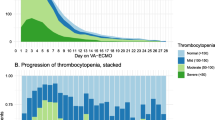
Abstract
Despite increasing improvement in extracorporeal membrane oxygenation (ECMO) technology and knowledge, thrombocytopenia and impaired platelet function are usual findings in ECMO patients and the underlying mechanisms are only partially elucidated. The purpose of this meta-analysis and systematic review was to thoroughly summarize and discuss the existing knowledge of platelet profile in adult ECMO population. All studies meeting the inclusion criteria (detailed data about platelet count and function) were selected, after screening literature from July 1975 to August 2019. Twenty-one studies from 1.742 abstracts were selected. The pooled prevalence of thrombocytopenia in ECMO patients was 21% (95% CI 12.9–29.0; 14 studies). Thrombocytopenia prevalence was 25.4% (95% CI 10.6–61.4; 4 studies) in veno-venous ECMO, whereas it was 23.2% (95% CI 11.8–34.5; 6 studies) in veno-arterial ECMO. Heparin-induced thrombocytopenia prevalence was 3.7% (95% CI 1.8–5.5; 12 studies). Meta-regression revealed no significant association between ECMO duration and thrombocytopenia. Platelet function impairment was described in 7 studies. Impaired aggregation was shown in 5 studies, whereas loss of platelet receptors was found in one trial, and platelet activation was described in 2 studies. Platelet transfusions were needed in up to 50% of the patients. Red blood cell transfusions were administered from 46 to 100% of the ECMO patients. Bleeding events varied from 16.6 to 50.7%, although the cause and type of haemorrhage was not consistently reported. Thrombocytopenia and platelet dysfunction are common in ECMO patients, regardless the type of ECMO mode. The underlying mechanisms are multifactorial, and understanding and management are still limited. Further research to design appropriate strategies and protocols for its monitoring, management, or prevention should be matter of thorough investigations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Platelet count and function seem to be severely impaired in extracorporeal membrane oxygenation (ECMO) patients. Thrombocytopenia and platelet dysfunction are common in extracorporeal membrane oxygenation (ECMO) patients, regardless the type of ECMO mode. |
Introduction
Extracorporeal membrane oxygenation (ECMO) is a temporary support able to provide short-term mechanical support to the heart, lungs, or both. Despite its first clinical use in 1970s, extracorporeal membrane oxygenation treatment has been exponentially increasing over the last 2 decades and is now considered a life-sustaining treatment in critical care medicine [1,2,3]. Haemorrhagic and thromboembolic complications are common during ECMO treatment, resulting in considerable morbidity and mortality [4, 5]. Platelet activation and aggregation play a major role in this context. The contact with foreign circuit surfaces and the high shear stress, during ECMO treatment, have been shown to cause enhanced platelet activation, which may lead to an increased thrombotic predisposition [6,7,8]. On the other side, high shear stress has also been shown to cause loss of platelet surface receptors, important for platelet adhesion, as well as loss of high-molecular-weight von Willebrand factor (vWF) multimers, resulting in decreased binding of vWF to platelets [8, 9]. Moreover, anticoagulant therapy, necessary to preserve the patency of the circuit and to avoid thrombotic complications, plays as a contributing factor to haemorrhagic complications [10]. Although anticoagulation guidelines vary widely among ECMO centres, unfractionated heparin (UFH) is the most administered anticoagulant because of its rapid onset of action, a promptly available antagonist (protamine) that can reverse its effect, and its low cost [11].
Moreover, heparin-induced thrombocytopenia (HIT) is an immune-mediated complication of UFH, characterized by moderate thrombocytopenia few days after initial heparin exposure, and, paradoxically, an increased risk of thrombosis [10]. The diagnosis of HIT in ECMO patients is even challenging and requires awareness and a high index of suspicion [12]. Limited data exist on thrombocytopenia during ECMO treatment in adult patients.
The aim of the present systematic review and meta-analysis is to highlight the occurrence of thrombocytopenia and to summarize the current knowledge about platelet function during ECMO in adult patients. We hypothesized that we could identify the prevalence of thrombocytopenia, platelet dysfunction and major complications in different ECMO mode.
Material and methods
Systematic review and meta-analysis were performed according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) and Meta-Analysis of Observational Studies in Epidemiology (MOOSE) guidelines. Complete details, including electronic search strategy, objectives, criteria for study selection, eligibility, data collection, and assessment of study quality, were registered and published online in PROSPERO International prospective register of systematic reviews (CRD42019129037) on 15 April 2019 (https://www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42019129037).
Literature search and data extraction
Eligible studies were identified by consulting the Cochrane Central Register of Controlled Trials (CENTRAL; Internet), MEDLINE, EMBASE, without date or language restriction. Keywords and MeSH terms pertinent to the exposure of interest were used in relevant combinations: “platelet”, “thrombocytopenia”, “platelet function”, “platelet count”, “ECMO”, “ECLS”, “extracorporeal life support”, “extracorporeal membrane oxygenation”, “bleeding”. The literature search was run from July 1975 to August 2019. In addition, we searched in trial registries and reference lists were carefully analysed for pertinent studies.
