Abstract
Purpose
This scoping review aims to identify and describe knowledge gaps and research priorities in veno-arterial extracorporeal membrane oxygenation (VA-ECMO).
Methods
An expert panel was recruited consisting of eight international experts from different backgrounds. First, a list of priority topics was made. Second, the panel developed structured questions using population, intervention, comparison and outcomes (PICO) format. All PICOs were scored and prioritized. For every selected PICO, a structured literature search was performed.
Results
After an initial list of 49 topics, eight were scored as high-priority. For most of these selected topics, current literature is limited to observational studies, mainly consisting of retrospective cohorts. Only for ECPR and anticoagulation, randomized controlled trials (RCTs) have been performed or are ongoing. Per topic, a summary of the literature is stated including recommendations for further research.
Conclusions
This scoping review identifies and presents an overview of knowledge gaps and research priorities in VA-ECMO. Current literature is mostly limited to observational studies, although with increasing attention for this patient population, more RCTs are finishing or ongoing. Translational research, from preclinical trials to high-quality or randomized controlled trials, is important to improve the standard practices in this critically ill patient population.
Take-home message
This scoping review identifies and presents an overview of research gaps and priorities in VA-ECMO. Translational research, from preclinical trials to high-quality or randomized controlled trials, is important to improve the standard practices in this critically ill patient population.
Similar content being viewed by others
Introduction
Veno-arterial extracorporeal membrane oxygenation (VA-ECMO) is a mechanical circulatory support (MCS) used for refractory cardio-circulatory failure, in case conventional therapies prove insufficient [1]. The past decades, the use and range of indications for VA-ECMO has been increasing worldwide. In 2021, almost 5000 VA-ECMO runs were recorded in the ELSO registry, of which 48% survived [2]. Despite the promising role of VA-ECMO as a cornerstone supportive treatment, complication rates remain high and should be better evaluated in daily practice. Available guidelines are mainly based on expert opinion, resulting in a high variance in local protocols worldwide. It is important to identify topics which require further attention in this complex, critically ill patient population. Therefore, the aim of this study is to identify and describe research gaps in VA-ECMO, and to form recommendations for future research.
Methods
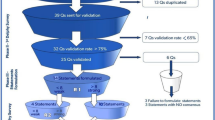
This scoping review was performed in several steps. Firstly, an expert panel was developed consisting of eight international experts with research lines and backgrounds in different medical specialties involved in VA-ECMO practices (Additional file 1 p.2). Secondly, an initial list of topics was developed by JR, CV and AV. The expert panel was invited to submit additional topics to this list and give feedback on the topics stated. Thirdly, per topic, structured questions using population, intervention, comparison and outcomes (PICO) format were created. Fourthly, the PICOs were prioritized by rating the importance of every PICO on a scale of 0–10 using a cloud-based survey tool. The eight PICOs with the highest ratings were considered as highest priority. Fifthly, search strategies were developed for every selected PICO, whereafter searches in MEDLINE, EMBASE, Web of Science, Google Scholar, and Cochrane databases were performed up to September 2022, along with trial databases (clinicaltrials.gov, ISRCTN) to identify in-progress trials. Per topic, the search results were screened by two experts (Additional file 1 p. 9–40).
Results
The initial list of 49 topics can be found in the Additional file 1 (p. 3–8). After rating the degree of importance, eight topics were selected as depicted in Table 1 and shown in Fig. 1.
Priority topics further elaborated in this review. From left to right: a percutaneous versus surgical methods of cannulation, b anticoagulant therapies, c blood transfusion regimen, d daily therapy goals, e ECPR selection criteria, f cardiogenic shock, g optimal balance of blood pressure and vasoactive medication, h endothelial activation and damage.
Part I: indication for ECMO
#1 Cardiogenic shock: definition, degree and timing of cardiogenic shock as VA-ECMO indication
Timing of ECMO
Clinical benefits of VA-ECMO for cardiogenic shock (CS) due to acute myocardial infarction (AMI) have been demonstrated in observational studies [3]. For defining the degree of severity of cardiogenic shock, different classification systems are available. Most commonly used classifications include the INTERMACS (Interagency Registry for Mechanically Assisted Circulatory Support) and Society for Cardiovascular Angiography and Interventions (SCAI). VA-ECMO may play a role in either INTERMACS’ level 1 and 2 (1: “crash and burn” vs. 2:“early” phase, “sliding on inotropes”). Initiation is usually employed for patients refractory to usual resuscitative techniques (i.e., inotropes and vasopressors [4]. Guidelines recommend that MCS, including VA-ECMO, may be considered in patients with either any type of CS (Heart Failure Guidelines [5]) or due to acute coronary syndrome (ACS, European Guidelines [6]). However, there is no consensus on optimal timing. ‘The sooner the better’ seems likely to assume, but its relation with the timing of reperfusion, and to the risk benefit of any MCS device remains unclear.
Before or after percutaneous coronary intervention (PCI)
In patients with CS from different etiologies, shorter time from shock onset to ECMO insertion is associated with lower risk of mortality in observational studies [7]. In selected patients with CS due to AMI undergoing PCI, early ECMO initiation prior to PCI resulted in better short- and long-term outcomes, even though this resulted in a longer door-to-balloon time [7,8,9,10]. However, respective studies included small numbers of patients, of whom up to 60% experienced a cardiac arrest. In a single cohort of AMI-CS patients that excluded post-cardiac arrest patients, no association between time of CS onset to VA-ECMO start time and 6-month survival was found [11]. Moreover, no conclusion can yet be drawn regarding which MCS could be most beneficial. Available studies show divergent results, whereas crucial variables as timing and severity of disease may be insufficiently corrected for RCTs on this topic are lacking.
Extracorporeal cardiopulmonary resuscitation (ECPR)
In case of refractory cardiac arrest, ECPR combined with intra-arrest PCI leads to better outcomes in terms of survival as shown in the highly selected ARREST trial [12]. In the larger PRAGUE-OHCA trial such a mortality benefit could not be observed, although it does suggest a favorable neurological outcome in patients receiving ECPR [13]. Contrarily, observational studies suggest that a delay in the start of ECPR is an independent predictor of poor neurological outcome and should ideally be minimalized to < 40 min [14].
