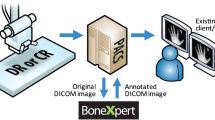
Abstract
This narrative review focuses on clinical applications of artificial intelligence (AI) in musculoskeletal imaging. A range of musculoskeletal disorders are discussed using a clinical-based approach, including trauma, bone age estimation, osteoarthritis, bone and soft-tissue tumors, and orthopedic implant-related pathology. Several AI algorithms have been applied to fracture detection and classification, which are potentially helpful tools for radiologists and clinicians. In bone age assessment, AI methods have been applied to assist radiologists by automatizing workflow, thus reducing workload and inter-observer variability. AI may potentially aid radiologists in identifying and grading abnormal findings of osteoarthritis as well as predicting the onset or progression of this disease. Either alone or combined with radiomics, AI algorithms may potentially improve diagnosis and outcome prediction of bone and soft-tissue tumors. Finally, information regarding appropriate positioning of orthopedic implants and related complications may be obtained using AI algorithms. In conclusion, rather than replacing radiologists, the use of AI should instead help them to optimize workflow, augment diagnostic performance, and keep up with ever-increasing workload.
Relevance statement This narrative review provides an overview of AI applications in musculoskeletal imaging. As the number of AI technologies continues to increase, it will be crucial for radiologists to play a role in their selection and application as well as to fully understand their potential value in clinical practice.
Key points
• AI may potentially assist musculoskeletal radiologists in several interpretative tasks.
• AI applications to trauma, age estimation, osteoarthritis, tumors, and orthopedic implants are discussed.
• AI should help radiologists to optimize workflow and augment diagnostic performance.
Graphical Abstract

Similar content being viewed by others
Introduction
The term “artificial intelligence” (AI) refers to a field of computer science focused on allowing computers to mimic human cognitive functions [1]. This includes machine learning, which is a domain of AI enabling computers to learn and detect patterns in data without being explicitly programmed [2]. In turn, deep learning is a domain of machine learning (and therefore of AI) which can perform superior classification tasks [3]. In musculoskeletal imaging, AI, machine learning, and deep learning may assist radiologists in every step of the workflow, including both interpretative—such as detection and characterization of abnormalities—and non-interpretative tasks. The latter group includes (but is not limited to) the management of radiologic requests [4], protocoling [5], and production of images [6], similarly to what happens in any other imaging subspecialty. As they are not specific for musculoskeletal imaging, a detailed discussion on non-interpretative tasks is beyond the scope of this review. Thus, this narrative review mainly focuses on interpretative tasks and provides the reader with an up-to-date overview of AI applications in musculoskeletal imaging. A range of musculoskeletal disorders are discussed using a clinical-based approach, including those most often addressed in AI literature, such as trauma, bone age estimation, osteoarthritis, tumors, and orthopedic implants. A general overview of the clinical tasks achieved through AI in these fields of musculoskeletal imaging is offered in Table 1.
Musculoskeletal trauma
Trauma is one of the most common reasons for patients presenting to emergency department. It represents a high cost for health care systems and missed or delayed diagnosis may lead to increased mortality and morbidity [36]. With the increasing growth of imaging utilization in the emergency setting, radiologists are constantly under pressure. It is estimated that there is approximately 4% error rate in imaging interpretation by a trained radiologist [37]. The risk of misinterpretation is higher when radiological exams are interpreted by non-radiology clinicians [38], which often occurs at night, when a consulting radiologist is not always available in every hospital. AI has the potential to reduce workload and improve diagnosis in the emergency settings [36].
AI has been applied to different imaging modalities, such as radiography, computed tomography (CT), and magnetic resonance imaging (MRI), with a special focus on radiography. To improve case collection and development of algorithms, deidentified radiography datasets have been created, for example MURA (musculoskeletal radiographs). MURA includes close to 41,000 radiographs of the upper extremity, which are all labeled as “normal” or “abnormal” by expert radiologists [39]. Several AI algorithms have been applied to fracture detection and classification. Lindsey et al. developed a deep neural network to detect and localize fractures on radiographs, which resulted in an improved diagnostic accuracy in fracture identification by emergency medicine clinicians with the assistance of AI [40]. Krogue et al. developed a deep learning-based hip fracture detection and classification model, which improved residents’ performance approximating that of unaided fellowship-trained attendings [41]. Gale et al. developed a DenseNet based architecture to detect hip fractures from frontal pelvic radiographs, which achieved equivalent performance compared to a human radiologist [7]. Chen et al. developed a ResNeXt architecture which was transferred to abdominal radiographs to identify vertebral fractures, with only slightly inferior performance compared to physicians such as radiologists and orthopedic surgeons (average accuracy of 76.8% for the physicians versus accuracy of 73% for the model) [8]. Thus, this model could be useful to assist physicians in the identification of vertebral fractures as incidental findings. Overall, in the mentioned studies, radiography-based algorithms had comparable performance to trained musculoskeletal radiologists. Thus, healthcare professionals without radiology training or residents in training may benefit the most in fracture detection, especially in centers with limited access to specialized musculoskeletal radiologists.
Most of these algorithms are specific to a single anatomic area or body part. However, to be useful in practice, they will need to be combined in one interconnected software module that is capable to detect a fracture in any anatomic region. In a multicenter study, Jones et al. used 715,343 radiographs across 16 anatomic regions and an ensemble of 10 convolutional neural networks for fracture detection, with a mean area under the curve (AUC) above 0.98 in half of the anatomic sites [9]. Ma et al. developed a two-step approach to first detect the anatomic region among 20 different bones and thereafter to classify fractures, with an accuracy of 90% [42]. Regarding fracture classification, Chung et al. developed a proximal humerus fracture classification model based on Neer criteria using antero-posterior radiographs [12]. Tanzi et al. and Lind et al. developed deep learning models to classify proximal femur fractures [11] and fractures around the knee [14], respectively, based on the AO-OTA (Arbeitsgemeinschaft für Osteosynthesefragen-Orthopaedic Trauma Association) classification system. Olczak et al. trained a deep learning model to classify ankle fractures over a dataset of 4,941 patients achieving an average AUC of 0.90 [15]. Li et al. developed a deep learning model to classify vertebral fractures based on the Genant classification using plain lateral radiographs from 941 patients and achieved an AUC of 0.919−0.99 [13]. An example of fracture detection/classification method based on deep learning is shown in Fig. 1.
