Jian T, Zhao H, et al. Blueberry and blackberry anthocyanins ameliorate metabolic syndrome by modulating gut microbiota and short-chain fatty acids metabolism in high-fat diet-fed C57BL/6J mice. J Agric Food Chem. 2023 Oct 11;71(40):14649–65. Available from: https://pubs.acs.org/doi/10.1021/acs.jafc.3c04606. Cited 17 Jun 2024
Xu Y, Curtasu MV, Bendiks Z, Marco ML, Nørskov NP, Knudsen KEB, et al. Effects of dietary fibre and protein content on intestinal fibre degradation, short-chain fatty acid and microbiota composition in a high-fat fructose-rich diet induced obese Göttingen Minipig model. Food Funct. 2020;11(12):10758–73. Available from: https://pubs.rsc.org/en/content/articlehtml/2020/fo/d0fo02252g. Cited 17 Jun 2024
Chen AS, Liu DH, Hou HN, Yao JN, Xiao SC, Ma XR, et al. Dietary pattern interfered with the impacts of pesticide exposure by regulating the bioavailability and gut microbiota. Sci Total Environ. 2023;858:159936. Available from: https://www.sciencedirect.com/science/article/pii/S004896972207036X. Cited 17 Jun 2024
Kimura I, Miyamoto J, Ohue-Kitano R, Watanabe K, Yamada T, Onuki M, et al. Maternal gut microbiota in pregnancy influences offspring metabolic phenotype in mice. Science. 2020 Feb 28;367(6481):eaaw8429. Available from: https://www.science.org/doi/10.1126/science.aaw8429. Cited 17 Jun 2024
Wu X, Unno T, Kang S, Park S. A Korean-Style Balanced Diet Has a Potential connection with Ruminococcaceae enterotype and reduction of metabolic syndrome incidence in Korean adults. Nutrients. 2021;13(2):495. Available from: https://www.mdpi.com/2072-6643/13/2/495. Cited 17 Jun 2024
Kuo CH, Liu CH, Wang JH, Hsu BG. Serum trimethylamine N-oxide levels correlate with metabolic syndrome in coronary artery disease patients. Int J Environ Res Public Health. 2022;19(14):8710. Available from: https://www.mdpi.com/1660-4601/19/14/8710. Cited 17 Jun 2024
Mirzababaei A, Mahmoodi M, Keshtkar A, Ashraf H, Abaj F, Soveid N, et al. Serum levels of trimethylamine N-oxide and kynurenine novel biomarkers are associated with adult metabolic syndrome and its components: a case-control study from the TEC cohort. Front Nutr. 2024;11:1326782. Available from: https://www.frontiersin.org/articles/10.3389/fnut.2024.1326782/full. Cited 17 Jun 2024
Sun L, Guo X, Qin Y, Li P, Yu C, Gao X, et al. Serum intestinal metabolites are raised in patients with psoriasis and metabolic syndrome. Clin Cosmet Investig Dermatol. 2022 May;Volume 15:879–86. Available from: https://www.dovepress.com/serum-intestinal-metabolites-are-raised-in-patients-with-psoriasis-and-peer-reviewed-fulltext-article-CCID. Cited 17 Jun 2024
Alam MJ, Kamboj P, Sarkar S, Gupta SK, Kasarla SS, Bajpai S, et al. Untargeted metabolomics and phenotype data indicate the therapeutic and prophylactic potential of Lysimachia candida Lindl. towards high-fat high-fructose-induced metabolic syndrome in rats. Mol Omics. 2023;19(10):787–99. Available from: https://pubs.rsc.org/en/content/articlehtml/2023/mo/d3mo00104k. Cited 17 Jun 2024
Chen H, Li Y, Yi P, Cao H, Wang Q, Zhao X. Dietary interventions of salmon and silver carp phospholipids on mice with metabolic syndrome based on lipidomics. Cells. 2022;11(20):3199. Available from: https://www.mdpi.com/2073-4409/11/20/3199. Cited 17 Jun 2024
Ocaña-Wilhelmi L, Cardona F, Garrido-Sanchez L, Fernandez-Garcia D, Tinahones FJ, Ramos-Molina B. Change in serum polyamine metabolome pattern after bariatric surgery in obese patients with metabolic syndrome. Surg Obes Relat Dis. 2020;16(2):306–11. Available from: https://www.sciencedirect.com/science/article/pii/S1550728919310780. Cited 17 Jun 2024
Mancera‐Hurtado Y, Lopez‐Contreras BE, Flores‐Lopez R, Villamil‐Ramirez H, Del Rio‐Navarro BE, Canizales‐Quinteros S, et al. A dysregulated bile acids pool is associated with metabolic syndrome and gut microbial dysbiosis in early adolescence. Obesity. 2023 Aug;31(8):2129–38. Available from: https://onlinelibrary.wiley.com/doi/10.1002/oby.23797. Cited 17 Jun 2024