Randomized controlled trials (RCTs), or prospective as well as retrospective observational cohort studies of adult ECMO patients were included in our analysis. Studies were not included in the analysis if they met one of the following exclusion criteria: (1) reviews, case reports, animal and in vitro experiments, and conference abstracts; (2) incomplete information about study objectives; (3) studies in which outcomes were expressed as continuous variable; and (4) case reports. Inclusion and exclusion criteria for qualitative/quantitative analyses were summarized according to the PICOS (population, intervention, comparator, outcomes, and study design) approach (Supplemental Data). Two investigators (F.J. and G.F.S.) independently screened titles and abstracts. A third investigator (R.L.) helped to solve any disagreement. After excluding non-relevant studies, full texts of potentially relevant articles were then screened for inclusion in the final analysis. Supplementary documents of selected studies were also assessed, if available. A standardized form was used to extract data from included studies for assessment of study quality and evidence synthesis. Extracted information included year of publication, study design, sample size, number of patients, ECMO/ECLS configuration, and study parameters (thrombocytopenia, severe thrombocytopenia, HIT, platelet dysfunction, platelet and red cell transfusions, in-hospital bleeding, and mortality).
Outcomes and analyses
The primary outcome of this meta-analysis was the occurrence of thrombocytopenia and the platelet dysfunction. Thrombocytopenia definition was according to agreed guidelines criteria: moderate thrombocytopenia: platelet count < 150 × 103/uL; severe thrombocytopenia; and platelet count < 50 × 103/uL [13, 14]. Platelet dysfunction was considered and recorded in the study analysis according to the report in the selected studies of impaired aggregation, adhesion, and decreased activation.
Secondary outcomes included (1) the occurrence of confirmed HIT, defined as an iatrogenic disorder mediated by immunoglobulin G antibodies targeting multimolecular complexes of platelet factor 4 (PF4) and heparin; (2) the rate of platelet transfusion; (3) the occurrence of bleeding (major haemorrhage/major bleeding or > 2 red blood cell units over a 24-h period, during ECMO run, according to the Extracorporeal Life Support Organization (ELSO) definition [15, 16]) and re-exploration for bleeding; and (4) the in-hospital mortality [17]. The review has been performed in accordance with instructions given in the Cochrane Handbook for Systematic Reviews of Intervention [17].
Risk of bias
Quality was assessed using the Cochrane Collaboration’s tool for assessing risk of bias for RCT. For non-RCT, modified Newcastle–Ottawa quality assessment scale for cross-sectional studies was used to assess the quality of the study for inclusion. The total score for the modified Newcastle–Ottawa scale for cross-sectional studies is nine stars as a maximum for the overall scale with the minimum of zero. A study was considered high quality if it achieved 7 out 9 and medium if it achieved 5 out of 9 (Supplemental Data). Overall quality was independently determined by each reviewer with discrepancies solved by consensus.
Statistical analysis
OpenMeta-Analyst software for Macintosh, Review Manager (RevMan), version 5.2, the MetaXL add-in for Microsoft Excel MetaXL v5.2 (EpiGear International, Sunrise Beach, Australia), and the R Package for Windows (version 3.0) (metaprop command) were used to perform all the meta-analyses of prevalence of thrombocytopenia and HIT [17, 18]. Random-effects meta-analyses were performed using the proportions of patients who experienced an adverse event (i.e. thrombocytopenia, HIT) as the outcome of interest. Because of the anticipated high degree of heterogeneity, predominantly among non-RCTs, an inverse variance (DerSimonian–Laird) random-effects model was applied. Following the identification of each study to be included, precise event rates were noted from the reported results in all cases, without requiring back-transformation of the event parameters [18,19,20]. Pooled effect estimates were expressed as risk ratios with 95% confidence interval (CI). Heterogeneity within each meta-analysis using a Chi-squared test with significance set at a P < 0.10 was explored. We expressed the percentage of heterogeneity attributable to variation rather than to chance as I2 [21]. We defined heterogeneity as follows: I2 = 25–49%, low heterogeneity; I2 = 50–74%, moderate heterogeneity; and I2 > 75%, severe heterogeneity [21]. Based on the results of the analysis of Cochrane Q and Higgin’s I2, in order to be as conservative as possible, the random-effects method was used to take into account the variability among included studies. A sub-group analysis was performed to investigate the association between TCP and veno-arterial (V-A) ECMO, and thrombocytopenia and veno-venous (V-V) ECMO. Meta-regression models were performed applying an inverse variance weighting with a mixed-effects model to explore potential association with ECMO duration and thrombocytopenia occurrence. Publication bias was assessed by the visual assessment of funnel plots [22]. Sensitivity analysis excluded trials with high risk of bias for any of the following: random sequence generation; allocation concealment; blinding of participants; health care providers or outcome assessors; incomplete outcome data; attrition; and other sources of bias, including source of funder, Newcastle–Ottawa scale score < 7 for non-RCT, and studies with less than 10 enrolled patients (Supplemental Material) [17, 23].