Example of fracture detection and classification method based on neural networks. Image areas where the network focuses on are shown as colored dots. Colored dots seem to cluster close to fracture lines, suggesting that the network appropriately finds these areas to contain relevant information. Adapted from: Lind A et al. [14] [open-access article distributed under the terms of the Creative Commons Attribution License (CC BY)]
In CT, Jin et al. developed a deep learning model to identify rib fractures with a sensitivity of 92.9% [43]. Zhou et al. developed a convolutional neural network model which combined clinical information with CT to detect and classify rib fractures into recent, healing, and old fractures [44]. Pranata et al. built a model to identify and precisely localize calcaneal fractures [45]. Farda et al. built a convolutional neural network model to classify calcaneal fractures into the four Sanders categories with 72% accuracy [10]. Other models based on CT images focused on classification of osteoporotic vertebral compression and femoral neck fractures [46, 47].
Regarding MRI, Bien et al. showed a deep learning model which could perform multiple functions, such as detection of anterior cruciate ligament and meniscal tears, with however lower sensitivity for anterior cruciate ligament tears and lower specificity for meniscal tears compared to radiologists, respectively [16]. Liu et al. developed a deep learning model to improve sensitivity for anterior cruciate ligament tear detection [48], without significant difference compared to radiologists. Liu et al. developed a deep learning-based cartilage lesion model, with a high diagnostic performance and good intra-observer agreement for detecting cartilage degeneration and acute cartilage injury [20]. Kim et al. developed a deep learning algorithm to detect rotator cuff tear with 87% accuracy [49]. Hong et al. developed an AI model to analyze the efficacy of knee ligament trauma repair [50].
Ultrasound-based AI algorithms have been only mildly investigated compared to other imaging modalities. AI in ultrasound is mainly linked to classification, segmentation and diagnosis [51].
Beyond pure imaging interpretation, AI offers other applications in musculoskeletal trauma that can impact the management of patients. For example, it helps to predict the probability for occult posterior malleolar fracture in patients with known tibial shaft fracture [52] or to identify patients with tibial shaft fracture at risk for infection after operative treatment [53]. Despite the great potential of AI, further studies are needed to implement the use of AI in clinical practice, considering it as a diagnostic support for radiologists and clinicians and not a substitute of them.
Bone age estimation
Correct assessment of bone age is important for different clinical fields, such as pediatric endocrinology, orthodontics, and orthopedics, as well as for legal medicine issues [54]. Bone age assessment is currently based on two main different methods: the Greulich-Pyle and the Tanner-Whitehouse methods, both focused on the analysis of the epiphyses and diaphysis morphology on hands radiographs. The Greulich-Pyle method is an atlas that contains reference images from hand radiographs, which were collected from 1931 to 1942 from upper-middle class Caucasian children in Ohio, USA [54, 55]. Bone age attribution is made by comparing a child hand radiographs with reference images in the atlas. The Tanner-Whitehouse method is based on data collected between 1950 and 1960 from children of average socioeconomic class in the UK, with further update in 2001 [56]. It evaluates maturity scores from the radius, ulna, carpals, and 13 hand short bones. Some of these bones are evaluated and categorized into stages ranging from A to I; then, a total score is calculated and converted into bone age [54, 56]. However, these conventional methods are time-consuming and prone to intra- and inter-observer variability.
Bone age assessments have become a major target of the machine learning community. The task is a typical object detection and classification problem of deep learning, with promising results [57]. Most of the studies are focused on left hand and wrist radiographs, with very few papers dealing with MRI, CT, and ultrasound. Radiographs in fact are faster, and radiation exposure is relatively low and considered safe [58]. In the history of bone age assessment, the first radiography-based automated techniques were introduced without the support of AI algorithms, such as HANDX system [59] and computer-based skeletal aging scoring system (CASAS) [60] introduced in 1989 and 1994, respectively. More recently, with the advancements of AI, many studies were conducted on AI-based bone age assessment solutions. BoneXpert (2008) is an automatic AI system which is widely used in Europe [17]. It uses feature extraction techniques and calculates bone age by analyzing the left-hand radiograph based on 13 bones, with improvement of time efficiency in daily clinical practice. Similarly, VUNO Med-BoneAge [18] and HH-boneage.io solution [61] are respectively semi-automatic and fully automatic AI systems to assess bone age on hand radiographs. MediAI-BA solution analyzes seven epiphysis-metaphysis growth regions [62].
Although different AI methods exist, the assessment of bone age in different ethnicities still represent a limit in most cases. In fact, different populations show different rates of skeletal maturation [63]. Therefore, most AI bone age assessment methods, particularly those based on Greulich-Pyle method, might be inaccurate with population of different ethnicity [57]. In addition, patients with congenital or acquired bone abnormalities or with previous surgery still necessitate manual assessment.
In conclusion, AI in bone age assessment is a useful tool that can assist radiologists, reducing workload and inter- and intra-observer variability. AI-assisted interpretation of bone age can also improve accuracy among junior readers [64]. However, multi-center and multi-national clinical trials are warranted to overcome the limitations of currently available AI methods.
Osteoarthritis
AI research studies have been mainly focused on classification and prediction tasks in osteoarthritis [65]. Automated classification tasks may be highly beneficial to perform quantitative or semiquantitative analysis, which are essential to clinical routine but time-consuming for radiologists and subject to interobserver variability. However, the main challenge lies in creating individualized prediction models for osteoarthritis progression or development. Particularly, treatment plans may be targeted to the needs of individual patients prior to irreversible morphologic joint degeneration, including lifestyle changes (such as weight loss) at a timeframe during which disease progression may still be reversible [65].
