Abstract
Background
Recent advances on cardiorespiratory monitoring applied in ARDS patients undergoing invasive mechanical ventilation and noninvasive ventilatory support are available in the literature and may have potential prognostic implication in ARDS treatment.
Main body
The measurement of oxygen saturation by pulse oximetry is a valid, low-cost, noninvasive alternative for assessing arterial oxygenation. Caution must be taken in patients with darker skin pigmentation, who may experience a greater incidence of occult hypoxemia. Dead space surrogates, which are easy to calculate, have important prognostic implications. The mechanical power, which can be automatically computed by intensive care ventilators, is an important parameter correlated with ventilator-induced lung injury and outcome. In patients undergoing noninvasive ventilatory support, the use of esophageal pressure can measure inspiratory effort, avoiding possible delays in endotracheal intubation. Fluid responsiveness can also be evaluated using dynamic indices in patients ventilated at low tidal volumes (< 8 mL/kg). In patients ventilated at high levels of positive end expiratory pressure (PEEP), the PEEP test represents a valid alternative to passive leg raising. There is growing evidence on alternative parameters for evaluating fluid responsiveness, such as central venous oxygen saturation variations, inferior vena cava diameter variations and capillary refill time.
Conclusion
Careful cardiorespiratory monitoring in patients affected by ARDS is crucial to improve prognosis and to tailor treatment via mechanical ventilatory support.
Keypoints
-
1.
The SpO2/FiO2 ratio, corrected minute ventilation and ventilatory ratio are valid surrogates for estimating gas exchange in ARDS patients, and caution should be taken in patients with darker skin pigmentation and moderate–severe ARDS.
-
2.
Changes in esophageal pressure during noninvasive respiratory support and mechanical power must be carefully monitored to estimate PSILI and VILI in ARDS patients undergoing mechanical ventilation.
-
3.
The use of dynamic indexes of fluid responsiveness should be encouraged in ARDS patients. Pulse pressure variation and stroke volume variation have also been validated in mechanically ventilated patients with low tidal volume.
-
4.
A possible alternative to passive leg raising in a mechanically ventilated patient is the PEEP test. Two minimally invasive alternatives to predict fluid responsiveness are changes in central venous oxygen saturation and capillary refill time after a passive leg raising or a fluid challenge.
Similar content being viewed by others
Introduction
Patients with acute respiratory distress syndrome (ARDS) exhibit inflammatory pulmonary edema resulting from changes in endothelial and epithelial permeability, leading to organ damage. The severity of ARDS determines the application of different types of mechanical support. Superimposed hemodynamic impairment may complicate patient management, worsening outcomes. Therefore, a comprehensive evaluation of ARDS patients involves careful respiratory and hemodynamic monitoring, encompassing both invasive and noninvasive technologies, along with clinical and laboratory data. This approach is crucial for tailoring therapeutic strategies to individual patients and minimizing lung injury.
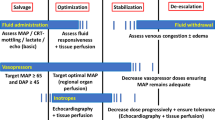
This manuscript reviews strategies for respiratory and hemodynamic monitoring in ARDS patients, highlighting the most recent data and clinical utility in daily management, as synthesized in Fig. 1.
Respiratory and hemodynamic monitoring in patients affected by ARDS. Recent evidence about respiratory and hemodynamic monitoring in mechanically ventilated patients is available. VeCORR corrected minute ventilation, EtCO2 end-tidal CO2, SpO2 peripheral oxygen saturation, FiO2 fraction of inspired oxygen, PaO2 arterial oxygen partial pressure, mL milliliters, kg kilograms, IBW ideal body weight, TVc tidal volume challenge, PEEP positive end expiratory pressure, ΔScvO2 central venous oxygen saturation increase
Respiratory monitoring
Careful respiratory monitoring is essential in patients affected by ARDS. This approach allows the application of an adequate intensity of treatment and reduces injuries caused by mechanical ventilation (MV).
Gas exchange efficiency
Gas exchange is directly affected by pulmonary alterations induced by ARDS. In this section, we review the renewed role of pulse oximetry and useful surrogate indices of dead space.
Pulse oximetry
Pulse-oximetry exploits the principle of spectrophotometry to quantify the amount of oxygenated hemoglobin in blood, allowing continuous noninvasive monitoring of arterial saturation [1]. The difference between arterial oxygen saturation (SaO2) measured via blood gas analysis and oxygen saturation measured via pulse-oximetry (SpO2) is normally less than 3% [2]. However, the accuracy of SpO2 may be lower among patients with darker skin pigmentation, thus overestimating arterial oxygen saturation. This phenomenon, as recently demonstrated by Henry et al., possibly increases the incidence of occult hypoxemia, i.e., patients in which SaO2 is lower than 88% with an SpO2 higher than 92% [3]. The clinical consequences of occult hypoxemia have also been investigated during the recent pandemic. In COVID-19 patients, occult hypoxemia is more frequent in Asian, Black and non-Black Hispanic patients than in White patients, with lower treatment eligibility for these three ethnicities [4].
The ratio of pulse-oximetric oxygen saturation to the fraction of inspired oxygen (SpO2/FiO2) is an acceptable surrogate of the ratio of the partial pressure of arterial oxygen to FiO2 (PaO2/FiO2). Its use has been described both in invasively and noninvasively ventilated patients [5,6,7,8,9]. The SpO2/FiO2 ratio is a good outcome predictor both in patients with coronavirus disease (COVID-19) and non-COVID-19 ARDS patients [10, 11]. In patients with COVID-19-associated pneumonia requiring oxygen therapy, the SpO2/FiO2 ratio at admission showed an area under the curve (AUC) of 85% for the prediction of ARDS occurrence [12]. Kim et al. showed that the SpO2/FiO2 ratio can predict high-flow nasal cannula (HFNC) failure [13]. Moreover, SpO2/FiO2 shows a good correlation with PaO2/FiO2 in invasively ventilated COVID-19 ARDS patients, and when computed on day 2 and day 3, it is associated with outcome [11]. These data confirm the reliability of pulse oximetry for evaluating gas exchange in ARDS patients and for following this trend, as pulse oximetry is continuously measurable. It is easy to measure and is thus especially valid in contexts in which a blood gas analyzer is not promptly available.
The optimal SpO2 concentration for ARDS treatment is still a matter of debate, ranging from 88% to 96–100% to balance the risk of hyperoxia and hypoxia. In a recent large randomized controlled trial (RCT), Semler et al. showed that, in mechanically ventilated patients, the use of a lower (90%, range from 88 to 92%), intermediate (94%, 92–96%) or higher (98%, 96–100%) SpO2 target does not affect either ventilator-free days or hospital outcomes [14].
Dead space
Physiological dead space is the inspired volume of air that does not participate in gas exchange. It includes anatomic and alveolar dead space [15]. In mechanically ventilated patients, the anatomic dead space remains relatively constant, while the alveolar dead space can significantly increase according to alterations in the ventilation/perfusion (V/Q) ratio [16, 17].