Results
Study selection
Figure 1 shows the PRISMA flow diagram, describing the study selection process along with the reasons for exclusion. After removal of reports not pertinent to the meta-analysis and systematic review design, 21 studies were finally included in the data assessment [6,7,8, 16, 24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40]. Two studies enrolling less than 10 patients were excluded from the meta-analysis and included in a sensitivity analysis [supplemental references 1,2] (Supplemental material). Key characteristics of individual studies and enrolled patients are described in the supplemental material.
Four trials that met our inclusion criteria were excluded after review of the full manuscript (reason for exclusion mentioned in Table 3, Supplemental Data) [supplemental references 3–6].
Study description
Tables 1 and 2 summarize the main characteristics and outcomes of the included studies. All 21 selected papers were published after 2005. The number of included patients for each trial ranged from 13 to 5.797, with a total of 7190 patients. There were 6.698 V-A ECMO patients, of whom 459 were post-cardiotomy. In 6 studies, authors did not report about the ECMO setting (post-cardiotomy, post-acute myocardial infarction, or other aetiologies) [8, 16, 25, 26, 28, 39]. V-V ECMO was present in 477 patients. Patient age ranged from 41 to 60 years. ECMO duration averaged from 4 to 15 days. Two prospective RCTs were included in the analysis [29, 37]. Seven studies analysed the platelet function during ECMO (Table 3) [7, 16, 27, 30, 33, 35, 38]. Platelet aggregometry was tested in 5 studies, platelet receptor quantification was assessed in one trial, and testing for monoclonal antibodies, expression of impaired platelet granule secretion, was performed in one study [7, 16, 27, 30, 38]. Impaired platelet activation, assessed through PF4 and ß-thromboglobulin secretion, was tested only in one investigation [35].
Risk of bias and study quality
A summary of the risk of biases of included trials is reported in the Supplemental Data.
Quality assessment for observational studies showed no low-quality study (Supplemental Data). One RCT was classified as low risk of bias [37]. There was, instead, an unclear risk of allocation bias, randomization bias, and blinding for the other RCT [29]. The individual bias domains are presented in the risk of bias supplemental material.
Publication bias
A funnel plot for all the included outcomes showed an asymmetrical distribution indicating likely publication bias (Supplemental material).
Primary Outcome
-
Thrombocytopenia
Fourteen studies reported the number of patients with thrombocytopenia during ECMO [6, 8, 24,25,26, 28, 29, 31, 32, 34,35,36,37, 40]. The lowest prevalence of thrombocytopenia was 1.9%, while the highest was 80% [25, 29]. The I2 test result showed severe heterogeneity. Using the random-effects analysis, the pooled prevalence of thrombocytopenia in patients with ECMO was 21% (95% CI 12.9–29.0; 14 studies) (Fig. 2). Severe thrombocytopenia was reported only in 6 studies, and its prevalence ranged from 6.3 to 26.6% [8, 24, 32, 35, 37, 40]. Five studies reported the timeline of platelet count after ECMO start [7, 8, 16, 24, 29]. The time to lowest platelet count varied from 2 to 7 days after ECMO initiation. The percentage of decreasing of the platelet count was mentioned in 7 papers [6,7,8, 16, 28, 29, 31]. Thrombocytopenia in V-A ECMO patients has been reported in 6 studies [8, 24, 25, 31, 36, 40]. The I2 test result showed high heterogeneity also in this sub-group analysis. Among selected studies, the lowest prevalence of thrombocytopenia in V-A ECMO patients was 7.3%, whereas the highest prevalence was 90.9% [25, 31]. The pooled prevalence was 23.2% (95% CI 11.8–34.5; 6 studies) (Fig. 2). The subgroup analysis of thrombocytopenia in V-V ECMO patients included four studies and showed a similar pooled prevalence, 25.4% (95% CI 10.6–61.4; 4 studies), with a high heterogeneity (Fig. 2) [29, 32, 35, 37].
a Forest plot of the overall prevalence of thrombocytopenia in adult ECMO patients; b forest plot of prevalence of thrombocytopenia in V-A ECMO patients; c forest plot of prevalence of thrombocytopenia in V-V ECMO patients; d forest plot of the overall prevalence of heparin-induced thrombocytopenia in adult ECMO patients
-
Platelet dysfunction
Seven studies assessed the platelet function in ECMO patients [7, 16, 27, 28, 33, 35, 38]. Platelet aggregation tests were performed in 5 studies, showing a substantial reduction in platelet function [7, 16, 27, 30, 38]. One study reported reduced levels of adhesion receptors for collagen and vWF (GPVI and GPIbα) on circulating platelets during ECMO assistance [33]. Platelet function was investigated in one study by measuring soluble markers of platelet activation [35]. This study reported a decline in platelet activation markers, β-thromboglobulin and PF4, during the first 3 days of ECMO support compared with baseline [35]. Seven days after ECMO start, β-thromboglobulin levels remained low, while PF4 levels had returned to baseline values [35]. Another study examined platelet activation analysing the expression of activation-dependent platelet surface markers (CD62 and CD63) [38]. Both biomarkers were decreased during the ECMO run [38].