These prediction models are multifactorial, and AI may help combining clinical risk factors for osteoarthritis with imaging biomarkers. The workflow for building AI models includes several steps. First, the clinical problem must be defined, including the definition of predictors (such as imaging biomarkers and clinical risk factors) and outcomes. Second, data are extracted and prepared for AI analysis, including dataset partition into training and test cohorts for model tuning and testing, respectively, as well as dimensionality reduction and class balancing in the training cohort to reduce the number of predictors and compensate for unbalanced datasets, respectively. Third, model training and hyperparameter optimization are performed to obtain AI models which predict the associations between predictor variables and outcomes. Finally, model performance is evaluated on the test cohort, which can be either internal if a single dataset is split into training and test cohorts or independent if two separate datasets are available for analysis. The latter approach is preferred as it improves the generalizability of the model [65].
In research studies dealing with osteoarthritis, AI-based classifications and predictions have been performed using deep learning, conventional machine learning, or ensemble machine learning approaches. Deep learning has been employed to analyze imaging data not only for binary classifications but also for quantitative or semiquantitative grading. Particularly, radiograph-based convolutional neural networks were used to automatically determine knee osteoarthritis grade with the Kellgren-Lawrence system, achieving an AUC of 0.93 [19]. On MRI, an automated method for cartilage lesion detection using convolutional neural networks showed sensitivity and specificity of 84% and 85%, respectively [20]. Conventional machine learning models have been built upon preidentified imaging data and demographics to predict future development or progression of osteoarthritis. For instance, radiographic and pain progression of knee osteoarthritis could be predicted with high accuracy (AUC of 0.86 and 0.95, respectively) when clustering subjects based on radiographic and pain progressive abnormalities and then using clinical variables to build machine learning models for predicting the probabilities of belonging to each cluster [66]. Finally, ensemble models incorporating both deep learning and conventional machine learning approaches based on imaging data have been employed for prediction purposes. Particularly, convolutional neural networks were used to evaluate the probability of knee osteoarthritis progression according to the Kellgren-Lawrence grade on radiographs [21]. In the same study, prognosis estimation was improved by combining deep learning prediction with clinical information using a gradient boosting machine, resulting in an AUC of 0.79 [21].
In conclusion, AI may potentially aid radiologists in identifying and grading abnormal findings of osteoarthritis more quickly and efficiently, also resulting in higher reproducibility. Additionally, by integrating clinical and imaging data, AI may help radiologists to predict the onset of osteoarthritis and its progression, thus enabling the implementation of preventive treatment strategies at early stages of the disease and resulting in decreased disability [65].
Bone and soft-tissue tumors
Computer-aided diagnosis of bone tumors has been of interest for more than 50 years [67]. The first studies described a probabilistic approach based on patients’ demographics and imaging findings [67]. Recently, research studies have shifted away from radiologists entering imaging findings into computers, and towards direct presentation of medical images to AI models. The use of AI has been investigated for several applications in musculoskeletal tumor imaging, including primary or metastatic bone and soft-tissue lesions, although it is still at research stage [68]. Particularly, while skeletal metastases are relatively common, bone and soft-tissue sarcomas or primary malignant tumors are rare and highly heterogeneous, thus representing a challenge for AI model development.
Unsurprisingly, a recent systematic review focused on AI applied to musculoskeletal oncology showed that machine learning papers rapidly increased over the years [68]. Conventional machine learning and deep learning accounted for 77% and 23% of the included studies, respectively [68]. The bulk of conventional machine learning papers was related to radiomics applied to CT and MRI [68]. Nowadays, attention is focused on AI and radiomics as emerging tools to noninvasively provide information regarding diagnosis and outcome [69, 70].
Radiomics refers to the extraction and analysis of quantitative features from medical images, known as radiomic features, which may be used to support decision-making algorithms [71]. In musculoskeletal oncology, most AI-based radiomic studies focused on prediction of diagnosis—such as benign versus malignant tumor discrimination [72] or tumor grading [73]—and outcome—such as therapy response [27, 74], recurrence [28, 75], and survival [29]. In particular, several diagnosis-related studies dealt with benign versus malignant (or intermediate, like atypical cartilaginous or lipomatous tumors) discrimination and grading in skeletal cartilaginous tumors [23,24,25, 76], lipomatous soft-tissue tumors [77, 78] and soft-tissue sarcomas [26]. Most outcome-related studies dealt with therapy response, recurrence, or survival prediction in osteosarcoma [27,28,29] and soft-tissue sarcomas [30].
Radiomics involves a series of discrete steps, from image collection and segmentation to radiomic feature extraction and selection and, finally, classification model development, as summarized in Fig. 2. First, collected images are segmented using manual, semiautomated, or automated methods. In most studies, segmentation was manually performed by expert radiologists/clinicians or trainees under experts’ supervision [79]. Although the influence of interobserver variability on image segmentation can be evaluated as part of every radiomic workflow [80], semiautomated and automated approaches would ideally achieve higher reliability than manual segmentation. Second, several first-order, shape, and texture features are extracted and possibly combined with clinical information. Next, as most radiomic features are redundant and not informative [81], radiomic features are selected to create datasets which can be later mined. Finally, machine learning is used to perform classification tasks. Ideally, machine learning models are trained and validated using cross-validation on training datasets and then tested on independent datasets from different institutions. A clinical validation of the models against completely independent datasets is highly desirable to achieve clinical transferability. However, this independent or external validation is lacking in most radiomic studies dealing with musculoskeletal tumors [79], thus hampering generalizability of results. As musculoskeletal tumors are relatively rare entities, in particular sarcomas, free public repositories such as The Cancer Imaging Archive (https://www.cancerimagingarchive.net) may grant opportunities for research groups around the world to access data from different institutions and validate their models against independent datasets.