Ávila-Román J, Arreaza-Gil V, Cortés-Espinar AJ, Soliz-Rueda JR, Mulero M, Muguerza B, et al. Impact of gut microbiota on plasma oxylipins profile under healthy and obesogenic conditions. Clin Nutr. 2021;40(4):1475–86. Available from: https://www.sciencedirect.com/science/article/pii/S0261561421001230. Cited 17 Jun 2024
Mirzaei S, DeVon HA, Cantor RM, Cupido AJ, Pan C, Ha SM, et al. Relationships and mendelian randomization of gut microbe-derived metabolites with metabolic syndrome traits in the METSIM cohort. Metabolites. 2024;14(3):174. Available from: https://www.mdpi.com/2218-1989/14/3/174. Cited 17 Jun 2024
Sheng S, Yan S, Chen J, Zhang Y, Wang Y, Qin Q, et al. Gut microbiome is associated with metabolic syndrome accompanied by elevated gamma-glutamyl transpeptidase in men. Front Cell Infect Microbiol. 2022;12:946757. Available from: https://www.frontiersin.org/articles/10.3389/fcimb.2022.946757/full. Cited 17 Jun 2024
Capel F, Bongard V, Malpuech-Brugère C, Karoly E, Michelotti GA, Rigaudière JP, et al. Metabolomics reveals plausible interactive effects between dairy product consumption and metabolic syndrome in humans. Clin Nutr. 2020;39(5):1497–509. Available from: https://www.sciencedirect.com/science/article/pii/S0261561419302687. Cited 17 Jun 2024
Qin Q, Yan S, Yang Y, Chen J, Li T, Gao X, et al. A metagenome-wide association study of the gut microbiome and metabolic syndrome. Front Microbiol. 2021 Jul 16;12:682721. Available from: https://www.frontiersin.org/articles/10.3389/fmicb.2021.682721/full cited 2024 Jun 17
Yan H, Qin Q, Chen J, Yan S, Li T, Gao X, et al. Gut microbiome alterations in patients with visceral obesity based on quantitative computed tomography. Front Cell Infect Microbiol. 2022;11:823262. Available from: https://www.frontiersin.org/articles/10.3389/fcimb.2021.823262/full. Cited 17 Jun 2024
de Groot P, Scheithauer T, Bakker GJ, Prodan A, Levin E, Khan MT, et al. Donor metabolic characteristics drive effects of faecal microbiota transplantation on recipient insulin sensitivity, energy expenditure and intestinal transit time. Gut. 2020;69(3):502–12. Available from: https://gut.bmj.com/content/69/3/502.short. Cited 17 Jun 2024
Hernández-Flores T de J, Pedraza-Brindis EJ, Cárdenas-Bedoya J, Ruíz-Carrillo JD, Méndez-Clemente AS, Martínez-Guzmán MA, et al. Role of micronutrients and gut microbiota-derived metabolites in COVID-19 recovery. Int J Mol Sci. 2022 Oct 14;23(20):12324. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9604189/. Cited 17 Jun 2024
Pi X, Teng W, Fei D, Zhao G, Liu W. Effects of live combined Bacillus subtilis and Enterococcus faecium on gut microbiota composition in C57BL/6 mice and in humans. Front Cell Infect Microbiol. 2022;12:821662. Available from: https://www.frontiersin.org/articles/10.3389/fcimb.2022.821662/full cited 2024 Jun 17
Portincasa P, Bonfrate L, Vacca M, De Angelis M, Farella I, Lanza E, et al. Gut microbiota and short chain fatty acids: implications in glucose homeostasis. Int J Mol Sci. 2022 Jan;23(3):1105. Available from: https://www.mdpi.com/1422-0067/23/3/1105. Cited 17 Jun 2024
Day-Walsh P, Shehata E, Saha S, Savva GM, Nemeckova B, Speranza J, et al. The use of an in-vitro batch fermentation (human colon) model for investigating mechanisms of TMA production from choline, l-carnitine and related precursors by the human gut microbiota. Eur J Nutr. 2021 Oct 1;60(7):3987–99. Available from: https://doi.org/10.1007/s00394-021-02572-6. Cited 17 Jun 2024
Gatarek P, Kaluzna-Czaplinska J. Trimethylamine N-oxide (TMAO) in human health. EXCLI J. 2021 Feb 11;20:301–19. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7975634/. Cited 17 Jun 2024
Johri AM, Hétu MF, Heyland DK, Herr JE, Korol J, Froese S, et al. Progression of atherosclerosis with carnitine supplementation: a randomized controlled trial in the metabolic syndrome. Nutr Metab. 2022 Dec;19(1):26. Available from: https://nutritionandmetabolism.biomedcentral.com/articles/10.1186/s12986-022-00661-9. Cited 17 Jun 2024
Nurwanti E, Bai CH. Increased gut microbiota-dependent trimethylamine-N-oxide and body mass index associated with metabolic syndrome risk in a community-dwelling elders. Curr Dev Nutr. 2021;5:171. Available from: https://www.sciencedirect.com/science/article/pii/S2475299123106895 cited 2024 Jun 17