Secondary outcomes
Considering only the studies reporting a confirmed diagnosis of HIT, the lowest prevalence of HIT was 0.8%, whereas the highest was 22.2% (I2 = 81.4%) [6, 8, 24,25,26, 28, 29, 31, 34,35,36, 39]. The pooled prevalence of confirmed HIT was 3.7% (Fig. 2) (95% CI 1.8–5.5; 12 studies). Eight trials reported about alternative therapy to heparin when HIT was suspected [8, 24, 26, 28, 29, 31, 36, 39]. Argatroban was utilized in 6 studies, whereas bivalirudin was administered only in 2 studies [8, 26, 28, 29, 31, 36, 39]. Fondaparinux was used in one study [24]. Meta-regression analysis investigating potential effects of ECMO duration with the occurrence of thrombocytopenia revealed no significant relationship (P = 0.199) (Supplemental Data).
Bleeding events were reported only in 6 studies [7, 24,25,26, 35, 37]. The lowest and the highest prevalence of bleeding was 16.6% and 50.7%, respectively [7, 24]. One study, comparing ECMO patients primarily supported with bivalirudin versus heparin, showed that bivalirudin patients experienced fewer bleeding events than the heparin patients [26]. The re-exploration rate because of bleeding was described in 2 studies and ranged from 33.3 to 34.6% [25, 31]. Only 2 studies reported the rate of need of platelet transfusion, which varied from none to 21.8% of patients [6, 24]. In-hospital mortality was also evaluated as secondary outcome. It was reported in all but five studies and ranged from 6 to 88.3% of ECMO patients [6,7,8, 24,25,26, 28, 29, 31, 34, 35, 37, 38, 40].
Sensitivity analyses
Fixed-effects models did not materially change the results of our primary analyses. We also conducted sensitivity analyses in 9 trials identified as being at low risk of bias [8, 26, 28, 31, 32, 35,36,37, 39]. These trials reported outcomes in 6.555 participants. They reported a thrombocytopenia pooled prevalence of 21.7% in overall ECMO (95% CI, 0.080–0.354; I2 = 96.25%; p < 0.001); 9.4% in V-A ECMO (95% CI, 0.048–0.140; I2 = 5174%; p = n.s.); 33.6% in V-V ECMO (95% CI, − 0.052 to 0.724; I2 = 9660%; p < 0.001). Moreover, they reported an HIT pooled prevalence of 5% in ECMO patients (95% CI, 0.019–0.082; I2 = 8730%, p < 0.001).
Including the two studies with less than 10 enrolled patients, we did not find any difference for all the outcomes analysed [supplemental references 1, 2]. The thrombocytopenia pooled prevalence in overall ECMO was 23% (95% CI, 0.150–0.309, I2 = 9525%, p < 0.001); in V-A ECMO was 28.9% (95% CI, 0.169–0.409, I2 = 9539%, p < 0.001); in V-V ECMO was 26% (95% CI, − 0.029 to 0.549; I2 = 9756%; p < 0.001). The HIT pooled prevalence was 5% (95% CI, 0.027–0.073; I2 = 8764; p < 0.001) (Supplemental material).
Discussion
This systematic review and meta-analysis showed that thrombocytopenia has a considerable prevalence (up to 21%) in ECMO patients. Thrombocytopenia has been demonstrated in patients undergoing different mechanical circulatory support (cardiopulmonary bypass, intra-aortic balloon pump, left ventricular assist device (LVAD)) [41,42,43,44,45,46] (Figure 4 in Supplemental material). The origin of thrombocytopenia is likely multifactorial (contact with foreign surfaces, platelet activation, inflammatory and coagulative cascade activation), with sepsis, medications, surgery, bleeding, intravascular devices, and blood transfusions as contributing factors [41,42,43] (Figure 3 and Figure 4 in Supplemental material). We found that platelet count usually continues to decline over the first 2–3 days up to 7 days after ECMO implantation. Although in the literature strong evidence is still lacking, possible causes for a quick decrease in platelet count are patient’s primary disease, toxic drug effects, anticoagulation (HIT) and ECMO system used [29]. Besides the rapid occurrence of the phenomenon, one of the main observations in the present systematic review and meta-analysis is that thrombocytopenia is common in ECMO patients, regardless the type of ECMO mode. Malfertheiner et al. [29] showed in their RCT that platelet count decrease is independent by the applied ECMO technology. Although thrombocytopenia pooled prevalence is similar between V-A ECMO (23.2%) and V-V ECMO (25.4%) patients, we could speculate that the original mechanism is different in the two ECMO modes. Patients undergoing V-A ECMO represent a population with cardiorespiratory failure and associated diseases that may predispose them to have a pre-ECMO thrombocytopenia. Patients in refractory cardiac arrest, in fact, often have platelets and coagulation disorders that enhance during V-A ECMO run [40]. Similarly, post-cardiotomy ECMO is more prone to develop thrombocytopenia [31]. In our analysis, 7% and 3% of V-A ECMO were implanted after open-heart surgery and cardiac arrest, respectively. Moreover it is also likely that hemodynamic differences in perfusion rates and pressures (i.e. high flow required to guarantee a good oxygenation during V-V ECMO [11]) lead to a variable shear stress and consequent platelet activation and aggregation. Therefore, we could suppose that platelet impairment may vary also according to different ECMO configurations (i.e. central vs. peripheral cannulation) [4, 5, 47].