In a seminal study, Nuckton et al. demonstrated that physiological dead space is significantly higher in non-ARDS survivors than in survivors [18]. Like in ARDS patients, COVID-19 pneumonia is characterized by an increase in minute ventilation and an increase in the dead space fraction [19, 20]. Additionally, in COVID-19 ARDS patients, there is a significant association between the amount of dead space computed in the first 7 days and mortality [21]. According to a secondary analysis of the PRoVENT COVID-19 study, the dead space fraction is significantly greater in nonsurvivors and increases more during the first four days than in survivors, suggesting that dynamic changes during the initial week in the intensive care unit (ICU) are crucial for evaluating outcomes [22]. These recent data underline the strong prognostic role of dead space and strengthen the rationale for its use in ARDS patients.
Corrected minute ventilation
The corrected minute ventilation (VEcorr) is a simple and easy-to-calculate surrogate of the dead space fraction that does not require the expired carbon dioxide (CO2) measurement. VEcorr is calculated as the ventilation required to achieve a PaCO2 value of 40 mmHg. In mechanically ventilated COVID-19 ARDS patients, Fusina et al. found a strong correlation between VEcorr and the dead space fraction, with a higher VEcorr in nonsurvivors, which was independently associated with mortality [23].
Ventilatory ratio
In recent years, in addition to dead space fraction computation, the ventilatory ratio (VR) has been proposed as an additional, easy-to-calculate estimation of ventilation efficiency [24]. VR is computed as the product of minute ventilation and arterial carbon dioxide weighed on the patient’s predicted body weight [24]. It is a unitless ratio, being approximately one in healthy subjects. In ARDS patients, Sinha et al. reported a positive relationship between VR and alveolar dead space. Furthermore, VR is more common in nonsurvivors than in survivors [24] and is associated with increased odds of hospital mortality (OR 2.07, confidence interval [CI] 1.53–2.83). As recently shown by Siegel et al., the ventilatory ratio, in association with the APACHE III score at admission, has an area under the curve (AUC) of 0.81 (95% CI 0.68–0.92) in predicting hospital mortality and is significantly better than the APACHE III score alone [25]. Changes in VR within the first 4 h after prone positioning in ARDS patients predict weaning from mechanical ventilation, with an AUC of 0.64 (95% CI 0.53–0.75) [26].
VR reliability can be affected by venous admixture (Qva/Q) and the amount of patient CO2 produced (VCO2). Indeed, these two factors may increase the difference between alveolar and arterial PCO2, with the latter being used for VR calculations. Maj et al. showed that the predictive value of the VR decreases in most severe patients, who are affected by greater Qva/Q impairment [27]. To investigate the effect of VCO2, Monteiro et al. performed a post hoc analysis of the PETAL-ROSE trial [28]. The authors showed that the presence of neuromuscular blockade, a factor influencing skeletal muscle CO2 production, did not significantly affect the relationship between VR and mortality [29].
In mechanically ventilated patients, dead space should be continuously assessed as an additional measurement of gas exchange impairment, together with the PaO2/FiO2 or SpO2/FiO2 ratio. The use of surrogates, which are easier to calculate, seems to be reliable and should encourage the use of such measures to predict patient outcomes. Caution must be used in moderate and severe ARDS patients with major Qva/Q impairment when assessing VR. In these situations, dead space might be overestimated.
ETCO2 to arterial PCO2
A further parameter to estimate gas exchange efficiency is the computation of the end-tidal-to-arterial PCO2 ratio (PETCO2/PaCO2), which measures the influence of venous admixture and alveolar dead space on lung performance. Ideally, this ratio should be equal to one. Bonifazi et al. showed that the PETCO2/PaCO2 ratio significantly decreases from mild to severe ARDS [30]. Additionally, PETCO2/PaCO2 is strongly correlated with the amount of nonaerated tissue measured via computed tomography (CT) and respiratory compliance [30]. A subsequent study revealed a relationship between the PETCO2/PaCO2 ratio, alveolar ventilation and hospital mortality [31]. For every 0.01 increase in the PETCO2/PaCO2 ratio, the risk for mortality decreases by 1%.
Currently, weaning from venous extracorporeal membrane oxygenation (VV-ECMO) lacks well-defined criteria and is often based on acceptable blood gas analysis and the absence of excessive inspiratory effort. In a recent multicenter study, Lazzari et al. showed that the PETCO2/PaCO2 ratio, with a cutoff of 0.83, is able to predict weaning [32].
Ventilation and patient self-induced lung injury
Mechanical ventilation and spontaneous inspiratory effort may be harmful. The mechanical power, its normalization (i.e., the mechanical power ratio) and the measurement of the esophageal pressure are crucial to minimize these sources of lung injury in patients affected by ARDS. Indices of recruitment are helpful for adequately establishing mechanical ventilation.
The mechanical power
Mechanical power refers to the energy dissipated in the respiratory system while moving a specific volume at a given PEEP. It is typically expressed in Joules per minute (J/min) [33]. This energy dissipation within the respiratory system plays a crucial role in modulating and potentially promoting ventilator-induced lung injury (VILI). The mechanical power is a unifying indicator computed considering the major ventilatory variables generated from the interaction between the patient and ventilator. It can be assessed under passive conditions and categorized based on ventilation modality (pressure or volume-controlled ventilation) using algebraic equations [34]. The newest intensive care mechanical ventilators now offer the possibility to directly measure mechanical power, with acceptable accuracy compared to traditional algebraic methods [35].
Recent studies have demonstrated that mechanical power at admission is associated with hospital mortality across a heterogeneous range of patients [36,37,38]. Urner et al. further explored the relationship between the intensity of mechanical power throughout the intensive care stay and mortality, revealing an increased risk of death with each additional day of exposure to mechanical power equal to or greater than 17 J/min [39]. Pozzi et al. analyzed the clinical course of ventilatory variables in ARDS patients during the initial three days of MV and identified the mechanical power ratio at admission as the only variable associated with intensive care mortality [40]. By day 3, the mechanical power ratio, alveolar dead space, and PaO2/FiO2 were associated with the outcome. Therefore, in ARDS patients, assessing ventilatory variables during the initial days of mechanical ventilation seems to be crucial for predicting outcomes.
Concerning the different components of mechanical power, Costa et al. showed a stronger association with mortality for the dynamic component (i.e., the respiratory rate and the driving pressure) than for the total mechanical power [41]. However, the impact of similar values of mechanical power on lung injury can vary significantly based on factors such as ventilated lung size, respiratory system compliance, or the amount of aerated tissue at a given PEEP. Coppola et al. demonstrated that normalizing the mechanical power at admission to the compliance of the respiratory system and the amount of ventilated tissue, as computed by lung CT, provides a better predictive measure for outcomes in ARDS patients [36].
Esophageal pressure and diaphragmatic ultrasound
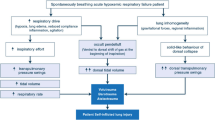
Preserving spontaneous breathing over invasive ventilation offers advantages [42, 43]. However, elevated inspiratory efforts are associated with high negative esophageal pressure (Pes) swing and positive transpulmonary pressure, which may lead to patient self-inflicted lung injury (PSILI). PSILI is associated with organ dysfunction and increased mortality [44,45,46]. Additionally, excessive inspiratory effort cannot be detected simply by monitoring airway pressure [47].