Ringel C, Dittrich J, Gaudl A, Schellong P, Beuchel CF, Baber R, et al. Association of plasma trimethylamine N-oxide levels with atherosclerotic cardiovascular disease and factors of the metabolic syndrome. Atherosclerosis. 2021;335:62–7. Available from: https://www.sciencedirect.com/science/article/pii/S0021915021013708. Cited 17 Jun 2024
Vu V, Kim YM, Cho M. Effects of SCFAs and TMAO on non-alcoholic fatty liver disease indicating the therapeutic benefits of plant-based diet, and supplemental prebiotics, probiotics and synbiotics. Appl Biol Chem. 2023 Feb 17;66(1):11. Available from: https://applbiolchem.springeropen.com/articles/10.1186/s13765-022-00755-1. Cited 17 Jun 2024
Collins SL, Stine JG, Bisanz JE, Okafor CD, Patterson AD. Bile acids and the gut microbiota: metabolic interactions and impacts on disease. Nat Rev Microbiol. 2023 Apr;21(4):236–47. Available from: https://www.nature.com/articles/s41579-022-00805-x. Cited 17 Jun 2024
A Mukherjee C Lordan RP Ross PD Cotter Gut microbes from the phylogenetically diverse genus Eubacterium and their various contributions to gut health. Gut Microbes. 2020 Nov 9;12(1):1802866 Available from: https://doi.org/10.1080/19490976.2020.1802866. Cited 17 Jun 2024
Pushpass RAG, Alzoufairi S, Jackson KG, Lovegrove JA. Circulating bile acids as a link between the gut microbiota and cardiovascular health: impact of prebiotics, probiotics and polyphenol-rich foods. Nutr Res Rev. 2022 Dec;35(2):161–80. Available from: https://www.cambridge.org/core/journals/nutrition-research-reviews/article/circulating-bile-acids-as-a-link-between-the-gut-microbiota-and-cardiovascular-health-impact-of-prebiotics-probiotics-and-polyphenolrich-foods/F8FA2A5B2F01954F445D15C22F12BDFB. Cited 17 Jun 2024
Xue R, Su L, Lai S, Wang Y, Zhao D, Fan J, et al. Bile acid receptors and the gut–liver axis in nonalcoholic fatty liver disease. Cells. 2021 Nov;10(11):2806. Available from: https://www.mdpi.com/2073-4409/10/11/2806. Cited 17 Jun 2024
Ma L, Ni Y, Wang Z, Tu W, Ni L, Zhuge F, et al. Spermidine improves gut barrier integrity and gut microbiota function in diet-induced obese mice. Gut Microbes. 2020 Nov 9;12(1):1832857. Available from: https://www.tandfonline.com/doi/full/10.1080/19490976.2020.1832857. Cited 17 Jun 2024
Madella AM, Van Bergenhenegouwen J, Garssen J, Masereeuw R, Overbeek SA. Microbial-derived tryptophan catabolites, kidney disease and gut inflammation. Toxins. 2022 Sep;14(9):645. Available from: https://www.mdpi.com/2072-6651/14/9/645. Cited 17 Jun 2024
Nakamura A, Kurihara S, Takahashi D, Ohashi W, Nakamura Y, Kimura S, et al. Symbiotic polyamine metabolism regulates epithelial proliferation and macrophage differentiation in the colon. Nat Commun. 2021;12(1):2105. Available from: https://www.nature.com/articles/s41467-021-22212-1. Cited 17 Jun 2024
Tari Selcuk K, Atan RM, Arslan S, Dal N, Sahin K. The relationship between dietary polyamine levels, metabolic risk parameters and anthropometric measurements in postmenopausal women. Nutr Food Sci . 2024; Available from: https://www.emerald.com/insight/content/doi/10.1108/NFS-11-2023-0248/full/html. Cited 17 Jun 2024
Huang Z, Boekhorst J, Fogliano V, Capuano E, Wells JM. Impact of high-fiber or high-protein diet on the capacity of human gut microbiota to produce tryptophan catabolites. J Agric Food Chem. 2023 May 10;71(18):6956–66. Available from: https://pubs.acs.org/doi/10.1021/acs.jafc.2c08953. Cited 17 Jun 2024
Ma X, Yang J, Yang G, Li L, Hao X, Wang G, et al. A tryptophan metabolite of the microbiota improves neovascularization in diabetic limb ischemia. Front Cardiovasc Med. 2022;9:910323. Available from: https://www.frontiersin.org/articles/10.3389/fcvm.2022.910323/full. Cited 17 Jun 2024
Min BH, Devi S, Kwon GH, Gupta H, Jeong JJ, Sharma SP, et al. Gut microbiota-derived indole compounds attenuate metabolic dysfunction-associated steatotic liver disease by improving fat metabolism and inflammation. Gut Microbes. 2024 Dec 31;16(1):2307568. Available from: https://www.tandfonline.com/doi/full/10.1080/19490976.2024.2307568. Cited 17 Jun 2024
