Abstract
Background
Since oxygen content and oxygen consumption typically remain unchanged within a short period, variation in central venous oxygen saturation (ΔScvO2) during fluid challenge can theoretically track the changes in cardiac output (CO). We conducted this meta-analysis to systematically assess the diagnostic performance of ΔScvO2 during a fluid challenge for fluid responsiveness in mechanically ventilated patients receiving volume expansion.
Methods
Electronic databases were systematically searched to identify relevant studies published before October 24, 2022. As the cutoff value of ΔScvO2 was expected to vary across the included studies, we estimated the area under the hierarchical summary receiver operating characteristic curve (AUHSROC) as the primary measure of diagnostic accuracy. The optimal threshold of ΔScvO2 and the corresponding 95% confidential interval (CI) were also estimated.
Results
This meta-analysis included 5 observational studies comprising 240 participants, of whom 133 (55%) were fluid responders. Overall, the ΔScvO2 during the fluid challenge exhibited excellent performance for defining fluid responsiveness in mechanically ventilated patients receiving volume expansion, with an AUHSROC of 0.86 (95% CI 0.83–0.89), a pooled sensitivity of 0.78 (95% CI 0.69–0.85), a pooled specificity of 0.84 (95% CI 0.72–0.91), and a pooled diagnostic odds ratio of 17.7 (95% CI 5.9–53.2). The distribution of the cutoff values was nearly conically symmetrical and concentered between 3 and 5%; the mean and median cutoff values were 4% (95% CI 3–5%) and 4% (95% CI not estimable), respectively.
Conclusions
In mechanically ventilated patients receiving volume expansion, the ΔScvO2 during the fluid challenge is a reliable indicator of fluid responsiveness.
Clinical trial registration PROSPERO, https://www.crd.york.ac.uk/prospero/, registry number: CRD42022370192.
Similar content being viewed by others
Background
Volume expansion is one of the first-line treatments of hypotension and hypoperfusion in critically ill patients. Fluid administration is anticipated to increase cardiac output (CO) and oxygen delivery (DO2), and finally restore tissue perfusion, while accompanied by a high risk of fluid overloading if cardiac performance has reached the plateau of the Frank–Starling curve. Thus, the assessment of fluid responsiveness is a crucial procedure before fluid infusion. Seeking reliable surrogate indicators of fluid responsiveness has always been an important research issue in critical care medicine. As fluid responsiveness is usually defined by a significant increase (≥ 15%) in CO after receiving a certain amount of fluid within a short period [1, 2], parameters associated with the changes of CO or CO-derived indices, theoretically, possess the potential to be surrogate markers of fluid responsiveness.
Given the close relationship between blood flow (i.e., CO) and energy metabolism, parameters related to oxygen metabolism or carbon dioxide production are widely studied to define fluid responsiveness [3,4,5,6]. Mixed venous oxygen saturation (SvO2), which is clinically substituted by central venous oxygen saturation (ScvO2) in adequate circumstances due to the equivalent change trend of the two variables [7, 8], has historically been considered as an oxygen metabolic variable that may reflect the balance between DO2 and oxygen consumption (VO2). Since VO2 typically remains unchanged during a fluid challenge, the variation in ScvO2 (ΔScvO2) during the fluid challenge can theoretically track the change of DO2, which was confirmed in human and animal subjects with different cardiovascular conditions [9, 10]. As a consequence, the ΔScvO2 during the fluid challenge may also reflect the change of CO because oxygen content is, at least theoretically, constant within a short time. These rationales underlie the ability of ΔScvO2 to evaluate fluid responsiveness. Currently, the diagnostic performance of ΔScvO2 for fluid responsiveness remains inconclusive, even though the relationship between the changes of ScvO2 and CO during fluid challenge has been widely studied [3,4,5]. Therefore, we conducted this meta-analysis to systematically assess the diagnostic accuracy of ΔScvO2 for evaluating fluid responsiveness.
Method
This meta-analysis was reported following the Preferred Reporting Items for a Systematic Review and Meta-analysis of Diagnostic Test Accuracy [11]. We registered the study protocol at the international prospective register of systematic reviews (PROSPERO, CRD42022370192) before initiating the study.