We found that several studies have shown impairment in platelet function during ECMO including in platelet aggregation [7, 16, 27, 30, 38].
At the same time, platelets also show receptor shedding [33]. Lukito et al. [33] demonstrated a significant reduction in the expression of adhesion receptors (GPIbα and GPVI). This process decreases the binding capacity of platelets to vWF and collagen, leading to impaired platelet function [33]. Chung et al. [35] reported reduced levels of biomarkers of platelet activation, PF4 and ß-thromboglobulin, during ECMO as compared to baseline. Kalbhenn et al. [38] showed a reduced expression of CD62 and CD63, biomarkers of impaired granule secretions. Functional and structural thrombocytes integrity in ECMO patients, therefore, still represents a relevant field of investigation to enhance patient management and impact complication rates, in both thromboembolic and bleeding disorders.
Unexpectedly, we found that ECMO duration has not a significant relationship with the occurrence of thrombocytopenia. Abrams and associated reported the same results in their analysis [6]. Even considering and controlling potential confounding factors, they were unable to demonstrate that the number of days on ECMO was associated with a worsening in thrombocytopenia [6]. Conversely to our findings, Weingart [48] and Panigada [49] reported that platelet count is related to ECMO duration. However, their results might be affected by the patient illness, the lower baseline platelet count, and the development of hepatic or renal failure. After the initial activation of the inflammatory response, the endothelization process that occurs after few days on ECMO surfaces, especially in the oxygenator, could protect from a steady thrombocytopenia and platelet dysfunction [50, 51]; the new generated platelets after 8–9 days (platelet lifespan) might be not affected anymore by the inflammatory and coagulative cascade triggered by ECMO [52].
Heparin-induced thrombocytopenia (HIT), an immune-mediated coagulation side effect of heparin therapy characterized by thrombocytopenia and by a paradoxical prothrombotic state following heparin exposure, could complicate ECMO treatment.[26, 36, 39, 53]. In adult patients receiving heparin, the prevalence of HIT is reported to be 0.5–5% [54, 55]. We found the HIT pooled prevalence is 3.7%. However, tests required to diagnose HIT in ECMO patients are rarely performed, indicating that actual HIT rate in ECMO patients is highly underestimated and sometimes an approach to “diagnose” HIT is to switch to an alternative anticoagulant like Argatroban or Bivalirudin based on clinical suspicion and low platelet count [28, 29, 31]. In our review, seven studies report a confirmed diagnosis of HIT through laboratory tests [6, 8, 28, 31, 34, 36, 39]. All of them declared HIT occurrence as a primary outcome.
The clinical implication of thrombocytopenia during ECMO treatment is bleeding, a common event in this setting [4, 5]. The included studies in this systematic review and meta-analysis showed a high rate of bleeding (16.6–50.7%) in ECMO patients.
Despite the release in 2014 of ELSO anticoagulation guidelines with the aim to standardize the ECMO management, remarkable heterogeneity still persists among ECMO centres and no major advance has been observed in the prevention or reduction of bleeding complications [15, 56]. Since heparin represents the usual first-line anticoagulant, commonly, post-cardiotomy V-A ECMO is associated with a high rate of bleeding events and changes in the coagulation cascade [57, 58]. However, other anticoagulants have been shown less haemorrhagic complications than heparin. Pieri et al., indeed, demonstrated that ECMO patients on bivalirudin experienced fewer bleeding events than ECMO patients on heparin. Moreover, we found that re-explorations for bleeding (34%) are reported mostly for post-cardiotomy ECMO. This result could be justified by several points: (1) the heparin administration during cardiopulmonary bypass; (2) patients who have undergone cardiac surgery are prone to develop HIT [59]; (3) the inflammatory response after surgery; and (4) the perioperative rate of blood product transfusions.
However, the relationship between thrombocytopenia and bleeding is bidirectional and there is a positive feedback: bleeding frequently causes thrombocytopenia, which can subsequently increase bleeding risk [60, 61]. Nevertheless, multiple factor might be associated with bleeding events, besides thrombocytopenia and platelet impaired function, as like as elderly age, central cannulation, delayed sternal closure, and excessive anticoagulation [62]. Haemorrhagic complications, and blood product transfusions, are frequent and directly linked to patient mortality in up to 17% of supported patients [4, 5, 16, 62]. Even if we found a high in-hospital mortality prevalence (6–88%), this result is unlikely due solely to thrombocytopenia and platelet dysfunction. We could speculate that the high range of mortality is related to the different ECMO indications [62]. Despite advances in management and technology, mortality in ECMO patients remains substantial (V-V: 30–40%; V-A: 60–70%) [62].