Wrzosek L, Ciocan D, Hugot C, Spatz M, Dupeux M, Houron C, et al. Microbiota tryptophan metabolism induces aryl hydrocarbon receptor activation and improves alcohol-induced liver injury. Gut. 2021;70(7):1299–308. Available from: https://gut.bmj.com/content/70/7/1299.abstract. Cited 17 Jun 2024
Xavier-Santos D, Bedani R, Lima ED, Saad SMI. Impact of probiotics and prebiotics targeting metabolic syndrome. J Funct Foods. 2020 Jan 1 ;64:103666. Available from: https://www.sciencedirect.com/science/article/pii/S1756464619305900. Cited 17 Jun 2024
Bruzzone C, Gil-Redondo R, Seco M, Barragán R, De La Cruz L, Cannet C, et al. A molecular signature for the metabolic syndrome by urine metabolomics. Cardiovasc Diabetol. 2021 Dec;20(1):155. Available from: https://cardiab.biomedcentral.com/articles/10.1186/s12933-021-01349-9. Cited 17 Jun 2024
Thomas MS, DiBella M, Blesso CN, Malysheva O, Caudill M, Sholola M, et al. Comparison between egg intake versus choline supplementation on gut microbiota and plasma carotenoids in subjects with metabolic syndrome. Nutrients. 2022;14(6):1179. Available from: https://www.mdpi.com/2072-6643/14/6/1179. Cited 17 Jun 2024
Navarrete-Muñoz EM, Vioque J, Toledo E, Oncina-Canovas A, Martínez-González MÁ, Salas-Salvadó J, et al. Dietary folate intake and metabolic syndrome in participants of PREDIMED-Plus study: a cross-sectional study. Eur J Nutr. 2021 Mar;60(2):1125–36. Available from: https://link.springer.com/10.1007/s00394-020-02364-4. Cited 17 Jun 2024
Vajdi M, Farhangi MA, Nikniaz L. Diet-derived nutrient patterns and components of metabolic syndrome: a cross-sectional community- based study. BMC Endocr Disord. 2020 Dec;20(1):69. Available from: https://bmcendocrdisord.biomedcentral.com/articles/10.1186/s12902-020-0547-0. Cited 17 Jun 2024
Ganesan R, Gupta H, Jeong JJ, Sharma SP, Won SM, Oh KK, et al. A metabolomics approach to the validation of predictive metabolites and phenotypic expression in non-alcoholic fatty liver disease. Life Sci. 2023;322:121626. Available from: https://www.sciencedirect.com/science/article/pii/S0024320523002606. Cited 17 Jun 2024
Zhang Q, Hu W mu, Deng Y ling, Wan J jing, Wang Y jun, Jin P. Dysbiosis of gut microbiota and decreased propionic acid associated with metabolic abnormality in Cushing’s syndrome. Front Endocrinol. 2023;13:1095438. Available from: https://www.frontiersin.org/articles/10.3389/fendo.2022.1095438/full. Cited 17 Jun 2024
Nogal A, Louca P, Zhang X, Wells PM, Steves CJ, Spector TD, et al. Circulating levels of the short-chain fatty acid acetate mediate the effect of the gut microbiome on visceral fat. Front Microbiol. 2021;12:711359. Available from: https://www.frontiersin.org/articles/10.3389/fmicb.2021.711359/full. Cited 17 Jun 2024
Rosés C, Garcia-Ibañez P, Agudelo A, Viadel B, Tomás-Cobos L, Gallego E, et al. Effects of glucosinolate-enriched red radish ( Raphanus sativus ) on in vitro models of intestinal microbiota and metabolic syndrome-related functionalities. ACS Omega. 2023 Jul 4;8(26):23373–88. Available from: https://pubs.acs.org/doi/10.1021/acsomega.2c08128.Cited 17 Jun 2024
Wei G, Ye Y, Yan X, Chao X, Yang F, Wang M, et al. Effect of banana pulp dietary fibers on metabolic syndrome and gut microbiota diversity in high‐fat diet mice. J Food Biochem. 2020 Sep;44(9). Available from: https://onlinelibrary.wiley.com/doi/10.1111/jfbc.13362. Cited 17 Jun 2024
Du J, Zhang P, Luo J, Shen L, Zhang S, Gu H, et al. Dietary betaine prevents obesity through gut microbiota-drived microRNA-378a family. Gut Microbes. 2021 Jan 1;13(1):1862612. Available from: https://www.tandfonline.com/doi/full/10.1080/19490976.2020.1862612. Cited 17 Jun 2024
Li Y, Xia D, Chen J, Zhang X, Wang H, Huang L, et al. Dietary fibers with different viscosity regulate lipid metabolism via ampk pathway: roles of gut microbiota and short-chain fatty acid. Poult Sci. 2022;101(4):101742. Available from: https://www.sciencedirect.com/science/article/pii/S0032579122000475. Cited 17 Jun 2024
He L, Xing Y, Ren X, Zheng M, Yu S, Wang Y, et al. Mulberry leaf extract improves metabolic syndrome by alleviating lipid accumulation in vitro and in vivo. Molecules. 2022;27(16):5111. Available from: https://www.mdpi.com/1420-3049/27/16/5111. Cited 17 Jun 2024