Future research
Timing of VA-ECMO in CS needs further attention. In line with this, we would recommend to also focus on the simultaneous or consecutive use of alternative MCS such as Impella (“ECPELLA”).
#2 Patient selection criteria for ECPR
Patient selection for ECPR is a significant challenge for clinicians: on one hand, stringent selection criteria for ECPR would result in a higher proportion of patients with favorable outcome, however, this would also significantly limit its use in victims of refractory cardiac arrest [15]. Existing literature on ECPR effectiveness is mainly limited to cohort studies, with recently first RCTs published (Table 2) [12, 13, 16, 17].
Age and patients’ characteristics
Latest ELSO interim guidelines suggest a maximum age of 70 years for ECPR [18]. However, a large retrospective study found that in case of a low-flow state < 60 min, ECPR survival rates were independent of age, suggesting that some elderly patients could be still be considered [19]. At the time of ECPR assessment, patient’s medical history, laboratory values and severity scores are often unknown or difficult to obtain and can rarely be used into the decisional algorithm. Whether only patients with an initial shockable rhythm should be treated remains a matter of debate, as acceptable survival rates have been reported also for non-shockable rhythm, in particular pulseless activity [20, 21].
Time-to-ECMO and location of ECMO insertion
Time-to-ECMO is associated with neurological outcome and survival. The ELSO advises early assessment for ECPR and time from arrest to ECMO (i.e., “low-flow interval”) to be < 60 min [18]. Different cut-offs of no-flow or low-flow intervals have been evaluated in observational studies, varying from 30 to 60 min. Importantly, the longer the low-flow interval, the higher the difference in outcome between ECPR and conventional cardiopulmonary resuscitation (CPR) treated patients, whereas nearly 20% of patients undergoing ECPR with a time-to-ECMO > 60 min would eventually still experience favorable neurological outcome [22].
To reduce the low-flow time, which remains of approximately one hour even in RCTs [12, 13], there are two main strategies: (a) expedited transport to the hospital to reduce the time to hospital arrival [16]; or (b) pre-hospital ECMO implantation [23]. Currently, a stepped-wedge designed trial comparing on-scene initiation of ECPR and conventional CPR, is ongoing in the Netherlands (NCT04620070).
Future research
As ECPR is also dependent of country-specific logistics and infrastructure, identifying optimal patient selection criteria is an essential step to further evaluate the role of ECPR.
Part II: ECMO cannulation
#3 ECMO connecting: percutaneous vs surgical cannulation methods
Multiple cannulation techniques have been widely accepted for VA-ECMO. In case of post-cardiotomy patients, central placement of the cannulas might be considered, although recent data showed higher mortality rate as compared to peripheral access in such a setting [24, 25]. In non-cardiotomy patients, peripheral cannulation, either via percutaneous or surgical placement, is preferred due to the speed and easy accessibility [1]. Moreover, next to the indication, location (in-hospital or on-scene) can play a role in choice of cannulation technique. Despite their practical differences, limited data are available on the impact of placement methods on patients’ outcomes.
Percutaneous vs surgical cannulation
A recent analysis of the ELSO Registry including 12,592 patients receiving VA-ECMO showed a decreased in-hospital mortality in favor of percutaneous cannulation when compared to the surgical group [26]. This finding was supported by a propensity-score matched analysis comparing complication rates and survival in surgical versus percutaneous peripheral cannulation [27]. This can be explained by a lower degree of cannulation site bleeding and systematic infection in case of percutaneous cannulation [26]. However, percutaneous cannulation was found to be associated with an increased rate of vascular complications after decannulation, for which further attention is needed [27].
Both surgical as peripheral cannulation bring the risk of limb ischemia. As a result, following femoral cannulation, the adequacy of limb perfusion and absence of ischemia should be carried out by the use of near-infrared spectroscopy (NIRS). If NIRS is > 50–60% at both legs, a distal perfusion catheter (DPC) might be, theoretically, not necessary. However, distal limb perfusion is always recommended to avoid late intervention in case of leg ischemia, with the occurrence of potential irreversible injury. Either in case of larger cannulas (19–21 Fr), a NIRS-value < 50–60%, or a difference over 20% between the arterial and venous cannulated leg, DPCs are recommended [28].
Future research
Less invasive percutaneous approach appears to be favorable in terms of complications and patient outcome. However, this does not take into account the rate of cannulation failure, which may be higher using percutaneous methods. With the rising interest of ECPR, complication rates between different locations of cannulation (in-hospital vs. on-scene) should also be taken into account. Also, decannulation differs between the two methods, leading to different complication profiles. Future studies should focus on preventing cannulation failure and improving decannulation care, such as removal techniques, in a prospective setting.
Part III: ECMO support care
#4 Monitoring: daily therapy goals
Reducing blood flow
ELSO guidelines state the ideal situation in patients on VA-ECMO would be to decrease blood flow until the arterial pulse pressure is a minimum of 10 mmHg or to provide adequate support, according to other parameters of organ perfusion [1]. As over time the native cardiac function is expected to improve, there is rationale for evaluating on a daily basis whether blood flow can be reduced. However, few data have been reported about the course of ECMO blood flow and its manipulation over time in this setting.
Fluid balance
ECMO blood flow and patient’s volume status are intertwined, as on one hand sufficient intravascular volume is required to ensure adequate blood flow and organ perfusion, while on the other hand volume overload has to be prevented, despite the high occurrence rate of blood transfusions and the occurrence of acute kidney injury in these patients. Retrospective studies in VA-ECMO patients also showed a correlation between a higher fluid balance and mortality [29,30,31]. However, no recommendations regarding optimal fluid balances are described in the latest ELSO guideline, and no high-quality data on the optimal fluid strategy are available for such patients [32].
Reducing sedatives
Factors influencing sedative and analgesic management include additional treatments, such as the application of targeted temperature management, but also cannula related discomfort, and a possible change in drug pharmacokinetics due to the ECMO system (i.e., drug absorption) [33]. For sedation, it is commonly advised to provide light anesthesia in the first 24 h and then adjust therapy to relieve patient’s anxiety and discomfort, but still allowing a daily repeated neurological examination [1]. For some indications, such as bridge to lung transplant, awake support is also a possibility. No hard recommendations can be made based on current literature regarding the use of paralytics, whereas different factors should be taken into account (i.e., need for controlled mechanical ventilation, physical therapy). With regard to sedative management, different observational studies reported comparisons of different anesthetics and analgesics used, but otherwise literature is scarce.