Computing the changes in esophageal pressure during inspiration (ΔPes) as the difference between the esophageal pressure at the beginning of inspiration and its lowest value is the easiest way to measure inspiratory effort. In the presence of acute respiratory failure, several noninvasive respiratory support methods, such as HFNC therapy, continuous positive airway pressure (CPAP), and noninvasive ventilation (NIV), should improve gas exchange and decrease inspiratory effort. Menga et al., in a crossover study comparing noninvasive support, showed that only NIV delivered by a helmet is able to reduce delta pes [48]. In a large group of COVID-19 ARDS patients receiving helmet CPAP, total stress, defined as the sum of the transpulmonary pressure generated by the patient and the end expiratory airway pressure, is independently associated with a negative outcome [49].
Transpulmonary pressure measurements allow clinicians to evaluate lung recruitment efficacy. With this aim, it can be useful to evaluate the effects of awake prone positioning, as performed in COVID-19 ARDS patients. Prone positioning leads to a reduction in ventral alveolar hyperinflation and dorsal atelectasis, thus promoting homogenization of transpulmonary pressure and improvement in oxygenation. Additionally, as demonstrated in a cohort of COVID-19 ARDS patients assisted with helmet CPAP, prone positioning significantly reduces the amount of work involved in breathing [50]. The role of esophageal pressure manometry in evaluating inspiratory effort and preventing PSILI is increasingly recognized, and this technique is always recommended for ARDS patients.
Another possible way to evaluate inspiratory effort is the use of ultrasound. However, Steinberg et al. show poor correlation between diaphragmatic thickening fraction (DTI), diaphragmatic excursions and esophageal swing in a cohort of 46 mechanically ventilated patients affected by Covid-19 ARDS [51]. Similarly findings are available from Poulard et al. [52]. Delta Pes monitoring remains therefore essential to evaluate PSILI in patients undergoing assisted mechanical ventilation.
Nevertheless, diaphragmatic ultrasound remains a valid tool to predict weaning from MV, and recent studies strengthen this evidence. Mawla et al. find a possible cutoff of 13.5% for DTI as accurate to predict weaning from MV [53]. Another original investigation shows how the association of different diaphragmatic ultrasound indexes has an area under the curve of 0.77 in predicting extubation success [54].
Recruitment: the recruitment/inflation ratio and the EIT-based PEEP titration
Chen et al. proposed the recruitment-to-inflation ratio (R/I ratio) as a noninvasive method to compute the potential for lung recruitment at different PEEP levels [55]. Subsequently, the R/I ratio has been clinically validated to be accurate in detecting lung recruitment in ARDS patients in the supine position [56, 57]. In a secondary analysis of a previous study [58], the R/I ratio at two levels of PEEP, both in the supine and prone positions, correlated with lung recruitment computed by CT scan [59]. In addition, the overall data confirm high variability in lung recruitability among ARDS patients, with different effects on gas exchange, respiratory mechanics and hemodynamics. Zerbib et al. reported that an R/I ratio > 0.62 predicts lung recruitability with an AUC of 0.80 in COVID-19 ARDS patients [60]. Patients with high recruitability show an improvement in both oxygenation and respiratory system compliance, while in patients with low recruitability, an increase in oxygenation is associated with a decrease in cardiac output. These data confirm that the R/I ratio is a valuable aid for physicians to select an adequate level of PEEP, to improve respiratory mechanics and oxygenation, and to monitor hemodynamics and cardiac output.
New interesting evidences are available about electrical impedance tomography (EIT) as an effective tool to titrate PEEP in patients affected by ARDS. In an original article on 108 Covid-19 ARDS patients, PEEP titration was performed during EIT monitoring, via decrementing PEEP trials [61]. The authors identify the best PEEP as the one corresponding to the crossing point of the collapse–overdistension curves. They also determine the PEEP with the best regional distribution of ventilation. Interestingly, EIT-based PEEP found at the collapse-overdistension crossing point well correlates to the PEEP with the highest compliance, while PEEP with the best EIT ventilation distribution is higher than the previous ones [61]. Jimenez et al. show that EIT-based PEEP setting allows to decrease mechanical power in ARDS patients, thus being potentially able to reduce VILI in this population [62]. Robust data on clinical outcomes of PEEP titration techniques are still lacking in the literature. A multicenter randomized controlled trial is actually going on to find out differences on clinical outcomes in ARDS patients whose PEEP is titrated using either EIT-based techniques or PEEP/FiO2 tables [63].
Cardiac monitoring
In ARDS patients, hemodynamic instability and low cardiac output may further decrease oxygen delivery and promote tissue hypoxia [64]. Strategies aimed at increasing cardiac output often involve fluid administration and vasoactive agents [65]. Therefore, hemodynamic monitoring is crucial in ARDS patients to optimize fluid administration and cardiac output [66].
Dynamic indexes of fluid responsiveness
As also recently highlighted by the Surviving Sepsis Campaign, the intravenous fluids of choice for critically ill patients are balanced crystalloids [67]. The risks of net fluid accumulation in critically ill patients have also recently been advocated [68]. The deliberate choice of a liberal versus a restrictive fluid strategy fails to show benefits in terms of reducing mortality [69]. A possible decrease in terms of length of ICU stay and mechanical ventilation is demonstrated in patients receiving lower amounts of intravenous fluids [70]. These considerations underline the importance of fluid administration optimization in mechanically ventilated patients.
Pulse pressure variation and stroke volume variation (PPV, SVV) are established predictors of fluid responsiveness and are typically validated in patients ventilated with a tidal volume greater than 8 mL/kg. Indeed, as also recognized by the acronym LIMITS (low heart/respiratory rate ratio, irregular beats, mechanical ventilation at low tidal volume, increased abdominal pressure, thorax opening, spontaneous breathing), mechanical ventilation at low tidal volume may reduce the sensitivity of these tests [71]. This is a possible limitation for their application in patients ventilated with protective strategies (i.e., ARDS) [72]. However, Wang et al. recently demonstrated good PPV performance in patients ventilated with less than 8 mL/kg TVc [73]. Similarly, as highlighted in Table 1, Taccheri et al. [74] showed that, in patients ventilated with low tidal volume (6 mL/kg), a PPV or SVV increase of 20% or 1 point after the application of a TVc compared to the baseline is a good predictor of fluid responsiveness in patients ventilated with a low tidal volume.
As recently reported by Lai et al., in patients mechanically ventilated with high positive end expiratory pressure (> 10 cmH2O), a decrease in PEEP (the so-called PEEP test) is a possible alternative to passive leg raising (PLR) to demonstrate fluid responsiveness. The authors have shown that an increase in the cardiac index is evident after both a PLR or with a decrease in PEEP from 10 to 5 cmH2O, with high sensitivity and specificity [75] (Table 1). According to Perez et al., the validity of these results has to be further proven, as indicated by the low respiratory compliance of the study population in the original paper [76].