Maurer Sost M, Stevens Y, Salden B, Troost F, Masclee A, Venema K. Citrus extract high in flavonoids beneficially alters intestinal metabolic responses in subjects with features of metabolic syndrome. Foods. 2023;12(18):3413. Available from: https://www.mdpi.com/2304-8158/12/18/3413. cited 2024 Jun 17
Li Z, Zhou E, Liu C, Wicks H, Yildiz S, Razack F, et al. Dietary butyrate ameliorates metabolic health associated with selective proliferation of gut Lachnospiraceae bacterium 28–4. JCI Insight. 2023;8(4). Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9977501/. Cited 17 Jun 2024
Wang G, Zhu G, Chen C, Zheng Y, Ma F, Zhao J, et al. Lactobacillus strains derived from human gut ameliorate metabolic disorders via modulation of gut microbiota composition and short-chain fatty acids metabolism. Benef Microbes. 2021 Jun 15;12(3):267–82. Available from: https://brill.com/view/journals/bm/12/3/article-p267_7.xml. Cited 17 Jun 2024
Hoyles L, Pontifex MG, Rodriguez-Ramiro I, Anis-Alavi MA, Jelane KS, Snelling T, et al. Regulation of blood–brain barrier integrity by microbiome-associated methylamines and cognition by trimethylamine N-oxide. Microbiome. 2021 Dec;9(1):235. Available from: https://microbiomejournal.biomedcentral.com/articles/10.1186/s40168-021-01181-z. Cited 17 Jun 2024
Gao X, Sun C, Zhang Y, Hu S, Li D. Dietary supplementation of l-carnitine ameliorates metabolic syndrome independent of trimethylamine N-oxide produced by gut microbes in high-fat diet-induced obese mice. Food Funct. 2022;13(23):12039–50. Available from: https://pubs.rsc.org/en/content/articlehtml/2022/fo/d2fo02570a. Cited 17 Jun 2024
Franck M, de Toro-Martín J, Garneau V, Guay V, Kearney M, Pilon G, et al. Effects of daily raspberry consumption on immune-metabolic health in subjects at risk of metabolic syndrome: a randomized controlled trial. Nutrients. 2020;12(12):3858. Available from: https://www.mdpi.com/2072-6643/12/12/3858. Cited 17 Jun 2024
Fiorucci S, Baldoni M, Ricci P, Zampella A, Distrutti E, Biagioli M. Bile acid-activated receptors and the regulation of macrophages function in metabolic disorders. Curr Opin Pharmacol. 2020;53:45–54. Available from: https://www.sciencedirect.com/science/article/pii/S1471489220300175.Cited 17 Jun 2024
Zhang Y, Bobe G, Revel JS, Rodrigues R, Sharpton TJ, Fantacone ML, et al. Improvements in metabolic syndrome by xanthohumol derivatives are linked to altered gut microbiota and bile acid metabolism. Mol Nutr Food Res. 2020 Jan;64(1):e1900789. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7029812/. Cited 17 Jun 2024
Chen L, Wang XJ, Chen JX, Yang JC, Ling L, Cai XB et al (2023) Caffeine ameliorates the metabolic syndrome in diet-induced obese mice through regulating the gut microbiota and serum metabolism. Diabetol Metab Syndr 15(1):37
Juárez-Fernández M, Porras D, Petrov P, Román-Sagüillo S, García-Mediavilla MV, Soluyanova P, et al. The synbiotic combination of akkermansia muciniphila and quercetin ameliorates early obesity and NAFLD through gut microbiota reshaping and bile acid metabolism modulation. Antioxidants. 2021 Dec 15;10(12):2001. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8698339/. Cited 17 Jun 2024
Vasquez R, Oh JK, Song JH, Kang DK. Gut microbiome-produced metabolites in pigs: a review on their biological functions and the influence of probiotics. J Anim Sci Technol. 2022 Jul;64(4):671–95. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9353353/. Cited 13 Jul 2024
Xiao Y, Feng Y, Zhao J, Chen W, Lu W. Achieving healthy aging through gut microbiota-directed dietary intervention: focusing on microbial biomarkers and host mechanisms. J Adv Res. 2024 Mar 9; Available from: https://www.sciencedirect.com/science/article/pii/S2090123224000924. cited 2024 Jul 13
Melhem NJ, Taleb S. Tryptophan: from diet to cardiovascular diseases. Int J Mol Sci. 2021;22(18):9904. Available from: https://www.mdpi.com/1422-0067/22/18/9904. cited 14 Jun 2024
Shatova OP, Shestopalov AV. Tryptophan metabolism: a new look at the role of tryptophan derivatives in the human body. Biol Bull Rev. 2023 Apr;13(2):81–91. Available from: https://link.springer.com/10.1134/S2079086423020068. Cited 17 Jun 2024