Future research
In different critically ill patient populations, current studies focus more and more on how to prevent or treat fluid overload, for example using lung ultrasound. Prospective studies VA-ECMO patients, focusing on visualizing and evaluating fluid status and blood flow, are needed.
#5 Monitoring: optimal balance of blood pressure and vasoactive medication, “less is more?”
In all-cause shock patients, a target mean arterial pressure (MAP) of above 65 mm Hg is being aimed for. This is mostly based on settings of sepsis, in which below this threshold autoregulation fails and tissue perfusion becomes dependent on the MAP’s driving pressure [34]. Current MAP targets in other shock etiologies are based on the same principles, despite having a different pathophysiology. In cardiogenic shock, no clear MAP targets can be set due to limited supporting data [35].
Similarly, in (VA-)ECMO the optimal MAP target remains unknown. ELSO guidelines lack recommendations, except for when ‘left ventricle overloading’ occurs, in which it is recommended to reduce the MAP to the lowest acceptable value [36]. In case of ECPR, ELSO guidelines recommend a MAP between 60 and 80 mmHg [18]. Interestingly, a recent RCT studying blood pressure targets in comatose OHCA patients found equal survival and neurological outcomes in the low- (63 mm Hg) versus high-target group (77 mm Hg) [37]. Theoretically, as the heart has to eject against a continuous blood flow generated by the ECMO device, a lower MAP seems favorable as it reduces afterload, and therefore decreases myocardial oxygen demand, possibly optimizing native cardiac output in the already failing heart [38,39,40].
Currently, only one study addresses the question of optimal MAP targeting in adult patients on ECMO. In this retrospective observational study of 116 patients, a higher average MAP was associated with survival to discharge [38]. Furthermore, in patients with a lower MAP, a higher incidence of kidney injury was found. However, a lower MAP might also be the result of other factors contributing to the poor prognosis, rather than the cause. Due to its retrospective character and small cohort size, no definite conclusions can be drawn regarding the optimal MAP for ECMO.
Future research To fill this knowledge gap RCTs are needed. However, in the short future an RCT will start in the Netherlands, randomizing patients with AMI-CS to either a standard MAP or to a MAP ≥ 55 mmHg. This will be the first step towards determining optimal MAP targets in CS patients. Afterwards, the same study design might be considered to define MAP targets in patients on VA-ECMO.
#6: Adjuvant treatments: blood transfusion regimen
Transfusion guidelines for VA-ECMO patients are limited to expert-opinion statements in the ELSO guidelines [1]. Advised thresholds are quite liberal, resulting in a high inter-center variance in thresholds used [41]. Current literature consists mainly of observational studies.
Red blood cells (RBC)
RBC transfusion during VA-ECMO is common: 82–100% of patients receive RBC transfusion with a mean of 24 RBC units per run [42,43,44,45]. The hemoglobin (Hb) thresholds described are relatively liberal [46]. One of the hypotheses for these liberal thresholds in VA-ECMO is that patients with cardiac failure can develop tissue hypoxemia due to reduced cardiac output. The resulting decreased delivery of oxygen (DO2) can be compensated by providing a larger Hb buffer. This, however, does not consider that by the blood flow created by VA-ECMO, a fixed cardiac output, and thus DO2, can largely be maintained. Evidence to either confirm or refute this hypothesis is lacking.
Platelets
Thrombocytopenia and impaired platelet function are common during VA-ECMO [47, 48]. ELSO guidelines recommend platelet transfusion to maintain a platelet count over 80 × 109/L [1]. Platelet transfusion occurs in 20–50% of patients, while reasons for transfusion are not described [42, 49]. Both severity of thrombocytopenia and platelet transfusion have been associated with mortality [49, 50].
Plasma
ELSO guidelines state that indications for plasma transfusion include (i) (suspicion of) decreased antithrombin levels; (ii) correction of coagulation disturbances or (iii) system priming [1]. One-third of patients receive plasma transfusions; however, reasons and indications for transfusion are not properly recorded [42, 51]. As the role of plasma in treating hemorrhage is disputable, while increasing the risk of transfusion-related complications, routine use of plasma should be well considered [52, 53].
Hemorrhage
Hemorrhage is one of the main complications during VA-ECMO, occurring in up 60% of patients [54,55,56]. Central cannulation increases the risk of a hemorrhagic event, with an occurrence rate of 52% vs. 33% in peripheral cannulation [57]. Hemorrhage is associated with all types of transfusion and mortality on ECMO [58]. Therefore, preventing hemorrhage should be a priority in this vulnerable population.
Future research
Currently, no prospective studies focusing on transfusion thresholds have been announced. We recommend that reasons for transfusion of RBC, platelets and plasma should be further explored. In other non-ECMO patients, using a restrictive Hb threshold for RBC transfusion has been shown to be safe [59, 60]. As RBC transfusion is most commonly transfused in patients on ECMO, future research should first focus on the optimal Hb threshold for RBC transfusion in VA-ECMO.
#7 Adjuvant treatments: anticoagulant therapies
Hemostasis during ECMO is a precarious balance: While (systemic) anticoagulation is needed to prevent thrombotic complications, hemorrhage remains one of the main complications during ECMO. Different therapies, targets and monitoring options can be used, however, this PICO will focus on therapies only.
Unfractionated heparin
Unfractionated heparin (UFH) is one of the classic and most commonly used anticoagulant agents during ECMO [61]. However, it comes along with a risk of developing heparin-induced thrombocytopenia (HIT), and recommendations regarding the ideal targets are pending. Several cohort studies have described the safe use of lower UFH anticoagulation targets, showing a decrease of hemorrhagic events without an increase of thrombotic events [62,63,64].
Low-molecular weight heparin
Low-molecular weight heparin (LMWH) has been shown to be safe and effective in renal replacement therapy (RRT) [65]. The past years, a handful of retrospective studies appeared on LMWH in ECMO, showing an equal rate of hemorrhage and reduced thrombotic event rate in favor of LMWH [66]. Prospective studies, however, are lacking.