Example of machine learning radiomic workflow. A machine learning classifier can be employed to perform classification tasks based on radiomic features. Reproduced from: Fanciullo C et al. [69] [open-access article distributed under the terms of the Creative Commons Attribution License (CC BY)]
Deep learning can perform superior classification tasks compared to conventional machine learning. Particularly, deep learning models consist of neural network architectures which enable automated feature extraction (instead of manual extraction as in conventional machine learning), thus improving the efficiency of image analysis and providing assistance for nonexpert users [82]. However, deep learning models need to be trained using larger sets of labeled data compared to conventional machine learning, which is why their application to uncommon musculoskeletal tumors is still limited. First, images are preprocessed to obtain suitable quality annotated data and then split into training, validation, and test datasets with appropriate proportions. Second, the model is trained on the training dataset. Third, the model performance is evaluated on the test dataset [82]. In musculoskeletal oncology, most imaging-based deep learning models were developed using radiographs, CT, and MRI for diagnosis-related tasks, such as tumor classification—benign versus malignant discrimination [22, 83] or grading [84]—and segmentation [85]. In particular, deep learning showed similar and better accuracy compared to musculoskeletal fellowship-trained radiologists and radiology residents, respectively, in classifying primary bone tumors on radiographs [22]. In another study dealing with MRI of bone lesions, deep learning could differentiate benign from malignant tumors on a par with experts [83]. Deep learning was also used in combination with radiomics, for instance to differentiate lung from non-lung spine bone metastases on dynamic contrast-enhanced MRI [86] or benign from malignant sacral tumors on CT [87]. In addition to studies dealing with classification of lesions, deep learning was applied to musculoskeletal tumors like osteosarcoma for automated segmentation purposes [85]. Finally, a very few studies applied deep learning to musculoskeletal tumors for outcome-related tasks, such as recurrence prediction in giant cell tumor of the bone after curettage based on pre-operative MRI [88]. Overall, although promising results have been published, insufficient training data prevent most deep learning models from being implemented into clinical practice.
In conclusion, radiologists are asked to play a key role in moving AI—including both conventional machine learning and deep learning methods—and radiomics of bone and soft-tissue tumors from theory to clinical practice. The main limitations of current research studies, such as the relatively low number of patients and the lack of external/independent validation, need to be addressed in future investigations. Public repositories and institutional infrastructures for multi-center collaboration may allow to overcome these limitations and accelerate the process of clinical implementation.
Orthopedic implants and implant-related complication
Musculoskeletal radiologists routinely evaluate orthopedic implants for appropriate positioning and potential complications. With the increasing number of orthopedic implant surgeries being performed [89], AI-aided postoperative image analysis has the potential to reduce workload, minimize fatigue-related errors, increase speed, and improve efficiency.
A theoretical AI pipeline for implant evaluation may include several steps, from body part identification to implant assessment [90], as follows.
First, the body part of interest, laterality and radiographic views are identified. Deep learning showed up to 100% accuracy in classifying anatomic region based on musculoskeletal radiographs [91]. Similarly, deep learning algorithms had almost perfect accuracy in determining laterality on radiographs [92] as well as radiograph position [93].
Second, the orthopedic implant is identified. Deep learning models demonstrated the ability to detect the presence of implants with up to 100% accuracy, including knee [32] and shoulder [33] arthroplasties, spinal hardware [31], and fracture fixation devices [93].
Third, the orthopedic implant is characterized into design types and models. The task of design typing includes differentiating between anatomic types of orthopedic implants. Particularly, deep learning models were developed to differentiate total from unicompartmental knee arthroplasty [32] as well as total from reverse total shoulder arthroplasty [33]. The task of identifying specific implant models is less relevant for post-operative radiological evaluation but crucial for revision surgery planning, as implant-specific tool kits are required. Orthopedic surgeons often spend time and efforts to identify implant models before revision surgery, for instance using orthopedic implant atlases of post-operative radiographs, with the risk of failure and potential negative impacts on outcome. A recent systematic review reported good to excellent performance of deep learning in classifying orthopedic implant models on radiographs [94], and one study demonstrated better performance of deep learning compared to non-deep learning AI algorithms [95].
Fourth, the orthopedic implant position is evaluated. Deep learning algorithms could measure inclination and version of the acetabular component after total hip arthroplasty, and little difference in measurements was found between human reader and AI [34]. Other orthopedic measurements such as lumbar lordosis [96] and lower limb length [97] could be obtained from radiographs automatically using AI, while saving time compared to manual calculations. Additionally, some AI algorithms for implant position assessment are already incorporated into commercially available orthopedic software [98].
Fifth, implant-related complications are identified. An important consideration is the relative rarity of complications after orthopedic implant surgery, which is (luckily) observed in clinical practice and results in unbalanced classes, thus limiting AI analysis. Deep learning models achieved 70% accuracy in detecting loosening after total knee or total hip arthroplasty on radiographs, which was improved when combining imaging and clinical information [35]. AI may potentially help predicting other implant-related complications, such as periprosthetic fractures, dislocation, periprosthetic infection, and component wear, which would be beyond human perception. AI-aided prediction of post-operative complications is in its early stage of development, and, given their tremendous implications for surgical outcome, it deserves future investigation.
Further research is also needed to compare AI alone and as an adjunct with human experts in evaluating orthopedic implants [99].
Conclusion and future perspectives
This narrative review provided an overview of AI clinical applications in musculoskeletal imaging. Most studies evaluated the performance of AI algorithms compared to expert radiologists. Experts usually have very high accuracies, but many years of training and experience are required to achieve expert-level [100].
Thus, studies emulating real-life practice settings, including readers with different levels of expertise, are needed to fully understand the added value of AI in musculoskeletal diseases and bridge the gap between research and clinical application. It is important to mention that some AI technologies in musculoskeletal radiology are already commercially available, such as algorithms for fracture detection, bone age estimation, and osteoarthritis quantification [101].
As the number of AI products continues to increase, it will be crucial for radiologists to play a role in the selection and application of these technologies. Hence, rather than replacing radiologists, the use of AI may instead help them to optimize workflow, augment diagnostic performance, and keep up with ever-increasing workload. This also entails that legal liability is ultimately assigned to a human authority, namely the radiologist, who should take the responsibility [102].
Availability of data and materials
Data can be obtained upon request to the corresponding author.