Su X, Gao Y, Yang R. Gut microbiota-derived tryptophan metabolites maintain gut and systemic homeostasis. Cells. 2022;11(15):2296. Available from: https://www.mdpi.com/2073-4409/11/15/2296. cited 2024 Jun 17
Beaumont M, Roura E, Lambert W, Turni C, Michiels J, Chalvon-Demersay T. Selective nourishing of gut microbiota with amino acids: a novel prebiotic approach? Front Nutr. 2022;9:1066898. Available from: https://www.frontiersin.org/articles/10.3389/fnut.2022.1066898/full. Cited 13 Jul 2024
Li Q, Chen G, Zhu D, Zhang W, Qi S, Xue X, et al. Effects of dietary phosphatidylcholine and sphingomyelin on DSS-induced colitis by regulating metabolism and gut microbiota in mice. J Nutr Biochem. 2022 Jul 1;105:109004. Available from: https://www.sciencedirect.com/science/article/pii/S0955286322000754. Cited 13 Jul 2024
Peron G, Meroño T, Gargari G, Hidalgo-Liberona N, Miñarro A, Lozano EV et al (2022) A Polyphenol-rich diet increases the gut microbiota metabolite indole 3-propionic acid in older adults with preserved kidney function. Mol Nutr Food Res 66(21):e2100349
Wang S, Zuo Z, Ye B, Zhang L, Cheng Y, Xie S, et al. Microbiome–metabolomic analysis reveals beneficial effects of dietary kelp resistant starch on intestinal functions of hybrid snakeheads (Channa maculata♀\times Channa argus♂). Antioxidants. 2023;12(8):1631. Available from: https://www.mdpi.com/2076-3921/12/8/1631. Cited 18 Jun 2024
Zeng SL, Li SZ, Xiao PT, Cai YY, Chu C, Chen BZ, et al. Citrus polymethoxyflavones attenuate metabolic syndrome by regulating gut microbiome and amino acid metabolism. Sci Adv. 2020 Jan 3;6(1):eaax6208. Available from: https://www.science.org/doi/10.1126/sciadv.aax6208. Cited 18 Jun 2024
Li J, Song F. A causal relationship between antioxidants, minerals and vitamins and metabolic syndrome traits: a Mendelian randomization study. Diabetol Metab Syndr. 2023 Oct 10;15(1):194. Available from: https://dmsjournal.biomedcentral.com/articles/10.1186/s13098-023-01174-y. Cited 18 Jun 2024
Peng Z, Wang Y, Huang X, Zhu Q, Zhao Y, Xie H et al (2021) Dietary vitamin intake and risk of metabolic syndrome among centenarians in China. Exp Ther Med 21(2):105
Barzegar-Amini M, khorramruz F, Ghazizadeh H, Sahebi R, Mohammadi-Bajgiran M, Mohaddes Ardabili H, et al. Association between serum vitamin E concentrations and the presence of Metabolic Syndrome: A population-based cohort study. Acta Bio Medica Atenei Parm. 2021;92(3):e2021047. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8343740/. cited 2024 Jun 18
Kim T, Kang J (2020) Association between serum retinol and α-tocopherol levels and metabolic syndrome in Korean General Population: analysis of population-based nationally representative data. Nutrients 12(6):1689
Zhu J, Chen C, Lu L, Shikany JM, D’Alton ME, Kahe K. Folate, vitamin B6, and vitamin B12 status in association with metabolic syndrome incidence. JAMA Netw Open. 2023;6(1):e2250621–e2250621. Available from: https://jamanetwork.com/journals/jamanetworkopen/article-abstract/2800209. Cited 18 Jun 2024
Boughanem H, Ruiz-Limón P, Pilo J, Lisbona-Montañez JM, Tinahones FJ, Moreno Indias I, et al. Linking serum vitamin D levels with gut microbiota after 1-year lifestyle intervention with Mediterranean diet in patients with obesity and metabolic syndrome: a nested cross-sectional and prospective study. Gut Microbes. 2023 Dec 18;15(2):2249150. Available from: https://www.tandfonline.com/doi/full/10.1080/19490976.2023.2249150. Cited 18 Jun 2024
Liu M, Park S. A Causal Relationship between Vitamin C Intake with hyperglycemia and metabolic syndrome risk: a two-sample Mendelian randomization study. Antioxidants. 2022 Apr 27;11(5):857. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9137888/. Cited 18 Jun 2024
Maroufi N, Pezeshgi P, Mortezania Z, Pourmohammad P, Eftekhari R, Moradzadeh M, et al. Association between vitamin D deficiency and prevalence of metabolic syndrome in female population: a systematic review. Horm Mol Biol Clin Invest. 2020;41(4):20200033. https://doi.org/10.1515/hmbci-2020-0033.