Direct thrombin inhibitors (DTIs)
The use of DTIs in ECMO has been increasing, despite the shortage of prospective studies, mainly indicated in case of suspected HIT. Reports of the use of argatroban in VA-ECMO are limited to case series [67]. More is known about bivalirudin, of which observational retrospective studies show the safe use with regard to hemorrhagic and thrombotic complications [68, 69].
Absent systemic anticoagulation
As a result of improved ECMO coating, rationale has shifted towards lower anticoagulation targets. Some have taken it even further, describing the safe withhold of anticoagulation in VA-ECMO [70]. However, limitations include a retrospective design and small sample sizes, thereby limiting generalizability.
Future research
One pilot study has been performed comparing UFH targets. Currently, one RCT is ongoing with expected results early 2024, comparing two systemic heparin regimes (high and low target) and LMWH (NCT04536272) [71]. Further work is required to evaluate the best fit anticoagulant therapy and targets in VA-ECMO.
#8 Complications: endothelial activation and damage
ECMO induces a systemic inflammatory response due to among others exposure of the patient’s blood to the foreign surface of the ECMO circuit. This results in a variety of coagulative and inflammatory cascades and complex interactions with the endothelium [72].
Adhesion molecules and selectins
Activated endothelium is characterized by overexpression of adhesion molecules, such as ICAM1 and VCAM1, and selectins (P-selectin and E-selectin) that facilitate leukocyte adhesion, rolling, and transmigration of activated neutrophils. So far, neither adhesion molecules nor selectins have been studied in adult patients on VA-ECMO.
von Willebrand factor
Upon endothelial activation, von Willebrand factor (vWF) is released from the Weibel–Palade bodies [73]. In VA-ECMO patients, vWF antigen levels were high compared to values in healthy controls and remained high within the first 5 days after initiation of ECMO [74].
Angiopoietin-2 and VEGF
Also released from Weibel–Palade bodies is angiopoietin-2. Angiopoietin-2 is a growth factor and associated with increased endothelial permeability and organ dysfunction in patients on cardiopulmonary bypass (CPB) [75, 76]. Although angiopoietin-2 levels remained stable within the first three days on VA-ECMO, angiopoietin-2 levels were significantly higher in non-survivors compared to survivors [77]. Within the same study, vascular endothelial growth factor (VEGF), a pro-inflammatory growth factor that enhances endothelial permeability, increased over the first three days of VA-ECMO support. Interestingly, VEGF was lower in non-survivors compared survivors of VA-ECMO support [77].
Thrombomodulin
Thrombomodulin is a thrombin receptor on endothelial cells and released after injury [78]. In patients on VA-ECMO, no differences were found in soluble thrombomodulin levels over time nor between survivors and non-survivors [77].
Extracellular vesicles
Upon endothelial activation, extracellular vesicles (EVs) are released, which can mediate intercellular communication. Patients on VA-ECMO had increased levels of endothelial-derived EVs after ECMO initiation compared to healthy controls [79]. A follow-up study showed that endothelial-derived EVs did not differ between survivors and non-survivors [80]. Interestingly, EVs derived from leukocytes were associated with outcome [80]. These specific EVs are suggested to induce endothelial dysfunction [81].
Future research
Activation of the endothelium seems a less well recognized complication during ECMO, even though it is known from patients on CPB that it is associated with organ dysfunction [76]. The above-described results show preliminary evidence of endothelial activation in patients on VA-ECMO. Although inflammation and endothelial activation might be prices to pay for the benefits of ECMO, we do however recommend further exploration to possibly counteract the detrimental effects. Lastly, in other critically ill patient populations, recently successful evaluation of pharmacological interventions in a translational matter have been performed (i.e., protein kinase inhibitors in acute respiratory distress syndrome). This approach may also be applied to identify and prevent the negative effects of endothelial activation and damage.
Discussion and conclusion
This scoping review describes a selection of eight high-priority topics in which further research should be performed in patients receiving VA-ECMO, as identified by an expert panel. The expert panel primarily identified almost fifty topics for further elucidation, thereby emphasizing the many current knowledge gaps and research priorities. Available guidelines specific to patients receiving VA-ECMO are scarce, and in the ones available, many topics are based on expert-opinion only. This does not come as a surprise, as for some topics, only one study on the subject could be found. There is a strong need for further evidence-based research in this critically ill patient population.
For all sub-topics, the majority of studies consisted of an observational design. These observational studies often had a retrospective design, and were performed in a single-center setting. This comes along with different disadvantages, including a high risk of different types of bias (i.e., selection, immortal time), confounding and even methodological errors. Moreover, the single-center design impedes translation to other centers or countries, even more when taking into account the lack of evidence-based guidelines. For example, in case of ECPR, due to a different hospital occupancy per country, time-to-ECMO can differ drastically and thereby influence the result either too positive (short time-to-ECMO, high amount of expert centers and resources) or too negative.
Only a handful of RCTs have been performed on patients receiving ECMO. However, those are limited to either respiratory support (i.e., venovenous ECMO) or ECPR. Of these RCTs, stopping early due to meeting the stopping criteria, either for futility or superiority, is not uncommon [12, 13]. RCTs in VA-ECMO can face multiple important obstacles. Firstly, due to the wide range of indications, the patient population receiving VA-ECMO shows a high amount of heterogeneity: for example, ECPR and failure to wean cardiopulmonary bypass may come with different aspects of clinical attention and prognoses. Secondly, multicenter cooperation is key for a feasible study, whereas the number of runs performed by a center can range from a few to high-output. Lastly, ethical considerations play a large role, as patients are per definition unconscious and thus informed consent is dependent on by-proxy or deferred consent strategies.
As a result of the lack of studies in patients receiving VA-ECMO, the next best option consists of the translation and transposition of studies performed in similar patient populations. Although this may be sufficient in some cases, this does not always apply. Different research strategies are required to answer the topics as stated in this review. For example, a translational approach is key in further studying endothelial activation, wherein an essential step is the forming and testing of hypotheses in in vitro and animal models. To study the more general topics, such as cannulation method, it may be sufficient to focus primarily on large observational studies or RCTs as it involves all patients receiving VA-ECMO. Alternative subjects such as choice of anticoagulation on the other hand are preferred to be performed in a homogenous population, whereas a different profile of coagulation disturbances and thus bleeding risk may play an important role.