Abbreviations
- AI:
-
Artificial intelligence
- AUC:
-
Area under the curve
- CT:
-
Computed tomography
- MRI:
-
Magnetic resonance imaging
References
Russell S, Bohannon J (2015) Artificial intelligence. Fears of an AI pioneer. Science 349:252. https://doi.org/10.1126/science.349.6245.252
Erickson BJ, Korfiatis P, Akkus Z, Kline TL (2017) Machine learning for medical imaging. Radiographics 37:505–515. https://doi.org/10.1148/rg.2017160130
Chartrand G, Cheng PM, Vorontsov E et al (2017) Deep learning: a primer for radiologists. Radiographics 37:2113–2131. https://doi.org/10.1148/rg.2017170077
Shin Y, Kim S, Lee YH (2022) AI musculoskeletal clinical applications: how can AI increase my day-to-day efficiency? Skeletal Radiol 51:293–304. https://doi.org/10.1007/s00256-021-03876-8
Lee YH (2018) Efficiency improvement in a busy radiology practice: determination of musculoskeletal magnetic resonance imaging protocol using deep-learning convolutional neural networks. J Digit Imaging 31:604–610. https://doi.org/10.1007/s10278-018-0066-y
Galbusera F, Bassani T, Casaroli G et al (2018) Generative models: an upcoming innovation in musculoskeletal radiology? A preliminary test in spine imaging. Eur Radiol Exp 2:29. https://doi.org/10.1186/s41747-018-0060-7
Gale W, Oakden-Rayner L, Carneiro G, et al (2017) Detecting hip fractures with radiologist-level performance using deep neural networks. http://arxiv.org/abs/1711.06504
Chen H-Y, Hsu BW-Y, Yin Y-K et al (2021) Application of deep learning algorithm to detect and visualize vertebral fractures on plain frontal radiographs. PLoS One 16:e0245992. https://doi.org/10.1371/journal.pone.0245992
Jones RM, Sharma A, Hotchkiss R et al (2020) Assessment of a deep-learning system for fracture detection in musculoskeletal radiographs. NPJ Digit Med 3:144. https://doi.org/10.1038/s41746-020-00352-w
Aghnia Farda N, Lai J-Y, Wang J-C et al (2021) Sanders classification of calcaneal fractures in CT images with deep learning and differential data augmentation techniques. Injury 52:616–624. https://doi.org/10.1016/j.injury.2020.09.010
Tanzi L, Vezzetti E, Moreno R et al (2020) Hierarchical fracture classification of proximal femur X-Ray images using a multistage Deep Learning approach. Eur J Radiol 133:109373. https://doi.org/10.1016/j.ejrad.2020.109373
Chung SW, Han SS, Lee JW et al (2018) Automated detection and classification of the proximal humerus fracture by using deep learning algorithm. Acta Orthop 89:468–473. https://doi.org/10.1080/17453674.2018.1453714
Li Y-C, Chen H-H, Horng-Shing LuH et al (2021) Can a deep-learning model for the automated detection of vertebral fractures approach the performance level of human subspecialists? Clin Orthop Relat Res 479:1598–1612. https://doi.org/10.1097/CORR.0000000000001685
Lind A, Akbarian E, Olsson S et al (2021) Artificial intelligence for the classification of fractures around the knee in adults according to the 2018 AO/OTA classification system. PLoS One 16:e0248809. https://doi.org/10.1371/journal.pone.0248809
Olczak J, Emilson F, Razavian A et al (2021) Ankle fracture classification using deep learning: automating detailed AO Foundation/Orthopedic Trauma Association (AO/OTA) 2018 malleolar fracture identification reaches a high degree of correct classification. Acta Orthop 92:102–108. https://doi.org/10.1080/17453674.2020.1837420
Bien N, Rajpurkar P, Ball RL et al (2018) Deep-learning-assisted diagnosis for knee magnetic resonance imaging: development and retrospective validation of MRNet. PLoS Med 15:e1002699. https://doi.org/10.1371/journal.pmed.1002699
Thodberg HH, Kreiborg S, Juul A, Pedersen KD (2009) The BoneXpert method for automated determination of skeletal maturity. IEEE Trans Med Imaging 28:52–66. https://doi.org/10.1109/TMI.2008.926067
Kim JR, Shim WH, Yoon HM et al (2017) Computerized bone age estimation using deep learning based program: evaluation of the accuracy and efficiency. AJR Am J Roentgenol 209:1374–1380. https://doi.org/10.2214/AJR.17.18224
Tiulpin A, Thevenot J, Rahtu E et al (2018) Automatic knee osteoarthritis diagnosis from plain radiographs: a deep learning-based approach. Sci Rep 8:1727. https://doi.org/10.1038/s41598-018-20132-7
Liu F, Zhou Z, Samsonov A et al (2018) Deep learning approach for evaluating knee MR images: achieving high diagnostic performance for cartilage lesion detection. Radiology 289:160–169. https://doi.org/10.1148/radiol.2018172986
Tiulpin A, Klein S, Bierma-Zeinstra SMA et al (2019) Multimodal machine learning-based knee osteoarthritis progression prediction from plain radiographs and clinical data. Sci Rep 9:20038. https://doi.org/10.1038/s41598-019-56527-3
von Schacky CE, Wilhelm NJ, Schäfer VS et al (2021) Multitask deep learning for segmentation and classification of primary bone tumors on radiographs. Radiology 301:398–406. https://doi.org/10.1148/radiol.2021204531
Fritz B, Müller DA, Sutter R et al (2018) Magnetic resonance imaging–based grading of cartilaginous bone tumors. Invest Radiol 53:663–672. https://doi.org/10.1097/RLI.0000000000000486
Gitto S, Cuocolo R, Annovazzi A et al (2021) CT radiomics-based machine learning classification of atypical cartilaginous tumours and appendicular chondrosarcomas. EBioMedicine 68:103407. https://doi.org/10.1016/j.ebiom.2021.103407
Gitto S, Cuocolo R, van Langevelde K et al (2022) MRI radiomics-based machine learning classification of atypical cartilaginous tumour and grade II chondrosarcoma of long bones. EBioMedicine 75:103757. https://doi.org/10.1016/j.ebiom.2021.103757
Peeken JC, Spraker MB, Knebel C et al (2019) Tumor grading of soft tissue sarcomas using MRI-based radiomics. EBioMedicine 48:332–340. https://doi.org/10.1016/j.ebiom.2019.08.059