Zhang XL, Chen L, Yang J, Zhao SS, Jin S, Ao N, et al. Vitamin D alleviates non-alcoholic fatty liver disease via restoring gut microbiota and metabolism. Front Microbiol. 2023;14:1117644. Available from: https://www.frontiersin.org/articles/10.3389/fmicb.2023.1117644/full. Cited 18 Jun 2024
Nguyen HD, Oh H, Kim MS. Higher intakes of fruits, vegetables, and multiple individual nutrients is associated with a lower risk of metabolic syndrome among adults with comorbidities. Nutr Res. 2022;99:1–12. Available from: https://www.sciencedirect.com/science/article/pii/S0271531721000683. Cited 18 Jun 2024
Nguyen HD, Kim MS. Effects of heavy metal, vitamin, and curry consumption on metabolic syndrome during menopause: a Korean community-based cross-sectional study. Menopause. 2021;28(8):949–59. Available from: https://journals.lww.com/menopausejournal/fulltext/2021/08000/Effects_of_heavy_metal,_vitamin,_and_curry.16.aspx?context=LatestArticles. Cited 18 Jun 2024
Pei X, Yao J, Ran S, Lu H, Yang S, Zhang Y, et al. Association of serum water-soluble vitamin exposures with the risk of metabolic syndrome: results from NHANES 2003–2006. Front Endocrinol. 2023;14:1167317. Available from: https://www.frontiersin.org/articles/10.3389/fendo.2023.1167317/full. Cited 18 Jun 2024
Tang W, Zhan W, Wei M, Chen Q. Associations between different dietary vitamins and the risk of obesity in children and adolescents: a machine learning approach. Front Endocrinol. 2022 Feb 17;12:816975. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8893992/. Cited 18 Jun 2024
Zhang T, Sun P, Geng Q, Fan H, Gong Y, Hu Y, et al. Disrupted spermatogenesis in a metabolic syndrome model: the role of vitamin A metabolism in the gut–testis axis. Gut. 2022;71(1):78–87. Available from: https://gut.bmj.com/content/71/1/78.abstract. Cited 18 Jun 2024
Soto-Martin EC, Warnke I, Farquharson FM, Christodoulou M, Horgan G, Derrien M, et al. Vitamin biosynthesis by human gut butyrate-producing bacteria and cross-feeding in synthetic microbial communities. Relman DA, editor. mBio. 2020 Aug 25;11(4):e00886–20. Available from: https://journals.asm.org/doi/10.1128/mBio.00886-20. Cited 18 Jun 2024
Villatoro-Santos CR, Ramirez-Zea M, Villamor E. B-vitamins and metabolic syndrome in Mesoamerican children and their adult parents. Public Health Nutr. 2021;24(14):4537–45. Available from: https://www.cambridge.org/core/journals/public-health-nutrition/article/bvitamins-and-metabolic-syndrome-in-mesoamerican-children-and-their-adult-parents/751E640324E92A0DE38EA2CCC2A9250A. Cited 18 Jun 2024
Gholami S, Hazar N, Bagheri-Fahraji B, Azizi R, Ghadiri-Anari A, Nadjarzadeh A, et al. The Association between metabolic syndrome and the consumption of some supplements. J Nutr Food Secur. 2022 Aug 3; Available from: https://publish.kne-publishing.com/index.php/JNFS/article/view/10200. cCited 18 Jun 2024
Cano-Ibáñez N, Gea A, Ruiz-Canela M, Corella D, Salas-Salvadó J, Schröder H, et al. Diet quality and nutrient density in subjects with metabolic syndrome: Influence of socioeconomic status and lifestyle factors. A cross-sectional assessment in the PREDIMED-Plus study. Clin Nutr. 2020 Apr 1;39(4):1161–73. Available from: https://www.sciencedirect.com/science/article/pii/S0261561419302080. Cited 18 Jun 2024
Qiu S, Chen J, Bai Y, He J, Cao H, Che Q, et al. GOS ameliorates nonalcoholic fatty liver disease induced by high fat and high sugar diet through lipid metabolism and intestinal microbes. Nutrients. 2022;14(13):2749. Available from: https://www.mdpi.com/2072-6643/14/13/2749. Cited 18 Jun 2024
Zhang B, Liu K, Yang H, Jin Z, Ding Q, Zhao L. Gut microbiota: the potential key target of TCM’s therapeutic effect of treating different diseases using the same method—UC and T2DM as examples. Front Cell Infect Microbiol. 2022 Mar 30;12:855075. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9005880/. Cited 18 Jun 2024
Alhamoud Y, Ahmad MI, Abudumijiti T, Wu J, Zhao M, Feng F, et al. 6-Gingerol, an active ingredient of ginger, reshapes gut microbiota and serum metabolites in HFD-induced obese mice. J Funct Foods. 2023;109:105783. Available from: https://www.sciencedirect.com/science/article/pii/S1756464623003833. Cited 18 Jun 2024
Deng Z, Wu N, Wang J, Zhang Q. Dietary fibers extracted from Saccharina japonica can improve metabolic syndrome and ameliorate gut microbiota dysbiosis induced by high fat diet. J Funct Foods. 2021 Oct 1;85:104642. Available from: https://www.sciencedirect.com/science/article/pii/S1756464621002917. Cited 13 Jul 2024
Kant R, Chandra L, Verma V, Nain P, Bello D, Patel S, et al. Gut microbiota interactions with anti-diabetic medications and pathogenesis of type 2 diabetes mellitus. World J Methodol. 2022 Jul 20;12(4):246–57. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9350729/. Cited 13 Jul 2024