Central venous oxygen saturation
Central venous oxygen saturation (ScvO2) is a valuable indicator of oxygen delivery adequacy for patients who are not equipped with a pulmonary artery catheter and for whom mixed venous oxygen saturation (SvO2) is not available. Concerns have been recently raised about its reliability during mechanical ventilation: it has been shown that the difference between ScvO2 and SvO2 may increase when intrathoracic pressures grow, especially when high PEEPs are employed [77]. Nevertheless, beyond the ScvO2 absolute values, its variation responding to diagnostic maneuvers may provide useful hemodynamic insights. A recent prospective study showed that the increase in ScvO2 (ΔScvO2) after PLR is a good predictor of fluid responsiveness and is associated with an increase in cardiac index [78]. These findings and the cutoff found by the authors (+ 4%) are comparable to the results of a meta-analysis from Pan et al., in which the authors showed that ΔScvO2 after a fluid challenge (500 mL) may adequately predict fluid responsiveness [79]. Another possible indicator of fluid responsiveness and cardiac output adequacy is the veno-arterial carbon dioxide difference (Pv-aCO2), together with the arterial to venous oxygen content difference (Ca-vO2) [80]. In COVID-19 patients with ARDS, the ratio of Pv-aCO2 to Ca-vO2 was significantly associated with mortality, with an AUC of 0.89 (95% CI 0.598–0.774, P = 0.001). The best cutoff found by the authors was 2.1 mmHg/mL [81].
Ultrasound and fluid responsiveness
The use of the Velocity Time Integral (VTI) of the Left Ventricular Outflow Tract (LVOT) allows a semi-continuous measure of the stroke volume (SV) and its variation (ΔSV) after a fluid challenge or a passive leg raising. It is therefore a valuable and well-validated measure for evaluation of fluid responsiveness [82]. Recently, it has been demonstrated how LVOT-VTI time variation well correlates with other parameters of fluid responsiveness, such as PPV, in a cohort of surgical patients [83]. However, LVOT-VTI measure is not always easy to assess and might be influenced by inter-operator variability [84]. The measure of the carotid systodiastolic (CSD) flow has been recently proved to be a valid surrogate of the LVOT-VTI, easier to measure [85]. Further researches may be useful to validate this parameter and allow its use to evaluate fluid responsiveness.
Respiratory change in inferior vena cava diameter (ΔIVC) is easy to measure with little expertise on thoracic ultrasound. It may provide important information on cardiac preload [66]. However, its sensibility is lower in patients ventilated at low tidal volume and in spontaneously breathing patients [74]. In their report, Taccheri et al. show that, similarly to PPV and SVV, ΔIVC is potentially applicable to patients ventilated at low tidal volume, thus enhancing their applicability on ARDS patients. The authors find an AUC of 0.76 and 0.86 of ΔIVC in predicting fluid responsiveness, respectively, after a TVc or a PLR when the applied tidal volume is less than 6 mL/kg (Table 1) [74].
Capillary refill time
The capillary refill time (CRT) is a simple-to-evaluate index of peripheral tissue perfusion and microcirculation. Monitoring CRT in critically ill patients reduces organ dysfunction and mortality compared to monitoring lactate levels alone [86]. In a prospective observational study, Raia et al. showed a direct correlation between a decrease in CRT after a 500-mL fluid challenge and fluid responsiveness. A low percentage (0.5%) of the total variance of the measurements is due to operator dependence (intrareader variability), thus stressing the reliability of this noninvasive parameter for assessing fluid responsiveness [87]. Caution must be taken in the use of CRT in patients with vasodilatory shock. Fage et al. showed that in septic patients, the correlation between a decrease in CRT and fluid or vasoactive administration is consistent only when substantial increases in the mean arterial pressure (MAP) or cardiac index (CI) are recorded. In contrast, in patients with an increase in MAP and CI less than 15% compared to the baseline, CRT is poorly correlated with fluid and vasoactive responsiveness [88]. CRT is an indirect index of microcirculatory dysfunction. In 282 critically ill patients, a correlation between CRT and microcirculatory impairments has been highlighted using the sidestream dark field imaging technique. CRT is independently correlated with the microvascular flow index (MFI). Patients admitted to the ICU with a higher CRT have a higher mortality [89]. Similar findings have been described in patients with COVID-19-related ARDS, in which, despite hemodynamic stability and normal lactate levels, CRT and microcirculatory indices (such as the MFI) are impaired, resulting in altered tissue perfusion [90].
CRT ranks therefore among the useful and easily measurable parameters to assess fluid responsiveness, and its use must be encouraged, with a word of caution in patients with vasodilatory shock. In the latter, monitoring CRT to test fluid responsiveness may result in false negatives due to decreased test sensitivity.
Conclusions
In patients affected by ARDS, harmful mechanical ventilation associated with a positive fluid balance may worsen lung injury. Careful respiratory and hemodynamic monitoring is therefore crucial in these patients.
The SpO2/FiO2 ratio is a valid alternative to the PaO2/FiO2 ratio for evaluating gas exchange. Corrected minute ventilation and the ventilatory ratio are two valuable surrogates for estimating the dead space fraction, and their prognostic value is well recognized in ARDS patients. Mechanical power directly measures the energy delivered to the lungs by mechanical ventilation and is thus able to predict VILI in invasively ventilated patients. It is easy to calculate because algorithms can be directly implemented in mechanical ventilators. In patients undergoing noninvasive mechanical ventilation, the role of esophageal pressure is crucial for estimating the P-SILI.
In addition to respiratory variable monitoring, fluid stewardship is also important for detecting VILI. Dynamic indices of fluid responsiveness are also suitable for patients undergoing protective mechanical ventilation at low tidal volumes. There is increasing evidence about the validity of the ΔscvO2 and CRT after a PLR or a fluid challenge. The role of these easy-to-evaluate parameters may be increasingly important in patients affected by respiratory failure, especially in disadvantaged contexts in which enhanced monitoring is not available.
Availability of data and materials
Data sharing is not applicable to this article, as no datasets were generated or analyzed during the current study.
Abbreviations
- ARDS:
-
Acute respiratory distress syndrome
- PEEP:
-
Positive end expiratory pressure
- MV:
-
Mechanical ventilation
- SaO2 :
-
Arterial oxygen saturation
- SpO2 :
-
Oxygen saturation measured via pulse-oximetry
- SpO2/FiO2 :
-
Pulse-oximetric oxygen saturation to the fraction of inspired oxygen
- PaO2/FiO2 :
-
Partial pressure of arterial oxygen to FiO2
- COVID-19:
-
Coronavirus disease
- HFNC:
-
High-flow nasal cannula
- RCT:
-
Randomized controlled trial
- V/Q :
-
Ventilation/perfusion
- ICU:
-
Intensive care unit
- V Ecorr :
-
Corrected minute ventilation
- CO2 :
-
Carbon dioxide
- VR:
-
Ventilatory ratio
- AUC:
-
Area under the curve
- Q va/Q :
-
Venous admixture
- VCO2 :
-
Amount of CO2 produced
- PETCO2/PaCO2 :
-
Gas exchange efficiency is the computation of the end-tidal-to-arterial PCO2 ratio
- CT:
-
Computed tomography
- VV-ECMO:
-
Venous extracorporeal membrane oxygenation
- J/min:
-
Joules per minute
- VILI:
-
Ventilator-induced lung injury
- Pes:
-
Esophageal pressure
- PSILI:
-
Patient self-inflicted lung injury
- Δpes:
-
Changes in esophageal pressure during inspiration
- CPAP:
-
Continuous positive airway pressure
- NIV:
-
Noninvasive ventilation
- DTI:
-
Diaphragmatic thickening fraction
- R/I ratio:
-
Recruitment-to-inflation ratio
- EIT:
-
Electrical impedance tomography
- PPV:
-
Pulse pressure variation
- SVV:
-
Stroke volume variation
- LIMITS:
-
Low heart/respiratory rate ratio, irregular beats, mechanical ventilation at low tidal volume, increased abdominal pressure, thorax opening, spontaneous breathing
- TVc:
-
Tidal volume challenge
- PLR:
-
Passive leg raising
- ScvO2 :
-
Central venous oxygen saturation
- SvO2 :
-
Venous oxygen saturation
- ΔscvO2 :
-
Central venous oxygen saturation increase
- Pv-aCO2 :
-
Veno-arterial carbon dioxide difference
- Ca-vO2 :
-
Venous oxygen content difference
- VTI:
-
Velocity time integral
- LVOT:
-
Left ventricular outflow tract
- SV:
-
Stroke volume
- ΔSV:
-
Variation in stroke volume
- CSD:
-
Carotid systodiastolic
- ΔIVC:
-
Change in inferior vena cava diameter
- CRT:
-
Capillary refill time
- MAP:
-
Mean arterial pressure
References
Jubran A. Pulse oximetry. Crit Care. 2015;19:272. https://doi.org/10.1186/S13054-015-0984-8.