Lin P, Yang P-F, Chen S et al (2020) A delta-radiomics model for preoperative evaluation of neoadjuvant chemotherapy response in high-grade osteosarcoma. Cancer Imaging 20:7. https://doi.org/10.1186/s40644-019-0283-8
Chen H, Liu J, Cheng Z et al (2020) Development and external validation of an MRI-based radiomics nomogram for pretreatment prediction for early relapse in osteosarcoma: a retrospective multicenter study. Eur J Radiol 129:109066. https://doi.org/10.1016/j.ejrad.2020.109066
Wu Y, Xu L, Yang P et al (2018) Survival prediction in high-grade osteosarcoma using radiomics of diagnostic computed tomography. EBioMedicine 34:27–34. https://doi.org/10.1016/j.ebiom.2018.07.006
Gao Y, Kalbasi A, Hsu W et al (2020) Treatment effect prediction for sarcoma patients treated with preoperative radiotherapy using radiomics features from longitudinal diffusion-weighted MRIs. Phys Med Biol 65:175006. https://doi.org/10.1088/1361-6560/ab9e58
Kitamura G (2021) Hanging protocol optimization of lumbar spine radiographs with machine learning. Skeletal Radiol 50:1809–1819. https://doi.org/10.1007/s00256-021-03733-8
Yi PH, Wei J, Kim TK et al (2020) Automated detection & classification of knee arthroplasty using deep learning. Knee 27:535–542. https://doi.org/10.1016/j.knee.2019.11.020
Yi PH, Kim TK, Wei J et al (2020) Automated detection and classification of shoulder arthroplasty models using deep learning. Skeletal Radiol 49:1623–1632. https://doi.org/10.1007/s00256-020-03463-3
Rouzrokh P, Wyles CC, Philbrick KA et al (2021) A deep learning tool for automated radiographic measurement of acetabular component inclination and version after total hip arthroplasty. J Arthroplasty 36:2510–2517.e6. https://doi.org/10.1016/j.arth.2021.02.026
Shah RF, Bini SA, Martinez AM et al (2020) Incremental inputs improve the automated detection of implant loosening using machine-learning algorithms. Bone Joint J 102-B:101–106. https://doi.org/10.1302/0301-620X.102B6.BJJ-2019-1577.R1
Laur O, Wang B (2022) Musculoskeletal trauma and artificial intelligence: current trends and projections. Skeletal Radiol 51:257–269. https://doi.org/10.1007/s00256-021-03824-6
Bruno MA, Walker EA, Abujudeh HH (2015) Understanding and confronting our mistakes: the epidemiology of error in radiology and strategies for error reduction. Radiographics 35:1668–1676. https://doi.org/10.1148/rg.2015150023
Catapano M, Albano D, Pozzi G et al (2017) Differences between orthopaedic evaluation and radiological reports of conventional radiographs in patients with minor trauma admitted to the emergency department. Injury 48:2451–2456. https://doi.org/10.1016/j.injury.2017.08.054
Rajpurkar P, Irvin J, Bagul A, et al (2017) MURA: large dataset for abnormality detection in musculoskeletal radiographs. http://arxiv.org/abs/1712.06957
Lindsey R, Daluiski A, Chopra S et al (2018) Deep neural network improves fracture detection by clinicians. Proc Natl Acad Sci U S A 115:11591–11596. https://doi.org/10.1073/pnas.1806905115
Krogue JD, Cheng KV, Hwang KM et al (2020) automatic hip fracture identification and functional subclassification with deep learning. Radiol Artif Intell 2:e190023. https://doi.org/10.1148/ryai.2020190023
Ma Y, Luo Y (2021) Bone fracture detection through the two-stage system of Crack-Sensitive Convolutional Neural Network. Informatics Med Unlocked 22:100452. https://doi.org/10.1016/j.imu.2020.100452
Jin L, Yang J, Kuang K et al (2020) Deep-learning-assisted detection and segmentation of rib fractures from CT scans: development and validation of FracNet. EBioMedicine 62:103106. https://doi.org/10.1016/j.ebiom.2020.103106
Zhou Q-Q, Tang W, Wang J et al (2021) Automatic detection and classification of rib fractures based on patients’ CT images and clinical information via convolutional neural network. Eur Radiol 31:3815–3825. https://doi.org/10.1007/s00330-020-07418-z
Pranata YD, Wang K-C, Wang J-C et al (2019) Deep learning and SURF for automated classification and detection of calcaneus fractures in CT images. Comput Methods Programs Biomed 171:27–37. https://doi.org/10.1016/j.cmpb.2019.02.006
Mutasa S, Varada S, Goel A et al (2020) Advanced deep learning techniques applied to automated femoral neck fracture detection and classification. J Digit Imaging 33:1209–1217. https://doi.org/10.1007/s10278-020-00364-8
Tomita N, Cheung YY, Hassanpour S (2018) Deep neural networks for automatic detection of osteoporotic vertebral fractures on CT scans. Comput Biol Med 98:8–15. https://doi.org/10.1016/j.compbiomed.2018.05.011
Liu F, Guan B, Zhou Z et al (2019) Fully automated diagnosis of anterior cruciate ligament tears on knee MR images by using deep learning. Radiol Artif Intell 1:180091. https://doi.org/10.1148/ryai.2019180091
Kim M, Park H, Kim JY, et al (2020) MRI-based diagnosis of rotator cuff tears using deep learning and weighted linear combinations. In: Proceedings of the 5th Machine Learning for Healthcare Conference (PMLR). Vol. 126, p 292–308
Hong G, Zhang L, Kong X, Herbertl L (2021) Artificial intelligence image–assisted knee ligament trauma repair efficacy analysis and postoperative femoral nerve block analgesia effect research. World Neurosurg 149:492–501. https://doi.org/10.1016/j.wneu.2020.11.122
Shin Y, Yang J, Lee YH, Kim S (2021) Artificial intelligence in musculoskeletal ultrasound imaging. Ultrasonography 40:30–44. https://doi.org/10.14366/usg.20080
Hendrickx LAM, Sobol GL, Langerhuizen DWG et al (2020) A machine learning algorithm to predict the probability of (occult) posterior malleolar fractures associated with tibial shaft fractures to guide “malleolus first” fixation. J Orthop Trauma 34:131–138. https://doi.org/10.1097/BOT.0000000000001663