Chelakkot C, Choi Y, Kim DK, Park HT, Ghim J, Kwon Y et al (2018) Akkermansia muciniphila-derived extracellular vesicles influence gut permeability through the regulation of tight junctions. Exp Mol Med 50(2):e450
Gradisteanu Pircalabioru G, Liaw J, Gundogdu O, Corcionivoschi N, Ilie I, Oprea L, et al. Effects of the lipid profile, type 2 diabetes and medication on the metabolic syndrome—associated gut microbiome. Int J Mol Sci. 2022 Jan;23(14):7509. Available from: https://www.mdpi.com/1422-0067/23/14/7509. cited 2024 Jul 13
Lee CB, Chae SU, Jo SJ, Jerng UM, Bae SK. The relationship between the gut microbiome and metformin as a key for treating type 2 diabetes mellitus. Int J Mol Sci. 2021 Jan;22(7):3566. Available from: https://www.mdpi.com/1422-0067/22/7/3566. cited 13 Jul 2024
Kuka J, Videja M, Makrecka-Kuka M, Liepins J, Grinberga S, Sevostjanovs E, et al. Metformin decreases bacterial trimethylamine production and trimethylamine N-oxide levels in db/db mice. Sci Rep. 2020 Sep 3;10(1):14555. Available from: https://www.nature.com/articles/s41598-020-71470-4. cited 13 Jul 2024
Ahmadi S, Razazan A, Nagpal R, Jain S, Wang BO, Mishra SP, et al. Metformin reduces aging-related leaky gut and improves cognitive function by beneficially modulating gut microbiome/goblet cell/mucin axis. J Gerontol Ser A. 2020;75(7):e9–21. Available from: https://academic.oup.com/biomedgerontology/article-abstract/75/7/e9/5780100. Cited 13 Jul 2024
Huang YH, Wu YH, Tang HY, Chen ST, Wang CC, Ho WJ, et al. Gut microbiota and bile acids mediate the clinical benefits of YH1 in male patients with type 2 diabetes mellitus: A pilot observational study. Pharmaceutics. 2022;14(9):1857. Available from: https://www.mdpi.com/1999-4923/14/9/1857. Cited 18 Jun 2024
Koopen AM, Almeida EL, Attaye I, Witjes JJ, Rampanelli E, Majait S, et al. Effect of fecal microbiota transplantation combined with mediterranean diet on insulin sensitivity in subjects with metabolic syndrome. Front Microbiol. 2021 Jun 10;12:662159. Available from: https://www.frontiersin.org/articles/10.3389/fmicb.2021.662159/full. Cited 18 Jun 2024
Mocanu V, Zhang Z, Deehan EC, Kao DH, Hotte N, Karmali S et al (2021) Fecal microbial transplantation and fiber supplementation in patients with severe obesity and metabolic syndrome: a randomized double-blind, placebo-controlled phase 2 trial. Nat Med 27(7):1272–1279
Allegretti JR, Kassam Z, Mullish BH, Chiang A, Carrellas M, Hurtado J et al (2020) Effects of fecal microbiota transplantation with oral capsules in obese patients. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc 18(4):855-863.e2
Leong KS, Jayasinghe TN, Wilson BC, Derraik JG, Albert BB, Chiavaroli V, et al. Effects of fecal microbiome transfer in adolescents with obesity: the gut bugs randomized controlled trial. JAMA Netw Open. 2020;3(12):e2030415–e2030415. Available from: https://jamanetwork.com/journals/jamanetworkopen/article-abstract/2774355. cited 15 Jun 2024
Ng SC, Xu Z, Mak JWY, Yang K, Liu Q, Zuo T et al (2022) Microbiota engraftment after faecal microbiota transplantation in obese subjects with type 2 diabetes: a 24-week, double-blind, randomised controlled trial. Gut 71(4):716–723
Nemet I, Li XS, Haghikia A, Li L, Wilcox J, Romano KA, et al. Atlas of gut microbe-derived products from aromatic amino acids and risk of cardiovascular morbidity and mortality. Eur Heart J. 2023;44(32):3085–96. Available from: https://academic.oup.com/eurheartj/article-abstract/44/32/3085/7204162. Cited 18 Jun 2024
Sun XZ, Zhao DY, Zhou YC, Wang QQ, Qin G, Yao SK. Alteration of fecal tryptophan metabolism correlates with shifted microbiota and may be involved in pathogenesis of colorectal cancer. World J Gastroenterol. 2020;26(45):7173. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7723673/. Cited 18 Jun 2024
Ouyang Q, Xu Y, Ban Y, Li J, Cai Y, Wu B, et al. Probiotics and prebiotics in subclinical hypothyroidism of pregnancy with small intestinal bacterial overgrowth. probiotics antimicrob proteins . 2024 Apr;16(2):579–88. Available from: https://link.springer.com/10.1007/s12602-023-10068-4. Cited 18 Jun 2024
Acknowledgements
None
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
SOO conceived and designed the study. SOO wrote the initial version of the manuscript. OOB, IOO, and ASF reviewed the initial version of the manuscript. SOO, OOB, IOO, ASF, BOA, and ENE wrote the final draft of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All the authors of this manuscript willingly grant consent to publish this work in this journal.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Olalekan, S.O., Bakare, O.O., Osonuga, I.O. et al. Gut microbiota-derived metabolites: implications for metabolic syndrome and therapeutic interventions. Egypt J Intern Med 36, 72 (2024). https://doi.org/10.1186/s43162-024-00342-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43162-024-00342-4