Wick KD, Matthay MA, Ware LB. Pulse oximetry for the diagnosis and management of acute respiratory distress syndrome. Lancet Respir Med. 2022;10:1086–98. https://doi.org/10.1016/S2213-2600(22)00058-3.
Henry NR, Hanson AC, Schulte PJ, Warner NS, Manento MN, Weister TJ, et al. Disparities in hypoxemia detection by pulse oximetry across self-identified racial groups and associations with clinical outcomes. Crit Care Med. 2022;50:204–11. https://doi.org/10.1097/CCM.0000000000005394.
Fawzy A, Wu TD, Wang K, Robinson ML, Farha J, Bradke A, et al. Racial and ethnic discrepancy in pulse oximetry and delayed identification of treatment eligibility among patients with COVID-19. JAMA Intern Med. 2022;182:730–8. https://doi.org/10.1001/JAMAINTERNMED.2022.1906.
Babu S, Abhilash KPP, Kandasamy S, Gowri M. Association between SpO2/FiO2 ratio and PaO2/FiO2 ratio in different modes of oxygen supplementation. Indian J Crit Care Med. 2021;25:1001–5. https://doi.org/10.5005/JP-JOURNALS-10071-23977.
Bashar FR, Vahedian-Azimi A, Farzanegan B, Goharani R, Shojaei S, Hatamian S, et al. Comparison of non-invasive to invasive oxygenation ratios for diagnosing acute respiratory distress syndrome following coronary artery bypass graft surgery: a prospective derivation-validation cohort study. J Cardiothorac Surg. 2018;13:123. https://doi.org/10.1186/S13019-018-0804-8.
Pisani L, Roozeman JP, Simonis FD, Giangregorio A, van der Hoeven SM, Schouten LRA, et al. Risk stratification using SpO2/FiO2 and PEEP at initial ARDS diagnosis and after 24 h in patients with moderate or severe ARDS. Ann Intensive Care. 2017;7:108. https://doi.org/10.1186/S13613-017-0327-9.
Rice TW, Wheeler AP, Bernard GR, Hayden DL, Schoenfeld DA, Ware LB. Comparison of the SpO2/FIO2 ratio and the PaO2/FIO2 ratio in patients with acute lung injury or ARDS. Chest. 2007;132:410–7. https://doi.org/10.1378/CHEST.07-0617.
Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, et al. Report of the American–European consensus conference on ARDS: definitions, mechanisms, relevant outcomes and clinical trial coordination. The Consensus Committee. Intensive Care Med. 1994;20:225–32. https://doi.org/10.1007/BF01704707.
Fukuda Y, Tanaka A, Homma T, Kaneko K, Uno T, Fujiwara A, et al. Utility of SpO2/FiO2 ratio for acute hypoxemic respiratory failure with bilateral opacities in the ICU. PLoS ONE. 2021;16: e0245927. https://doi.org/10.1371/JOURNAL.PONE.0245927.
Roozeman JP, Mazzinari G, Serpa Neto A, Hollmann MW, Paulus F, Schultz MJ, et al. Prognostication using SpO2/FiO2 in invasively ventilated ICU patients with ARDS due to COVID-19—insights from the PRoVENT-COVID study. J Crit Care. 2022;68:31–7. https://doi.org/10.1016/J.JCRC.2021.11.009.
Choi KJ, Hong HL, Kim EJ. The Association between mortality and the oxygen saturation and fraction of inhaled oxygen in patients requiring oxygen therapy due to COVID-19-associated pneumonia. Tuberc Respir Dis (Seoul). 2021;84:125–33. https://doi.org/10.4046/TRD.2020.0126.
Kim JH, Baek AR, Lee SI, Kim WY, Na YS, Lee BY, et al. ROX index and SpO2/FiO2 ratio for predicting high-flow nasal cannula failure in hypoxemic COVID-19 patients: a multicenter retrospective study. PLoS ONE. 2022;17: e0268431. https://doi.org/10.1371/JOURNAL.PONE.0268431.
Semler MW, Casey JD, Lloyd BD, Hastings PG, Hays MA, Stollings JL, et al. Oxygen-saturation targets for critically ill adults receiving mechanical ventilation. N Engl J Med. 2022;387:1759–69. https://doi.org/10.1056/NEJMOA2208415.
Lumb Andrew. Nunn’s applied respiratory physiology. 5th edn. 2000.
Slobod D, Damia A, Leali M, Spinelli E, Mauri T. Pathophysiology and clinical meaning of ventilation-perfusion mismatch in the acute respiratory distress syndrome. Biology (Basel). 2022;12:67. https://doi.org/10.3390/BIOLOGY12010067.
Rossi A, Santos C, Roca J, Torres A, Félez MA, Rodriguez-Roisin R. Effects of PEEP on VA/Q mismatching in ventilated patients with chronic airflow obstruction. Am J Respir Crit Care Med. 1994;149:1077–84. https://doi.org/10.1164/AJRCCM.149.5.8173744.
Nuckton TJ, Alonso JA, Kallet RH, Daniel BM, Pittet J-F, Eisner MD, et al. Pulmonary dead-space fraction as a risk factor for death in the acute respiratory distress syndrome. N Engl J Med. 2002;346:1281–6. https://doi.org/10.1056/NEJMOA012835.
Farrow CE, Robles R, Prisk GK, Harbut P, Malhotra A, Amis TC, et al. Increased intrapulmonary shunt and alveolar dead space post-COVID-19. J Appl Physiol. 1985;2023:135. https://doi.org/10.1152/JAPPLPHYSIOL.00267.2023.
Bertelli M, Fusina F, Prezioso C, Cavallo E, Nencini N, Crisci S, et al. COVID-19 ARDS is characterized by increased dead space ventilation compared with non-COVID ARDS. Respir Care. 2021;66:1406–15. https://doi.org/10.4187/RESPCARE.08786.