Currently, several RCTs are ongoing and results are expected within the upcoming years (NCT04620070, NCT04536272). However, with the yearly increasing amount of ECMO centers, indications for VA-ECMO and thus runs and numbers of patients supported with VA-ECMO, more research is needed on a shorter notice. Alternative study designs, such as adaptive platform trials, may be of use in evaluating different adjuvant treatments in VA-ECMO simultaneously and efficiently, such as combining transfusion regimen and evaluating microcirculatory disturbances.
In conclusion, this scoping review identifies and presents an overview of research gaps and priorities in VA-ECMO. Gaining data from translational high-quality research, ranging from preclinical and animal studies to RCTs is important to improve the standard practices in this patient population.
Availability of data and materials
Not applicable.
Abbreviations
- ACS:
-
Acute coronary syndrome
- AMI:
-
Acute myocardial infarction
- CPB:
-
Cardiopulmonary bypass
- CPR:
-
Cardiopulmonary resuscitation
- CS:
-
Cardiogenic shock
- DPC:
-
Distal perfusion catheter
- DTI:
-
Direct thrombin inhibitor
- ECPR:
-
Extracorporeal cardiopulmonary resuscitation
- ED:
-
Emergency department
- ELSO:
-
Extracorporeal Life Support Organization
- Hb:
-
Hemoglobin
- HIT:
-
Heparin-induced thrombocytopenia
- ICAM-1:
-
Intercellular adhesion molecule-1
- INTERMACS:
-
Interagency Registry for Mechanically Assisted Circulatory Support
- LMWH:
-
Low-molecular weight heparin
- MAP:
-
Mean arterial pressure
- MCS:
-
Mechanical circulatory support
- NIRS:
-
Near-infrared spectroscopy
- PCI:
-
Percutaneous coronary intervention
- PICO:
-
Patient/population, intervention, comparison and outcomes
- RBC:
-
Red blood cell
- RCT:
-
Randomized controlled trial
- RRT:
-
Renal replacement therapy
- SCAI:
-
Society for Cardiovascular Angiography and Interventions
- UFH:
-
Unfractionated heparin
- VA-ECMO:
-
Veno-arterial extracorporeal membrane oxygenation
- VCAM1:
-
Vascular cell adhesion molecule 1
- VEGF:
-
Vascular endothelial growth factor
- vWF:
-
von Willebrand factor
References
ELSO (2017) General Guidelines for all ECLS Cases. ELSO Guidel 1–26
Extracorporeal Life Support Organization (2022) ELSO International Summary of Statistics | ECMO | ECLS. ECLS Regist Rep 2022:1–1
Ouweneel DM, Schotborgh JV, Limpens J et al (2016) Extracorporeal life support during cardiac arrest and cardiogenic shock: a systematic review and meta-analysis. Intensive Care Med 42:1922–1934. https://doi.org/10.1007/s00134-016-4536-8
Naidu SS, Baran DA, Jentzer JC et al (2022) SCAI SHOCK stage classification expert consensus update: a review and incorporation of validation studies: this statement was endorsed by the American College of Cardiology (ACC), American College of Emergency Physicians (ACEP), American Heart Association. J Am Coll Cardiol 79:933–946. https://doi.org/10.1016/j.jacc.2022.01.018
McDonagh TA, Metra M, Adamo M et al (2021) 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 42:3599–3726. https://doi.org/10.1093/eurheartj/ehab368
Collet JP, Thiele H, Barbato E et al (2021) 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J 42:1289–1367. https://doi.org/10.1093/eurheartj/ehaa575
Lee HH, Kim HC, Ahn CM et al (2021) Association between timing of extracorporeal membrane oxygenation and clinical outcomes in refractory cardiogenic shock. JACC Cardiovasc Interv 14:1109–1119. https://doi.org/10.1016/j.jcin.2021.03.048
Choi KH, Yang JH, Hong D et al (2020) Optimal timing of venoarterial-extracorporeal membrane oxygenation in acute myocardial infarction patients suffering from refractory cardiogenic shock. Circ J 84:1502–1510. https://doi.org/10.1253/circj.CJ-20-0259
Huang CC, Hsu JC, Wu YW et al (2018) Implementation of extracorporeal membrane oxygenation before primary percutaneous coronary intervention may improve the survival of patients with ST-segment elevation myocardial infarction and refractory cardiogenic shock. Int J Cardiol 269:45–50. https://doi.org/10.1016/j.ijcard.2018.07.023
Del Rio-Pertuz G, Benjanuwattra J, Juarez M et al (2022) Efficacy of mechanical circulatory support used before versus after primary percutaneous coronary intervention in patients with cardiogenic shock from ST-elevation myocardial infarction: a systematic review and meta-analysis. Cardiovasc Revascularization Med 42:74–83. https://doi.org/10.1016/j.carrev.2022.05.002
Szczanowicz L, Majunke N, de Waha-thiele S et al (2021) Predictors of clinical outcome after early veno-arterial extracorporeal oxygenation in cardiogenic shock complicating ST-elevation myocardial infarction. J invasive Cardiol 33:E329–E335
Yannopoulos D, Bartos J, Raveendran G et al (2020) Advanced reperfusion strategies for patients with out-of-hospital cardiac arrest and refractory ventricular fibrillation (ARREST): a phase 2, single centre, open-label, randomised controlled trial. Lancet 396:1807–1816. https://doi.org/10.1016/S0140-6736(20)32338-2
Belohlavek J, Smalcova J, Rob D et al (2022) Effect of intra-arrest transport, extracorporeal cardiopulmonary resuscitation, and immediate invasive assessment and treatment on functional neurologic outcome in refractory out-of-hospital cardiac arrest. JAMA J Am Med Assoc 327:737–747. https://doi.org/10.1001/jama.2022.1025
Kuroki N, Abe D, Iwama T et al (2017) Association between delay to coronary reperfusion and outcome in patients with acute coronary syndrome undergoing extracorporeal cardiopulmonary resuscitation. Resuscitation 114:1–6. https://doi.org/10.1016/j.resuscitation.2017.02.007