Machine learning consortium, on behalf of the SPRINT and FLOW Investigators (2021) A machine learning algorithm to identify patients with tibial shaft fractures at risk for infection after operative treatment. J Bone Joint Surg Am 103:532–40. https://doi.org/10.2106/JBJS.20.00903
Dallora AL, Anderberg P, Kvist O et al (2019) Bone age assessment with various machine learning techniques: a systematic literature review and meta-analysis. PLoS One 14:e0220242. https://doi.org/10.1371/journal.pone.0220242
Greulich WW, Pyle SI (1959) Radiographic atlas of skeletal development of the hand and wrist. Stanford University Press, Stanford
Tanner JM (2001) Assessment of skeletal maturity and prediction of adult height (TW3 method), 3rd edn. W.B. Saunders, London
Lee B-D, Lee MS (2021) Automated bone age assessment using artificial intelligence: the future of bone age assessment. Korean J Radiol 22:792. https://doi.org/10.3348/kjr.2020.0941
Mettler FA, Huda W, Yoshizumi TT, Mahesh M (2008) Effective doses in radiology and diagnostic nuclear medicine: a catalog. Radiology 248:254–263. https://doi.org/10.1148/radiol.2481071451
Michael DJ, Nelson AC (1989) HANDX: a model-based system for automatic segmentation of bones from digital hand radiographs. IEEE Trans Med Imaging 8:64–69. https://doi.org/10.1109/42.20363
Tanner JM, Oshman D, Lindgren G et al (1994) Reliability and validity of computer-assisted estimates of Tanner-Whitehouse skeletal maturity (CASAS): comparison with the manual method. Horm Res 42:288–294. https://doi.org/10.1159/000184211
Son SJ, Song Y, Kim N et al (2019) TW3-based fully automated bone age assessment system using deep neural networks. IEEE Access 7:33346–33358. https://doi.org/10.1109/ACCESS.2019.2903131
Bui TD, Lee J-J, Shin J (2019) Incorporated region detection and classification using deep convolutional networks for bone age assessment. Artif Intell Med 97:1–8. https://doi.org/10.1016/j.artmed.2019.04.005
Ontell FK, Ivanovic M, Ablin DS, Barlow TW (1996) Bone age in children of diverse ethnicity. AJR Am J Roentgenol 167:1395–1398. https://doi.org/10.2214/ajr.167.6.8956565
Zhang L, Chen J, Hou L et al (2022) Clinical application of artificial intelligence in longitudinal image analysis of bone age among GHD patients. Front Pediatr 10:986500. https://doi.org/10.3389/fped.2022.986500
Joseph GB, McCulloch CE, Sohn JH et al (2022) AI MSK clinical applications: cartilage and osteoarthritis. Skeletal Radiol 51:331–343. https://doi.org/10.1007/s00256-021-03909-2
Halilaj E, Le Y, Hicks JL et al (2018) Modeling and predicting osteoarthritis progression: data from the osteoarthritis initiative. Osteoarthritis Cartilage 26:1643–1650. https://doi.org/10.1016/j.joca.2018.08.003
Lodwick GS, Haun CL, Smith WE et al (1963) Computer diagnosis of primary bone tumors. Radiology 80:273–275. https://doi.org/10.1148/80.2.273
Li MD, Ahmed SR, Choy E et al (2022) Artificial intelligence applied to musculoskeletal oncology: a systematic review. Skeletal Radiol 51:245–256. https://doi.org/10.1007/s00256-021-03820-w
Fanciullo C, Gitto S, Carlicchi E et al (2022) Radiomics of musculoskeletal sarcomas: a narrative review. J Imaging 8:45. https://doi.org/10.3390/jimaging8020045
Richardson ML, Amini B, Kinahan PE (2022) Bone and soft tissue tumors: horizons in radiomics and artificial intelligence. Radiol Clin North Am 60:339–358. https://doi.org/10.1016/j.rcl.2021.11.011
Gillies RJ, Kinahan PE, Hricak H (2016) Radiomics: images are more than pictures, they are data. Radiology 278:563–577. https://doi.org/10.1148/radiol.2015151169
Chianca V, Cuocolo R, Gitto S et al (2021) Radiomic machine learning classifiers in spine bone tumors: a multi-software, multi-scanner study. Eur J Radiol 137:109586. https://doi.org/10.1016/j.ejrad.2021.109586
Gitto S, Cuocolo R, Albano D et al (2020) MRI radiomics-based machine-learning classification of bone chondrosarcoma. Eur J Radiol 128:109043. https://doi.org/10.1016/j.ejrad.2020.109043
Gitto S, Corino VDA, Annovazzi A et al (2022) 3D vs. 2D MRI radiomics in skeletal Ewing sarcoma: feature reproducibility and preliminary machine learning analysis on neoadjuvant chemotherapy response prediction. Front Oncol 12:1016123. https://doi.org/10.3389/fonc.2022.1016123
Casale R, Varriano G, Santone A et al (2023) Predicting risk of metastases and recurrence in soft-tissue sarcomas via radiomics and formal methods. JAMIA Open 6:ooad025. https://doi.org/10.1093/jamiaopen/ooad025
Lisson CS, Lisson CG, Flosdorf K et al (2018) Diagnostic value of MRI-based 3D texture analysis for tissue characterisation and discrimination of low-grade chondrosarcoma from enchondroma: a pilot study. Eur Radiol 28:468–477. https://doi.org/10.1007/s00330-017-5014-6
Pressney I, Khoo M, Endozo R et al (2020) Pilot study to differentiate lipoma from atypical lipomatous tumour/well-differentiated liposarcoma using MR radiomics-based texture analysis. Skeletal Radiol 49:1719–1729. https://doi.org/10.1007/s00256-020-03454-4
Gitto S, Interlenghi M, Cuocolo R et al (2023) MRI radiomics-based machine learning for classification of deep-seated lipoma and atypical lipomatous tumor of the extremities. Radiol Med 128:989–998. https://doi.org/10.1007/s11547-023-01657-y
Gitto S, Cuocolo R, Albano D et al (2021) CT and MRI radiomics of bone and soft-tissue sarcomas: a systematic review of reproducibility and validation strategies. Insights Imaging 12:68. https://doi.org/10.1186/s13244-021-01008-3