Mollura M, Baroncelli F, Mandelli G, Tricella G, Weissman GE, Poole D, et al. Physiologic dead space is independently associated with mortality and discharge of mechanically ventilated patients with COVID-19 ARDS: a retrospective study. Sci Rep. 2023;13:5719. https://doi.org/10.1038/S41598-023-31999-6.
Morales-Quinteros L, Serpa Neto A, Artigas A, Blanch L, Botta M, Kaufman DA, et al. Dead space estimates may not be independently associated with 28-day mortality in COVID-19 ARDS. Crit Care. 2021;25:171. https://doi.org/10.1186/S13054-021-03570-0.
Fusina F, Albani F, Bertelli M, Cavallo E, Crisci S, Caserta R, et al. Corrected minute ventilation is associated with mortality in ARDS caused by COVID-19. Respir Care. 2021;66:619–25. https://doi.org/10.4187/RESPCARE.08314.
Sinha P, Fauvel NJ, Singh S, Soni N. Ventilatory ratio: a simple bedside measure of ventilation. Br J Anaesth. 2009;102:692–7. https://doi.org/10.1093/BJA/AEP054.
Siegel ER, Zhuo H, Sinha P, Papolos AI, Ni SA, Vessel K, et al. Ventilatory ratio is a valuable prognostic indicator in an observational cohort of patients with ARDS. Respir Care. 2022;67:1075–81. https://doi.org/10.4187/RESPCARE.09854.
Wang Z, Xia F, Dai H, Chen H, Xie J, Qiu H, et al. Early decrease of ventilatory ratio after prone position ventilation may predict successful weaning in patients with acute respiratory distress syndrome: a retrospective cohort study. Front Med (Lausanne). 2022;9:1057260. https://doi.org/10.3389/FMED.2022.1057260.
Maj R, Palermo P, Gattarello S, Brusatori S, D’Albo R, Zinnato C, et al. Ventilatory ratio, dead space, and venous admixture in patients with acute respiratory distress syndrome. Br J Anaesth. 2023;130:360–7. https://doi.org/10.1016/J.BJA.2022.10.035.
Huang D, Brower R, Ferguson N, Ginde A, Gong M, Grisson GC, et al. Early neuromuscular blockade in the acute respiratory distress syndrome. N Engl J Med. 2019;380:1997–2008. https://doi.org/10.1056/NEJMOA1901686/SUPPL_FILE/NEJMOA1901686_DATA-SHARING.PDF
Monteiro ACC, Vangala S, Wick KD, Delucchi KL, Siegel ER, Thompson BT, et al. The prognostic value of early measures of the ventilatory ratio in the ARDS ROSE trial. Crit Care. 2022;26:297. https://doi.org/10.1186/S13054-022-04179-7.
Bonifazi M, Romitti F, Busana M, Palumbo MM, Steinberg I, Gattarello S, et al. End-tidal to arterial PCO2 ratio: a bedside meter of the overall gas exchanger performance. Intensive Care Med Exp. 2021;9:21. https://doi.org/10.1186/S40635-021-00377-9.
Kallet RH, Lipnick MS. End-tidal-to-arterial PCO2 ratio as signifier for physiologic dead-space ratio and oxygenation dysfunction in acute respiratory distress syndrome. Respir Care. 2021;66:263–8. https://doi.org/10.4187/RESPCARE.08061.
Lazzari S, Romitti F, Busana M, Vassalli F, Bonifazi M, Macrí MM, et al. End-tidal to arterial pco2 ratio as guide to weaning from venovenous extracorporeal membrane oxygenation. Am J Respir Crit Care Med. 2022;206:973–80. https://doi.org/10.1164/RCCM.202201-0135OC.
Gattinoni L, Tonetti T, Cressoni M, Cadringher P, Herrmann P, Moerer O, et al. Ventilator-related causes of lung injury: the mechanical power. Intensive Care Med. 2016;42:1567–75. https://doi.org/10.1007/S00134-016-4505-2.
Chiumello D, Gotti M, Guanziroli M, Formenti P, Umbrello M, Pasticci I, et al. Bedside calculation of mechanical power during volume- and pressure-controlled mechanical ventilation. Crit Care. 2020;24:417. https://doi.org/10.1186/S13054-020-03116-W.
Chiumello D, Coppola S, Formenti P, Ciabattoni A, Lucenteforte M, Liu G, et al. A validation study of a continuous automatic measurement of the mechanical power in ARDS patients. J Crit Care. 2022;67:21–5. https://doi.org/10.1016/J.JCRC.2021.09.009.
Coppola S, Caccioppola A, Froio S, Formenti P, De Giorgis V, Galanti V, et al. Effect of mechanical power on intensive care mortality in ARDS patients. Crit Care. 2020;24:246. https://doi.org/10.1186/S13054-020-02963-X.
Azizi BA, Munoz-Acuna R, Suleiman A, Ahrens E, Redaelli S, Tartler TM, et al. Mechanical power and 30-day mortality in mechanically ventilated, critically ill patients with and without Coronavirus Disease-2019: a hospital registry study. J Intensive Care. 2023;11:14. https://doi.org/10.1186/S40560-023-00662-7.
Van Meenen DMP, Algera AG, Schuijt MTU, Simonis FD, Van Der Hoeven SM, Neto AS, et al. Effect of mechanical power on mortality in invasively ventilated ICU patients without the acute respiratory distress syndrome: an analysis of three randomised clinical trials. Eur J Anaesthesiol. 2023;40:21–8. https://doi.org/10.1097/EJA.0000000000001778.
Urner M, Jüni P, Hansen B, Wettstein MS, Ferguson ND, Fan E. Time-varying intensity of mechanical ventilation and mortality in patients with acute respiratory failure: a registry-based, prospective cohort study. Lancet Respir Med. 2020;8:905. https://doi.org/10.1016/S2213-2600(20)30325-8.
Pozzi T, Fratti I, Tomarchio E, Bruno G, Catozzi G, Monte A, et al. Early time-course of respiratory mechanics, mechanical power and gas exchange in ARDS patients. J Crit Care. 2024;79: 154444. https://doi.org/10.1016/J.JCRC.2023.154444.
Costa ELV, Slutsky AS, Brochard LJ, Brower R, Serpa-Neto A, Cavalcanti AB, et al. Ventilatory variables and mechanical power in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2021;204:303–11. https://doi.org/10.1164/RCCM.202009-3467OC.
Gattinoni L, Marini JJ, Collino F, Maiolo G, Rapetti F, Tonetti T, et al. The future of mechanical ventilation: lessons from the present and the past. Crit Care. 2017;21:183. https://doi.org/10.1186/S13054-017-1750-X.
Dreyfuss D, Soler P, Basset G, Saumon G. High inflation pressure pulmonary edema: respective effects of high airway pressure, high tidal volume, and positive end-expiratory pressure. Am Rev Respir Dis. 2012;137:1159–64. https://doi.org/10.1164/AJRCCM/137.5.1159.
Brochard L, Slutsky A, Pesenti A. Mechanical ventilation to minimize progression of lung injury in acute respiratory failure. Am J Respir Crit Care Med. 2017;195:438–42. https://doi.org/10.1164/RCCM.201605-1081CP.