Lunz D, Calabrò L, Belliato M et al (2020) Extracorporeal membrane oxygenation for refractory cardiac arrest: a retrospective multicenter study. Intensive Care Med 46:973–982. https://doi.org/10.1007/s00134-020-05926-6
Hsu CH, Meurer WJ, Domeier R et al (2021) Extracorporeal cardiopulmonary resuscitation for refractory out-of-hospital cardiac arrest (EROCA): results of a randomized feasibility trial of expedited out-of-hospital transport. Ann Emerg Med 78:92–101. https://doi.org/10.1016/j.annemergmed.2020.11.011
Bol ME, Suverein MM, Lorusso R et al (2019) Early initiation of extracorporeal life support in refractory out-of-hospital cardiac arrest: design and rationale of the INCEPTION trial. Am Heart J 210:58–68. https://doi.org/10.1016/j.ahj.2018.12.008
Richardson ASC, Tonna JE, Nanjayya V et al (2021) Extracorporeal cardiopulmonary resuscitation in adults. Interim guideline consensus statement from the extracorporeal life support organization. ASAIO J 67:221–228. https://doi.org/10.1097/MAT.0000000000001344
Yu HY, Wang CH, Chi NH et al (2019) Effect of interplay between age and low-flow duration on neurologic outcomes of extracorporeal cardiopulmonary resuscitation. Intensive Care Med 45:44–54. https://doi.org/10.1007/s00134-018-5496-y
Debaty G, Babaz V, Durand M et al (2017) Prognostic factors for extracorporeal cardiopulmonary resuscitation recipients following out-of-hospital refractory cardiac arrest. A systematic review and meta-analysis. Resuscitation 112:1–10. https://doi.org/10.1016/j.resuscitation.2016.12.011
Tanimoto A, Sugiyama K, Tanabe M et al (2020) Out-of-hospital cardiac arrest patients with an initial non-shockable rhythm could be candidates for extracorporeal cardiopulmonary resuscitation: a retrospective study. Scand J Trauma Resusc Emerg Med 28:101. https://doi.org/10.1186/s13049-020-00800-2
Chen YS, Lin JW, Yu HY et al (2008) Cardiopulmonary resuscitation with assisted extracorporeal life-support versus conventional cardiopulmonary resuscitation in adults with in-hospital cardiac arrest: an observational study and propensity analysis. Lancet 372:554–561. https://doi.org/10.1016/S0140-6736(08)60958-7
Bougouin W, Dumas F, Lamhaut L et al (2020) Extracorporeal cardiopulmonary resuscitation in out-of-hospital cardiac arrest: a registry study. Eur Heart J 41:1961–1971. https://doi.org/10.1093/eurheartj/ehz753
Kowalewski M, Zieliński K, Brodie D et al (2021) Venoarterial extracorporeal membrane oxygenation for postcardiotomy shock—analysis of the extracorporeal life support organization registry∗. Crit Care Med 49:1107–1117. https://doi.org/10.1097/CCM.0000000000004922
Mariscalco G, Salsano A, Fiore A et al (2020) Peripheral versus central extracorporeal membrane oxygenation for postcardiotomy shock: multicenter registry, systematic review, and meta-analysis. J Thorac Cardiovasc Surg 160:1207-1216.e44. https://doi.org/10.1016/j.jtcvs.2019.10.078
Wang L, Yang F, Zhang S et al (2022) Percutaneous versus surgical cannulation for femoro-femoral VA-ECMO in patients with cardiogenic shock: results from the Extracorporeal Life Support Organization Registry. J Hear Lung Transplant 41:470–481. https://doi.org/10.1016/j.healun.2022.01.009
Danial P, Hajage D, Nguyen LS et al (2018) Percutaneous versus surgical femoro-femoral veno-arterial ECMO: a propensity score matched study. Intensive Care Med 44:2153–2161. https://doi.org/10.1007/s00134-018-5442-z
MacLaren G, Peek GJ, Lorusso R, et al (2022) The ELSO Red Book, 6th Edition, 6th ed. Extracorporeal Life Support Organization, Ann Arbor, United States
Schmidt M, Bailey M, Kelly J et al (2014) Impact of fluid balance on outcome of adult patients treated with extracorporeal membrane oxygenation. Intensive Care Med 40:1256–1266. https://doi.org/10.1007/s00134-014-3360-2
Staudacher DL, Gold W, Biever PM et al (2017) Early fluid resuscitation and volume therapy in venoarterial extracorporeal membrane oxygenation. J Crit Care 37:130–135. https://doi.org/10.1016/j.jcrc.2016.09.017
Besnier E, Boubèche S, Clavier T et al (2020) Early positive fluid balance is associated with mortality in patients treated with veno-arterial extra corporeal membrane oxygenation for cardiogenic shock: a retrospective cohort study. Shock 53:426–433. https://doi.org/10.1097/SHK.0000000000001381
Bridges BC, Dhar A, Ramanathan K et al (2022) Extracorporeal life support organization guidelines for fluid overload, acute kidney injury, and electrolyte management. ASAIO J 68:611–618. https://doi.org/10.1097/MAT.0000000000001702
Dzierba AL, Abrams DC, Muir J, Brodie D (2019) Ventilatory and pharmacotherapeutic strategies for management of adult patients on extracorporeal life support. Pharmacotherapy 39:355–368. https://doi.org/10.1002/phar.2230
Alhazzani W, Møller MH, Arabi YM et al (2020) Surviving Sepsis Campaign: guidelines on the management of critically ill adults with Coronavirus Disease 2019 (COVID-19). Intensive Care Med 46:854–887. https://doi.org/10.1007/s00134-020-06022-5
Van Diepen S, Katz JN, Albert NM, et al (2017) Contemporary Management of Cardiogenic Shock: A Scientific Statement from the American Heart Association
Lorusso R, Shekar K, MacLaren G et al (2021) ELSO interim guidelines for venoarterial extracorporeal membrane oxygenation in adult cardiac patients. ASAIO J 67:827–844. https://doi.org/10.1097/MAT.0000000000001510
Kjaergaard J, Møller JE, Schmidt H et al (2022) Blood-pressure targets in comatose survivors of cardiac arrest. N Engl J Med. https://doi.org/10.1056/nejmoa2208687