Strengths and limitations
The findings and interpretations of this meta-analysis and systematic review are limited by the quality of available evidence. The studies population showed high heterogeneity and thus must be interpreted with care. No sufficient information was available to perform other subgroup analysis besides the ECMO mode. Not enough data were reported about the technology used, the priming solution, and the antiplatelet medication taken. Moreover, the studies included in the meta-analysis are designed to specifically find out the incidence or suspicious of HIT and may affect our results, overestimating the occurrence of the disease. Additionally, of 21 studies included, 9 have a low risk of bias. Moreover, among these 9, only one is a RCT enrolling about 100 patients [37]. Therefore, large prospective randomized trials are required to better analyse and understand the phenomenon.
Notwithstanding these limitations, the present meta-analysis provides the most comprehensive evaluation of thrombocytopenia and platelet function in adult ECMO patients.
Conclusions
Thrombocytopenia and platelet dysfunction are common in ECMO patients, regardless the type of ECMO mode. Moreover, the ECMO duration does not seem to have any impact on the decrease of platelet count. The underlying mechanisms are multifactorial, and understanding and management are still limited. Further research to design appropriate strategies and protocols for its monitoring, management, or prevention should be a matter of thorough investigations.
References
Hill JD, O'Brien TG, Murray JJ et al (1972) Prolonged extracorporeal oxygenation for acute post-traumatic respiratory failure (shock-lung syndrome). Use of the Bramson membrane lung. N Engl J Med 286(12):629–634
Lindén V, Palmér K, Reinhard J et al (2000) High survival in adult patients with acute respiratory distress syndrome treated by extracorporeal membrane oxygenation, minimal sedation, and pressure supported ventilation. Intensive Care Med 26(11):1630–1637
Peek GJ, Mugford M, Tiruvoipati R, CESAR trial collaboration et al (2009) Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet 374(9698):1351–1363
Chen Z, Mondal NK, Zheng S et al (2017) High shear induces platelet dysfunction leading to enhanced thrombotic propensity and diminished hemostatic capacity. Platelets 2017:1–8
Chen Z, Mondal NK, Ding J et al (2016) Paradoxical effect of nonphysiological shear stress on platelets and von willebrand factor. Artif Organs 40:659–668
Abrams D, Baldwin MR, Champion M et al (2016) Thrombocytopenia and extracorporeal membrane oxygenation in adults with acute respiratory failure: a cohort study. Intensive Care Med 42(5):844–852
Laine A, Niemi T, Suojaranta-Ylinen R et al (2016) Decreased maximum clot firmness in rotational thromboelastometry (ROTEM®) is associated with bleeding during extracorporeal mechanical circulatory support. Perfusion 31(8):625–633
Sokolovic M, Pratt AK, Vukicevic V et al (2016) Platelet count trends and prevalence of heparin-induced thrombocytopenia in a cohort of extracorporeal membrane oxygenator patients. Crit Care Med 44(11):e1031–e1037
Yoshimoto Y, Hasebe T, Takahashi K et al (2013) Ultrastructural characterization of surface-induced platelet activation on artificial materials by transmission electron microscopy. Microsc Res Tech 76:342–349
Heilmann C, Geisen U, Beyersdorf F et al (2012) Acquired von Willebrand syndrome in patients with extracorporeal life support (ECLS). Intensive Care Med 38:62–68
Vaquer S, de Haro C, Peruga P et al (2017) Systematic review and meta-analysis of complications and mortality of veno-venous extracorporeal membrane oxygenation for refractory acute respiratory distress syndrome. Ann Intensive Care 7(1):51
Zangrillo A, Landoni G, Biondi-Zoccai G et al (2013) A meta-analysis of complications and mortality of extracorporeal membrane oxygenation. Crit Care Resusc 15:172–178
British Committee for Standards in Haematology, Blood Transfusion Task Force (2003) Guidelines for the use of platelet transfusions. Br J Haematol 122(1):10–23
https://www.labtestsonline.org.uk/understanding/analytes/platelet/tab/test. Accessed 14 Dec 2019
Lequier L, Annich G, Al-Ibrahim O et al (2014) ELSO anticoagulation guidelines. https://www.elso.org/Resources/Guidelines.aspx. Accessed 04 Jan 2020
Tauber H, Streif W, Fritz J et al (2016) Predicting transfusion requirements during extracorporeal membrane oxygenation. J Cardiothorac Vasc Anesth 30(3):692–701
Higgins JPT, Green S (2011) Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated March 2011]. The Cochrane Collaboration. www.handbook.cochrane.org. Accessed 14 Dec 2019