Yoshida T, Grieco DL, Brochard L, Fujino Y. Patient self-inflicted lung injury and positive end-expiratory pressure for safe spontaneous breathing. Curr Opin Crit Care. 2020;26:59–65. https://doi.org/10.1097/MCC.0000000000000691.
Goligher EC, Dres M, Patel BK, Sahetya SK, Beitler JR, Telias I, et al. Lung- and diaphragm-protective ventilation. Am J Respir Crit Care Med. 2020;202:950–61. https://doi.org/10.1164/RCCM.202003-0655CP.
Bellani G, Grassi A, Sosio S, Gatti S, Kavanagh BP, Pesenti A, et al. Driving pressure is associated with outcome during assisted ventilation in acute respiratory distress syndrome. Anesthesiology. 2019;131:594–604. https://doi.org/10.1097/ALN.0000000000002846.
Menga LS, Delle Cese L, Rosà T, Cesarano M, Scarascia R, Michi T, et al. Respective effects of helmet pressure support, continuous positive airway pressure, and nasal high-flow in hypoxemic respiratory failure: a randomized crossover clinical trial. Am J Respir Crit Care Med. 2023;207:1310–23. https://doi.org/10.1164/RCCM.202204-0629OC.
Coppola S, Chiumello D, Busana M, Giola E, Palermo P, Pozzi T, et al. Role of total lung stress on the progression of early COVID-19 pneumonia. Intensive Care Med. 2021;47:1130–9. https://doi.org/10.1007/S00134-021-06519-7.
Chiumello D, Chiodaroli E, Coppola S, Cappio Borlino S, Granata C, Pitimada M, et al. Awake prone position reduces work of breathing in patients with COVID-19 ARDS supported by CPAP. Ann Intensive Care. 2021;11:179. https://doi.org/10.1186/S13613-021-00967-6.
Steinberg I, Chiodaroli E, Gattarello S, Cappio Borlino S, Chiumello D. Diaphragmatic ultrasound and esophageal pressure in COVID-19 pneumonia during helmet CPAP. Intensive Care Med. 2022;48:1095–6. https://doi.org/10.1007/S00134-022-06785-Z.
Poulard T, Bachasson D, Fossé Q, Niérat MC, Hogrel JY, Demoule A, et al. Poor correlation between diaphragm thickening fraction and transdiaphragmatic pressure in mechanically ventilated patients and healthy subjects. Anesthesiology. 2022;136:162–75. https://doi.org/10.1097/ALN.0000000000004042.
Mawla TSA, Fattah SRA, Halim AMA, Elhefeny RA. Diaphragmatic function assessment using chest ultrasonography as a predictor for weaning from mechanical ventilation. Egypt J Crit Care Med. 2022;9:1–9. https://doi.org/10.1097/EJ9.0000000000000039.
Fossat G, Daillet B, Desmalles E, Boulain T. Does diaphragm ultrasound improve the rapid shallow breathing index accuracy for predicting the success of weaning from mechanical ventilation? Aust Crit Care. 2022;35:233–40. https://doi.org/10.1016/J.AUCC.2021.05.008.
Chen L, Del Sorbo L, Grieco DL, Junhasavasdikul D, Rittayamai N, Soliman I, et al. Potential for lung recruitment estimated by the recruitment-to-inflation ratio in acute respiratory distress syndrome a clinical trial. Am J Respir Crit Care Med. 2020;201:178–87. https://doi.org/10.1164/RCCM.201902-0334OC.
Stevic N, Chatelain E, Dargent A, Argaud L, Cour M, Guérin C. Lung recruitability evaluated by recruitment-to-inflation ratio and lung ultrasound in COVID-19 acute respiratory distress syndrome. Am J Respir Crit Care Med. 2021;203:1025–7. https://doi.org/10.1164/RCCM.202012-4447LE.
Cour M, Biscarrat C, Stevic N, Degivry F, Argaud L, Guérin C. Recruitment-to-inflation ratio measured with modern intensive care unit ventilators: how accurate is it? Crit Care. 2022;26:85. https://doi.org/10.1186/S13054-022-03961-X.
Cornejo RA, Diaz JC, Tobar EA, Bruhn AR, Ramos CA, Gonzalez RA, et al. Effects of prone positioning on lung protection in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2013;188:440–8. https://doi.org/10.1164/RCCM.201207-1279OC.
Del Sorbo L, Tisminetzky M, Chen L, Brochard L, Arellano D, Brito R, et al. Association of lung recruitment and change in recruitment-to-inflation ratio from supine to prone position in acute respiratory distress syndrome. Crit Care. 2023;27:140. https://doi.org/10.1186/S13054-023-04428-3.
Zerbib Y, Lambour A, Maizel J, Kontar L, De Cagny B, Soupison T, et al. Respiratory effects of lung recruitment maneuvers depend on the recruitment-to-inflation ratio in patients with COVID-19-related acute respiratory distress syndrome. Crit Care. 2022;26:12. https://doi.org/10.1186/S13054-021-03876-Z.
Jonkman AH, Alcala GC, Pavlovsky B, Roca O, Spadaro S, Scaramuzzo G, et al. Lung recruitment assessed by electrical impedance tomography (RECRUIT) a multicenter study of COVID-19 acute respiratory distress syndrome. Am J Respir Crit Care Med. 2023;208:25–38. https://doi.org/10.1164/RCCM.202212-2300OC/SUPPL_FILE/DISCLOSURES.PDF.
Jimenez JV, Munroe E, Weirauch AJ, Fiorino K, Culter CA, Nelson K, et al. Electric impedance tomography-guided PEEP titration reduces mechanical power in ARDS: a randomized crossover pilot trial. Crit Care. 2023;27:21. https://doi.org/10.1186/S13054-023-04315-X.
Yuan X, Zhang R, Wang Y, Chen D, Chao Y, Xu J, et al. Effect of EIT-guided PEEP titration on prognosis of patients with moderate to severe ARDS: study protocol for a multicenter randomized controlled trial. Trials. 2023;24:266. https://doi.org/10.1186/S13063-023-07280-6.
Vignon P, Evrard B, Asfar P, Busana M, Calfee CS, Coppola S, et al. Fluid administration and monitoring in ARDS: which management? Intensive Care Med. 2020;46:2252–64. https://doi.org/10.1007/S00134-020-06310-0.
Zampieri FG, Bagshaw SM, Semler MW. Fluid therapy for critically ill adults with sepsis: a review. JAMA. 2023;329:1967–80. https://doi.org/10.1001/JAMA.2023.7560.
Monnet X, Shi R, Teboul JL. Prediction of fluid responsiveness. What’s new? Ann Intensive Care. 2022;12:46. https://doi.org/10.1186/S13613-022-01022-8.
Evans L, Rhodes A, Alhazzani W, Antonelli M, Coopersmith CM, French C, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021;47:1181–247. https://doi.org/10.1007/S00134-021-06506-Y.
Messmer AS, Zingg C, Müller M, Gerber JL, Schefold JC, Pfortmueller CA. Fluid overload and mortality in adult critical care patients—a systematic review and meta-analysis of observational studies. Crit Care Med. 2020;48:1862–70. https://doi.org/10.1097/CCM.0000000000004617.