Tanaka D, Shimada S, Mullin M et al (2019) What is the optimal blood pressure on veno-arterial extracorporeal membrane oxygenation? Impact of mean arterial pressure on survival. ASAIO J 65:336–341. https://doi.org/10.1097/MAT.0000000000000824
Pyles LA, Gustafson RA, Fortney J, Einzig S (2010) Extracorporeal membrane oxygenation induced cardiac dysfunction in newborn lambs. J Cardiovasc Transl Res 3:625–634. https://doi.org/10.1007/s12265-010-9215-5
Choi MS, Sung K, Cho YH (2019) Clinical pearls of venoarterial extracorporeal membrane oxygenation for cardiogenic shock. Korean Circ J 49:657–677. https://doi.org/10.4070/kcj.2019.0188
De Bruin S, Scheeren TWL, Bakker J et al (2019) Transfusion practice in the non-bleeding critically ill: an international online survey-the TRACE survey. Crit Care 23:1–8. https://doi.org/10.1186/s13054-019-2591-6
Guimbretière G, Anselmi A, Roisne A et al (2019) Prognostic impact of blood product transfusion in VA and VV ECMO. Perfus (United Kingdom) 34:246–253. https://doi.org/10.1177/0267659118814690
Buscher H, Vukomanovic A, Benzimra M et al (2017) Blood and anticoagulation management in extracorporeal membrane oxygenation for surgical and nonsurgical patients: a single-center retrospective review. J Cardiothorac Vasc Anesth 31:869–875. https://doi.org/10.1053/j.jvca.2016.10.015
Hughes T, Zhang D, Nair P, Buscher H (2021) A systematic literature review of packed red cell transfusion usage in adult extracorporeal membrane oxygenation. Membranes (Basel) 11:1–19. https://doi.org/10.3390/membranes11040251
Raasveld SJ, Karami M, van den Bergh WM et al (2022) RBC transfusion in venovenous extracorporeal membrane oxygenation: a multicenter cohort study. Crit Care Med 50:224–234. https://doi.org/10.1097/CCM.0000000000005398
Abbasciano RG, Yusuff H, Vlaar A et al (2020) Blood transfusion threshold in patients receiving extracorporeal membrane oxygenation support for cardiac and respiratory failure—a systematic review and meta-analysis. J Cardiothorac Vasc Anesth. https://doi.org/10.1053/j.jvca.2020.08.068
Jiritano F, Serraino GF, ten Cate H et al (2020) Platelets and extra-corporeal membrane oxygenation in adult patients: a systematic review and meta-analysis. Intensive Care Med 46:1154–1169. https://doi.org/10.1007/s00134-020-06031-4
Granja T, Hohenstein K, Schüssel P et al (2020) Multi-modal characterization of the coagulopathy associated with extracorporeal membrane oxygenation. Crit Care Med 48:E400–E408. https://doi.org/10.1097/CCM.0000000000004286
Mazzeffi M, Rabin J, Deatrick K et al (2021) Platelet transfusion and in-hospital mortality in veno-arterial extracorporeal membrane oxygenation patients. ASAIO J Publish Ah. https://doi.org/10.1097/mat.0000000000001643
Opfermann P, Bevilacqua M, Felli A et al (2016) Prognostic impact of persistent thrombocytopenia during extracorporeal membrane oxygenation: a retrospective analysis of prospectively collected data from a cohort of patients with left ventricular dysfunction after cardiac surgery. Crit Care Med 44:e1208–e1218. https://doi.org/10.1097/CCM.0000000000001964
Ang AL, Teo D, Lim CH et al (2009) Blood transfusion requirements and independent predictors of increased transfusion requirements among adult patients on extracorporeal membrane oxygenation—a single centre experience. Vox Sang 96:34–43. https://doi.org/10.1111/j.1423-0410.2008.01110.x
Vlaar AP, Juffermans NP (2013) Transfusion-related acute lung injury: a clinical review. Lancet 382:984–994. https://doi.org/10.1016/S0140-6736(12)62197-7
Juffermans NP, Muller MM (2021) Prophylactic plasma: can we finally let go? Transfusion 61:1991–1992. https://doi.org/10.1111/trf.16546
Cheng R, Hachamovitch R, Kittleson M et al (2014) Complications of extracorporeal membrane oxygenation for treatment of cardiogenic shock and cardiac arrest: a meta-analysis of 1,866 adult patients. Ann Thorac Surg. https://doi.org/10.1016/j.athoracsur.2013.09.008
Mazzeffi M, Greenwood J, Tanaka K, et al (2016) Bleeding, Transfusion, and Mortality on Extracorporeal Life Support: ECLS Working Group on Thrombosis and Hemostasis. In: Annals of Thoracic Surgery. Elsevier USA, pp 682–689
Oude Lansink-Hartgring A, de Vries AJ, Droogh JM, van den Bergh WM (2019) Hemorrhagic complications during extracorporeal membrane oxygenation—the role of anticoagulation and platelets. J Crit Care 54:239–243. https://doi.org/10.1016/j.jcrc.2019.09.013
Raffa GM, Kowalewski M, Brodie D et al (2019) Meta-analysis of peripheral or central extracorporeal membrane oxygenation in postcardiotomy and non-postcardiotomy shock. Ann Thorac Surg 107:311–321. https://doi.org/10.1016/j.athoracsur.2018.05.063
Aubron C, Cheng AC, Pilcher D et al (2013) Factors associated with outcomes of patients on extracorporeal membrane oxygenation support: a 5-year cohort study. Crit Care. https://doi.org/10.1186/cc12681
Ducrocq G, Gonzalez-Juanatey JR, Puymirat E et al (2021) Effect of a restrictive vs liberal blood transfusion strategy on major cardiovascular events among patients with acute myocardial infarction and anemia: the REALITY randomized clinical trial. JAMA J Am Med Assoc 325:552–560. https://doi.org/10.1001/jama.2021.0135
Mazer CD, Whitlock RP, Fergusson DA et al (2017) Restrictive or liberal red-cell transfusion for cardiac surgery. N Engl J Med 377:2133–2144. https://doi.org/10.1056/nejmoa1711818
McMichael ABV, Ryerson LM, Ratano D et al (2022) 2021 ELSO adult and pediatric anticoagulation guidelines. ASAIO J 68:303–310. https://doi.org/10.1097/MAT.0000000000001652
Yeo HJ, Kim DH, Jeon D et al (2015) Low-dose heparin during extracorporeal membrane oxygenation treatment in adults. Intensive Care Med 41:2020–2021. https://doi.org/10.1007/s00134-015-4015-7