Byron CW, Dahabreh IJ, Trikalinos TA et al (2012) Closing the gap between methodologists and end-users: R as a computational back-end. J Stat Softw 49:5
Schwarzer G, Carpenter JR, Rücker G (2015) Meta-analysis with R. Springer, Berlin
Gordon M, Lumley T (2019) R package ‘forestplot’. Advanced forest plot using ‘grid’ graphics. The Comprehensive R Archive Network, Vienna
Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21:1539–1558
Sterne JAC, Egger M, Moher D. In: Higgins JPT, Green S (2011) Addressing reporting biases Cochrane handbook for systematic reviews of interventions version 5.1.0. The Cochrane Collaboration
Copas J, Shi JQ (2000) Meta-analysis, funnel plots and sensitivity analysis. Biostatistics 1(3):247–262
Loforte A, Montalto A, Ranocchi F et al (2012) Peripheral extracorporeal membrane oxygenation system as salvage treatment of patients with refractory cardiogenic shock: preliminary outcome evaluation. Artif Organs 36(3):E53–61
Cottini SR, Wenger U, Sailer S et al (2013) Extracorporeal membrane oxygenation: beneficial strategy for lung transplant recipients. J Extra Corpor Technol 45(1):16–20
Pieri M, Agracheva N, Bonaveglio E et al (2013) Bivalirudin versus heparin as an anticoagulant during extracorporeal membrane oxygenation: a case-control study. J Cardiothorac Vasc Anesth 27(1):30–34
Mutlak H, Reyher C, Meybohm P et al (2015) Multiple electrode aggregometry for the assessment of acquired platelet dysfunctions during extracorporeal circulation. Thorac Cardiovasc Surg 63(1):21–27
Glick D, Dzierba AL, Abrams D et al (2015) Clinically suspected heparin-induced thrombocytopenia during extracorporeal membrane oxygenation. J Crit Care 30(6):1190–1194
Malfertheiner MV, Philipp A, Lubnow M et al (2016) Hemostatic changes during extracorporeal membrane oxygenation: a prospective randomized clinical trial comparing three different extracorporeal membrane oxygenation systems. Crit Care Med 44(4):747–754
Nair P, Hoechter DJ, Buscher H et al (2015) Prospective observational study of hemostatic alterations during adult extracorporeal membrane oxygenation (ECMO) using point-of-care thromboelastometry and platelet aggregometry. J Cardiothorac Vasc Anesth 29(2):288–296
Opfermann P, Bevilacqua M, Felli A et al (2016) Prognostic impact of persistent thrombocytopenia during extracorporeal membrane oxygenation: a retrospective analysis of prospectively collected data from a cohort of patients with left ventricular dysfunction after cardiac surgery. Crit Care Med 44(12):e1208–e1218
Dzierba AL, Roberts R, Muir J et al (2016) Severe thrombocytopenia in adults with severe acute respiratory distress syndrome: impact of extracorporeal membrane oxygenation use. ASAIO J 62(6):710–714
Lukito P, Wong A, Jing J et al (2016) Mechanical circulatory support is associated with loss of platelet receptors glycoprotein Ibα and glycoprotein VI. J Thromb Haemost 14(11):2253–2260
Laverdure F, Louvain-Quintard V, Kortchinsky T et al (2016) PF4-heparin antibodies during ECMO: incidence, course, and outcomes. Intensive Care Med 42(6):1082–1083
Chung JH, Yeo HJ, Kim D et al (2017) Changes in the levels of beta-thromboglobulin and inflammatory mediators during extracorporeal membrane oxygenation support. Int J Artif Organs 40(10):575–580
Ljajikj E, Zittermann A, Morshuis M et al (2017) Bivalirudin anticoagulation for left ventricular assist device implantation on an extracorporeal life support system in patients with heparin-induced thrombocytopenia antibodies. Interact Cardiovasc Thorac Surg 25(6):898–904
Combes A, Hajage D, Capellier G, EOLIA Trial Group, REVA, and ECMONet et al (2018) Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N Engl J Med 378(21):1965–1975
Kalbhenn J, Schlagenhauf A, Rosenfelder S et al (2018) Acquired von Willebrand syndrome and impaired platelet function during venovenous extracorporeal membrane oxygenation: Rapid onset and fast recovery. J Heart Lung Transplant 37(8):985–991
Kimmoun A, Oulehri W, Sonneville R et al (2018) Prevalence and outcome of heparin-induced thrombocytopenia diagnosed under veno-arterial extracorporeal membrane oxygenation: a retrospective nationwide study. Intensive Care Med 44(9):1460–1469
Ruggeri L, Franco A, Alba AC et al (2018) Coagulation derangements in patients with refractory cardiac arrest treated with extracorporeal cardiopulmonary resuscitation. J Cardiothorac Vasc Anesth 33(7):1877–1882
Cavayas YA, Del Sorbo L, Fan E (2018) Intracranial hemorrhage in adults on ECMO. Perfusion 33(1_suppl):42–50
Tabata S, Yamaguchi S, Nagamine H et al (2004) Efficacy of fk633, an ultra-short acting glycoprotein iib/iiia antagonist on platelet preservation during and after cardiopulmonary bypass. Eur J Cardiothorac Surg 26:289–293