Meyhoff TS, Hjortrup PB, Wetterslev J, Sivapalan P, Laake JH, Cronhjort M, et al. Restriction of intravenous fluid in ICU patients with septic shock. N Engl J Med. 2022;386:2459–70. https://doi.org/10.1056/NEJMOA2202707.
Silversides JA, Major E, Ferguson AJ, Mann EE, McAuley DF, Marshall JC, et al. Conservative fluid management or deresuscitation for patients with sepsis or acute respiratory distress syndrome following the resuscitation phase of critical illness: a systematic review and meta-analysis. Intensive Care Med. 2017;43:155–70. https://doi.org/10.1007/S00134-016-4573-3.
Michard F, Chemla D, Teboul JL. Applicability of pulse pressure variation: how many shades of grey? Crit Care. 2015;19:144. https://doi.org/10.1186/S13054-015-0869-X.
Acute Respiratory Distress Syndrome Network; Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, Wheeler A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–1308. https://doi.org/10.1056/NEJM200005043421801.
Wang X, Liu S, Gao J, Zhang Y, Huang T. Does tidal volume challenge improve the feasibility of pulse pressure variation in patients mechanically ventilated at low tidal volumes? A systematic review and meta-analysis. Crit Care. 2023;27:45. https://doi.org/10.1186/S13054-023-04336-6.
Taccheri T, Gavelli F, Teboul JL, Shi R, Monnet X. Do changes in pulse pressure variation and inferior vena cava distensibility during passive leg raising and tidal volume challenge detect preload responsiveness in case of low tidal volume ventilation? Crit Care. 2021;25:10. https://doi.org/10.1186/S13054-021-03515-7.
Lai C, Shi R, Beurton A, Moretto F, Ayed S, Fage N, et al. The increase in cardiac output induced by a decrease in positive end-expiratory pressure reliably detects volume responsiveness: the PEEP-test study. Crit Care. 2023;27:136. https://doi.org/10.1186/S13054-023-04424-7.
Pérez C, Castillo L, Alvarado J. Can fluid responsiveness tests utilizing positive end-expiratory pressure changes be adapted to improve applicability in all mechanically ventilated patients? Crit Care. 2023;27:191. https://doi.org/10.1186/S13054-023-04483-W.
Fioccola A, Pozzi T, Fratti I, Nicolardi RV, Romitti F, Busana M, et al. Impact of mechanical power and positive end expiratory pressure on central vs. mixed oxygen and carbon dioxide related variables in a population of female piglets. Physiol Rep. 2024;12:e15954. https://doi.org/10.14814/PHY2.15954.
Giraud R, Vujovic B, Assouline B, Neto Silva I, Bendjelid K. Do ScvO2 variations induced by passive leg raising predict fluid responsiveness? A prospective study. Physiol Rep. 2021;9:e15015. https://doi.org/10.14814/PHY2.15012.
Pan J, Sun Y, Xu Z, Dong P, Zhou X. Variation in central venous oxygen saturation to evaluate fluid responsiveness: a systematic review and meta-analysis. Crit Care. 2023;27:203. https://doi.org/10.1186/S13054-023-04480-Z.
Monnet X, Julien F, Ait-Hamou N, Lequoy M, Gosset C, Jozwiak M, et al. Lactate and venoarterial carbon dioxide difference/arterial-venous oxygen difference ratio, but not central venous oxygen saturation, predict increase in oxygen consumption in fluid responders. Crit Care Med. 2013;41:1412–20. https://doi.org/10.1097/CCM.0B013E318275CECE.
Sánchez Díaz JS, Peniche Moguel KG, Reyes-Ruiz JM, Pérez Nieto OR, Escarramán Martínez D, Zamarrón López EI, et al. The ∆Pv-aCO2/∆Ca-vO2 ratio as a predictor of mortality in patients with severe acute respiratory distress syndrome related to COVID-19. PLoS ONE. 2023;18: e0290272. https://doi.org/10.1371/JOURNAL.PONE.0290272.
Lamia B, Ochagavia A, Monnet X, Chemla D, Richard C, Teboul JL. Echocardiographic prediction of volume responsiveness in critically ill patients with spontaneously breathing activity. Intensive Care Med. 2007;33:1125–32. https://doi.org/10.1007/S00134-007-0646-7.
Pérez-Manjarrez A, García-Cruz E, Gopar-Nieto R, Jiménez-Rodríguez GM, Lazcano-Díaz E, Rojas-Velasco G, et al. Usefulness of the velocity-time integral of the left ventricular outflow tract variability index to predict fluid responsiveness in patients undergoing cardiac surgery. Echo Res Pract. 2023;10:9. https://doi.org/10.1186/S44156-023-00022-Z.
Shaikh F, Kenny JE, Awan O, Markovic D, Friedman O, He T, et al. Measuring the accuracy of cardiac output using POCUS: the introduction of artificial intelligence into routine care. Ultrasound J. 2022;14:47. https://doi.org/10.1186/S13089-022-00301-6.
Cheong I, Otero Castro V, Sosa FA, Tort Oribe B, Merlo PM, Tamagnone FM. Carotid flow as a surrogate of the left ventricular stroke volume. J Clin Monit Comput. 2023;37:661–7. https://doi.org/10.1007/S10877-022-00938-7.
Hernandez G, et al. Effect of a resuscitation strategy targeting peripheral perfusion status vs serum lactate levels on 28-day mortality among patients with septic shock: the ANDROMEDA-SHOCK randomized clinical trial. JAMA. 2019;321:654–64. https://doi.org/10.1001/JAMA.2019.0071.
Raia L, Gabarre P, Bonny V, Urbina T, Missri L, Boelle PY, et al. Kinetics of capillary refill time after fluid challenge. Ann Intensive Care. 2022;12:74. https://doi.org/10.1186/S13613-022-01049-X.
Fage N, Moretto F, Rosalba D, Shi R, Lai C, Teboul JL, et al. Effect on capillary refill time of volume expansion and increase of the norepinephrine dose in patients with septic shock. Crit Care. 2023;27:429. https://doi.org/10.1186/S13054-023-04714-0.
Huang W, Xiang H, Hu C, Wu T, Zhang D, Ma S, et al. Association of sublingual microcirculation parameters and capillary refill time in the early phase of ICU admission. Crit Care Med. 2023;51:913–23. https://doi.org/10.1097/CCM.0000000000005851.
Kanoore Edul VS, Caminos Eguillor JF, Ferrara G, Estenssoro E, Siles DSP, Cesio CE, et al. Microcirculation alterations in severe COVID-19 pneumonia. J Crit Care. 2021;61:73–5. https://doi.org/10.1016/J.JCRC.2020.10.002.
Acknowledgements
Not applicable.
Funding
No funding was received from the authors for the present paper.
Author information
Authors and Affiliations
Contributions
Conceptualization, methodology, literature search, writing—original draft preparation, writing—review and editing, resources, visualization: DC, AF; validation, supervision: DC.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chiumello, D., Fioccola, A. Recent advances in cardiorespiratory monitoring in acute respiratory distress syndrome patients. j intensive care 12, 17 (2024). https://doi.org/10.1186/s40560-024-00727-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40560-024-00727-1