Aubron C, McQuilten Z, Bailey M et al (2019) Low-dose versus therapeutic anticoagulation in patients on extracorporeal membrane oxygenation: a pilot randomized trial. Crit Care Med 47:e563–e571. https://doi.org/10.1097/CCM.0000000000003780
Raman J, Alimohamed M, Dobrilovic N et al (2019) A comparison of low and standard anti-coagulation regimens in extracorporeal membrane oxygenation. J Hear Lung Transplant 38:433–439. https://doi.org/10.1016/j.healun.2019.01.1313
Joannidis M, Kountchev J, Rauchenzauner M et al (2007) Enoxaparin vs. unfractionated heparin for anticoagulation during continuous veno-venous hemofiltration: a randomized controlled crossover study. Intensive Care Med 33:1571–1579. https://doi.org/10.1007/s00134-007-0719-7
Gratz J, Pausch A, Schaden E et al (2020) Low molecular weight heparin versus unfractioned heparin for anticoagulation during perioperative extracorporeal membrane oxygenation: a single center experience in 102 lung transplant patients. Artif Organs 44:638–646. https://doi.org/10.1111/aor.13642
Geli J, Capoccia M, Maybauer DM, Maybauer MO (2022) Argatroban anticoagulation for adult extracorporeal membrane oxygenation: a systematic review. J Intensive Care Med 37:459–471. https://doi.org/10.1177/0885066621993739
Giuliano K, Bigelow BF, Etchill EW et al (2021) Extracorporeal membrane oxygenation complications in heparin- and bivalirudin-treated patients. Crit Care Explor 3:e0485. https://doi.org/10.1097/cce.0000000000000485
Sheridan EA, Sekela ME, Pandya KA et al (2022) Comparison of bivalirudin versus unfractionated heparin for anticoagulation in adult patients on extracorporeal membrane oxygenation. ASAIO J 68:920–924. https://doi.org/10.1097/MAT.0000000000001598
Wood KL, Ayers B, Gosev I et al (2020) Venoarterial-extracorporeal membrane oxygenation without routine systemic anticoagulation decreases adverse events. Ann Thorac Surg 109:1458–1466. https://doi.org/10.1016/j.athoracsur.2019.08.040
van Minnen O, Oude Lansink-Hartgring A, van den Boogaard B et al (2022) Reduced anticoagulation targets in extracorporeal life support (RATE): study protocol for a randomized controlled trial. Trials 23:1–11. https://doi.org/10.1186/s13063-022-06367-w
Millar JE, Fanning JP, McDonald CI et al (2016) The inflammatory response to extracorporeal membrane oxygenation (ECMO): a review of the pathophysiology. Crit Care 20:1–10. https://doi.org/10.1186/s13054-016-1570-4
Lip GYH, Blann A (1997) von Willebrand factor: a marker of endothelial dysfunction in vascular disorders? Cardiovasc Res 34:255–265. https://doi.org/10.1016/S0008-6363(97)00039-4
Mazzeffi M, Hasan S, Abuelkasem E et al (2019) Von Willebrand factor-GP1bα interactions in venoarterial extracorporeal membrane oxygenation patients. J Cardiothorac Vasc Anesth 33:2125–2132. https://doi.org/10.1053/j.jvca.2018.11.031
Dekker NAM, Van Leeuwen ALI, Van Strien WWJ et al (2019) Microcirculatory perfusion disturbances following cardiac surgery with cardiopulmonary bypass are associated with in vitro endothelial hyperpermeability and increased angiopoietin-2 levels. Crit Care 23:1–10. https://doi.org/10.1186/s13054-019-2418-5
Giacinto O, Satriano U, Nenna A et al (2019) Inflammatory response and endothelial dysfunction following cardiopulmonary bypass: pathophysiology and pharmacological targets. Recent Pat Inflamm Allergy Drug Discov 13:158–173. https://doi.org/10.2174/1872213x13666190724112644
Tsai TY, Tu KH, Tsai FC et al (2019) Prognostic value of endothelial biomarkers in refractory cardiogenic shock with ECLS: a prospective monocentric study. BMC Anesthesiol 19:1–8. https://doi.org/10.1186/s12871-019-0747-1
Lin SM, Wang YM, Lin HC et al (2008) Serum thrombomodulin level relates to the clinical course of disseminated intravascular coagulation, multiorgan dysfunction syndrome, and mortality in patients with sepsis. Crit Care Med 36:683–689. https://doi.org/10.1097/CCM.0B013E31816537D8
Siegel PM, Hentschel D, Bojti I et al (2021) Annexin V positive microvesicles are elevated and correlate with flow rate in patients receiving veno-arterial extracorporeal membrane oxygenation. Interact Cardiovasc Thorac Surg 31:884–891. https://doi.org/10.1093/icvts/ivaa198
Siegel PM, Bender I, Chalupsky J et al (2021) Extracellular vesicles are associated with outcome in veno-arterial extracorporeal membrane oxygenation and myocardial infarction. Front Cardiovasc Med 8:1–13. https://doi.org/10.3389/fcvm.2021.747453
Angelillo-Scherrer A (2012) Leukocyte-derived microparticles in vascular homeostasis. Circ Res 110:356–369. https://doi.org/10.1161/CIRCRESAHA.110.233403
Acknowledgements
Not applicable.
Funding
No funding or support was received for this study.
Author information
Authors and Affiliations
Contributions
Concept and design: SJR, APJV. Acquisition, analysis or interpretation of data: all authors. Drafting of the manuscript: all authors. Critical revision of the manuscript for important intellectual content: all authors. Statistical analysis: N/A. Supervision: APJV. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
No competing interests were stated by all authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Supplementary materials.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Raasveld, S.J., Volleman, C., Combes, A. et al. Knowledge gaps and research priorities in adult veno-arterial extracorporeal membrane oxygenation: a scoping review. ICMx 10, 50 (2022). https://doi.org/10.1186/s40635-022-00478-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40635-022-00478-z