Sniecinski RM, Chandler WL (2011) Activation of the hemostatic system during cardiopulmonary bypass. Anesth Analg 113:1319–1333
Varon D, Shai E (2015) Platelets and their microparticles as key players in pathophysiological responses. J Thromb Haemost 13(Suppl 1):S40–46
Vonderheide RH, Thadhani R, Kuter DJ (1998) Association of thrombocytopenia with the use of intra-aortic balloon pumps. Am J Med 105:27–32
Ho LTS, Lenihan M, McVey MJ et al (2019) Transfusion avoidance in cardiac surgery study investigators. The association between platelet dysfunction and adverse outcomes in cardiac surgical patients. Anaesthesia 74(9):1130–1137
Gu K, Zhang Y, Gao B et al (2016) Hemodynamic differences between central ECMO and peripheral ECMO: a primary CFD Study. Med Sci Monit 22:717–726
Weingart C, Lubnow M, Philipp A, Bein T, Camboni D, Müller T (2015) Comparison of coagulation parameters, anticoagulation, and need for transfusion in patients on interventional lung assist or veno-venous extracorporeal membrane oxygenation. Artif Organs 39(9):765–773
Panigada M, Artoni A, Passamonti SM et al (2016) Hemostasis changes during veno-venous extracorporeal membrane oxygenation for respiratory support in adults. Minerva Anestesiol 82(2):170–179
Ontaneda A, Annich GM (2018) Novel surfaces in extracorporeal membrane oxygenation circuits. Front Med (Lausanne) 5:321. https://doi.org/10.3389/fmed.2018.00321
Klein S, Hesselmann F, Djeljadini S et al (2020) EndOxy: dynamic long-term evaluation of endothelialized gas exchange membranes for a biohybrid lung. Ann Biomed Eng 48:747–756
Cho J (2015) A paradigm shift in platelet transfusion therapy. Blood 125(23):3523–3525
Porcelli R, Moskowitz BC, Cetta F et al (2003) Heparin-induced thrombocytopenia with associated thrombosis in children after the Fontan operation: report of two cases. Texas Heart Inst J 30(1):58–61
Warkentin TE, Sheppard JA, Horsewood P et al (2000) Impact of the patient population on the risk for heparin induced thrombocytopenia. Blood 96:1703–1708
Martel N, Lee J, Wells PS (2005) Risk for heparin-induced thrombocytopenia with unfractionated and low-molecular-weight heparin thromboprophylaxis: a meta-analysis. Blood 106:2710–2715
Vandenbriele C, Vanassche T, Price S (2020) Why we need safer anticoagulant strategies for patients on short-term percutaneous mechanical circulatory support. Intensive Care Med. https://doi.org/10.1007/s00134-019-05897-3
Lorusso R, Raffa GM, Alenizy K et al (2019) Structured review of post-cardiotomy extracorporeal membrane oxygenation: part 1-Adult patients. J Heart Lung Transplant 38(11):1125–1143
Yeo HJ, Kim DH, Jeon D, Kim YS, Cho WH (2015) Low-dose heparin during extracorporeal membrane oxygenation treatment in adults. Intensive Care Med 41(11):2020–2021
Selleng S, Selleng K (2016) Heparin-induced thrombocytopenia in cardiac surgery and critically ill patients. Thromb Haemost 116(5):843–851
Mazzeffi M, Tanaka K (2016) Platelets and ECMO: should we worry about count, function, or both? Intensive Care Med 42(7):1199–1200
Mazzeffi M, Gupta R, Lonergan T, Pasrija C, Kon Z, Tanaka K (2017) ABO type and bleeding during adult ECMO. Intensive Care Med 43(2):275–276
Mazzeffi M, Greenwood J, Tanaka K et al (2016) Bleeding, transfusion, and mortality on extracorporeal life support: ECLS working group on thrombosis and hemostasis. Ann Thorac Surg 101(2):682–689
Acknowledgements
None
Funding
None.
Author information
Authors and Affiliations
Contributions
FJ contributed to conception and design of the work; acquisition and interpretation of data; drafting the work; and final approval of the version to be published. GFS performed acquisition, analysis, and interpretation of data for the work; revision of the work; and final approval of the version to be published. HtC participated in interpretation of the data of the work; revision of the work; and final approval of the version to be published. DF and MM performed interpretation of the data of the work and final approval of the version to be published. PM and RL contributed to revision of the work and final approval of the version to be published.
Corresponding author
Ethics declarations
Conflicts of interest
Prof. Dr. Roberto Lorusso is consultant for LivaNova (principal investigator of PERSIST-AVR Trial) and Medtronic. Prof. Dr. Hugo ten Cate receives research funding from Bayer and Pfizer.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Jiritano, F., Serraino, G.F., ten Cate, H. et al. Platelets and extra-corporeal membrane oxygenation in adult patients: a systematic review and meta-analysis. Intensive Care Med 46, 1154–1169 (2020). https://doi.org/10.1007/s00134-020-06031-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-020-06031-4