Gitto S, Cuocolo R, Emili I et al (2021) Effects of interobserver variability on 2D and 3D CT- and MRI-based texture feature reproducibility of cartilaginous bone tumors. J Digit Imaging 34:820–832. https://doi.org/10.1007/s10278-021-00498-3
Gitto S, Bologna M, Corino VDA et al (2022) Diffusion-weighted MRI radiomics of spine bone tumors: feature stability and machine learning-based classification performance. Radiol Med 127:518–525. https://doi.org/10.1007/s11547-022-01468-7
Zhou X, Wang H, Feng C et al (2022) Emerging applications of deep learning in bone tumors: current advances and challenges. Front Oncol 12:908873. https://doi.org/10.3389/fonc.2022.908873
Eweje FR, Bao B, Wu J et al (2021) Deep learning for classification of bone lesions on routine MRI. EBioMedicine 68:103402. https://doi.org/10.1016/j.ebiom.2021.103402
Navarro F, Dapper H, Asadpour R et al (2021) Development and external validation of deep-learning-based tumor grading models in soft-tissue sarcoma patients using MR imaging. Cancers (Basel) 13:2866. https://doi.org/10.3390/cancers13122866
Zhang R, Huang L, Xia W et al (2018) Multiple supervised residual network for osteosarcoma segmentation in CT images. Comput Med Imaging Graph 63:1–8. https://doi.org/10.1016/j.compmedimag.2018.01.006
Lang N, Zhang Y, Zhang E et al (2019) Differentiation of spinal metastases originated from lung and other cancers using radiomics and deep learning based on DCE-MRI. Magn Reson Imaging 64:4–12. https://doi.org/10.1016/j.mri.2019.02.013
Yin P, Mao N, Chen H et al (2020) Machine and deep learning based radiomics models for preoperative prediction of benign and malignant sacral tumors. Front Oncol 10:564725. https://doi.org/10.3389/fonc.2020.564725
He Y, Guo J, Ding X et al (2019) Convolutional neural network to predict the local recurrence of giant cell tumor of bone after curettage based on pre-surgery magnetic resonance images. Eur Radiol 29:5441–5451. https://doi.org/10.1007/s00330-019-06082-2
Kurtz S, Ong K, Lau E et al (2007) Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am 89:780–785. https://doi.org/10.2106/JBJS.F.00222
Yi PH, Mutasa S, Fritz J (2022) AI MSK clinical applications: orthopedic implants. Skeletal Radiol 51:305–313. https://doi.org/10.1007/s00256-021-03879-5
Yi PH, Kim TK, Wei J et al (2019) Automated semantic labeling of pediatric musculoskeletal radiographs using deep learning. Pediatr Radiol 49:1066–1070. https://doi.org/10.1007/s00247-019-04408-2
Filice RW, Frantz SK (2019) Effectiveness of deep learning algorithms to determine laterality in radiographs. J Digit Imaging 32:656–664. https://doi.org/10.1007/s10278-019-00226-y
Kitamura G (2020) Deep learning evaluation of pelvic radiographs for position, hardware presence, and fracture detection. Eur J Radiol 130:109139. https://doi.org/10.1016/j.ejrad.2020.109139
Ren M, Yi PH (2022) Artificial intelligence in orthopedic implant model classification: a systematic review. Skeletal Radiol 51:407–416. https://doi.org/10.1007/s00256-021-03884-8
Urban G, Porhemmat S, Stark M et al (2020) Classifying shoulder implants in X-ray images using deep learning. Comput Struct Biotechnol J 18:967–972. https://doi.org/10.1016/j.csbj.2020.04.005
Cho BH, Kaji D, Cheung ZB et al (2020) Automated measurement of lumbar lordosis on radiographs using machine learning and computer vision. Global Spine J 10:611–618. https://doi.org/10.1177/2192568219868190
Zheng Q, Shellikeri S, Huang H et al (2020) Deep learning measurement of leg length discrepancy in children based on radiographs. Radiology 296:152–158. https://doi.org/10.1148/radiol.2020192003
Radlink surgical system. https://radlink.com/radlink-surgical-system/. Accessed 14 Aug 2022
Albano D, Gitto S, Messina C et al (2023) MRI-based artificial intelligence to predict infection following total hip arthroplasty failure. Radiol Med 128:340–346. https://doi.org/10.1007/s11547-023-01608-7
Fritz B, Fritz J (2022) Artificial intelligence for MRI diagnosis of joints: a scoping review of the current state-of-the-art of deep learning-based approaches. Skeletal Radiol 51:315–329. https://doi.org/10.1007/s00256-021-03830-8
Berson ER, Aboian MS, Malhotra A, Payabvash S (2023) Artificial intelligence for neuroimaging and musculoskeletal radiology: overview of current commercial algorithms. Semin Roentgenol 58:178–183. https://doi.org/10.1053/j.ro.2023.03.002
Harvey HB, Gowda V (2022) Clinical applications of AI in MSK imaging: a liability perspective. Skeletal Radiol 51:235–238. https://doi.org/10.1007/s00256-021-03782-z
Funding
Investigator Grant awarded by Fondazione AIRC per la Ricerca sul Cancro for the project “RADIOmics-based machine-learning classification of BOne and Soft Tissue Tumors (RADIO-BOSTT)” (L.M. Sconfienza). The funding source provided financial support without any influence on the study design, on the acquisition and analysis of data, and on the writing of the manuscript.
Author information
Authors and Affiliations
Contributions
Search and collection of papers: SG, FS, GR, and SF. Extraction of data: SG, FS, DA, and CM. Paper draft: SG and FS. Draft revision: DA, GR, SF, CM, and LMS. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
Luca Maria Sconfienza is a member of the European Radiology Experimental Editorial Board. He has not taken part in the review or selection process of this article.
The remaining authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gitto, S., Serpi, F., Albano, D. et al. AI applications in musculoskeletal imaging: a narrative review. Eur Radiol Exp 8, 22 (2024). https://doi.org/10.1186/s41747-024-00422-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41747-024-00422-8