Data sources and search strategy
Systematic literature searching was conducted by two independent reviewers (JP and YS) on October 24, 2022, in four electronic databases (PubMed, Web of Science, Embase, and Cochrane Library) to retrieve studies that assessed the diagnostic accuracy of the ΔScvO2 during the fluid challenge for fluid responsiveness in mechanically ventilated patients receiving volume expansion, without any date or language restriction. The bibliographies of relevant publications were reviewed to further identify relevant articles. The detailed search strategies are presented in Additional file 1: Table S1.
Eligibility criteria
Candidate studies were screened in compliance with the following eligibility criteria: (1) conducted on mechanically ventilated adults (age > 18 years), whose physicians in charge decided to administrate fluid to restore tissue hypoperfusion or hypotension; (2) assessing fluid responsiveness (the reference index) in the context of a fluid challenge; (3) measuring ScvO2 (the index test) before and immediately after fluid challenge, and calculating ΔScvO2 by the relative changes of ScvO2 from the baseline value; and (4) reporting sufficient information to construct a 2 × 2 contingency table. No restriction was applied to the definition of fluid responsiveness. We excluded studies that met anyone of the following criteria: (1) volume expansion was not conducted in the form of fluid challenge, that is, the volume of fluid was more than 500 mL or administrated for more than 30 min; (2) measuring SvO2 instead of ScvO2; or (3) conference abstracts without a full text.
Study selection, data extraction, and quality assessment
Initially, two independent reviewers (ZX and PD) checked all searched records for duplicates and performed deduplication. After that, they reviewed the title and abstract of the remaining records for relevance. Finally, the full text of candidate studies was carefully reviewed to determine their suitability for inclusion or exclusion. Any disagreement between the two reviewers was resolved through discussion with a third reviewer (XZ).
The same two reviewers (ZX and PD) pre-customized an extraction form to extract the study and patient characteristics from each included study. They also recorded the diagnostic accuracy of ScvO2 for fluid responsiveness in detail in Additional file 1: Table S2, including the area under the receiver operating characteristic (ROC) curve (AUROC), the sensitivity, the specificity, and its corresponding cutoff value. According to the diagnostic accuracy (sensitivity and specificity) and sample size in each included study, we calculated the true positive, false positive, false negative, and true negative values to construct a 2 × 2 contingency table. The corresponding authors would be contacted to inquire about the missing data if necessary. A joint review of articles was suggested to resolve any disagreements between the two reviewers.
The methodological quality of each included study was independently assessed by two reviewers (YS and XZ) using the Quality Assessment of Diagnostic Accuracy Studies (QUADAS)-2 tool [12]. If existing disagreements, a discussion would be required to achieve a consensus.
Statistical analysis
Due to the largely varied patient characteristics, we expected substantial between-study variations between the included studies. Therefore, before data synthesis, the derived estimates of sensitivity and specificity from each included study were plotted on forest plot and ROC space to explore the between-study variations in the diagnostic accuracy of ΔScvO2 for fluid responsiveness. We adopted the random-effect bivariate model to calculate the pooled sensitivity, pooled specificity, and pooled diagnostic odds ratio (DOR) [13, 14], where the bivariate model integrates a correlation parameter allowing for the expected trade-off in sensitivity and specificity due to the varied threshold values of ΔScvO2 across the included studies [13]. The DOR is an appropriate global measure of comparing the accuracy of different diagnostic tests, and it can take values between 0 and infinity. The higher the DOR value, the better the diagnostic performance. A value equal to 1 indicates that a test does not discriminate between patients with and those without the target condition [15]. However, we reported the area under the hierarchical summary ROC (HSROC) curve (AUHSROC), where the HSROC curve was fitted using the HSROC model [16], as the primary measure of diagnostic accuracy because of the expected threshold effect [13]. The pooled sensitivity and specificity cannot be reasonably suggested as main measures of diagnostic accuracy because they represent estimates of a certain notional unspecified average of different thresholds that cannot be clinically interpreted [13]. Consequently, we estimated the optimal threshold value of ΔScvO2 by calculating the mean and median values, while observing the distribution, dispersion, and central tendency of the cut-off values. Statistical analyses were performed using Stata/SE 15.0 software with the MIDAS and METANDI modules (Stata-Corp, College Station, TX, USA). A two-tailed P < 0.05 indicated statistical significance.
We assessed the between-study heterogeneity by using Cochran’s Q test and I2 statistics. The threshold effect was evaluated statistically by calculating the Spearman correlation coefficient between the logit of sensitivity and the logit of 1-specificity [17]. Meanwhile, a Bayesian nomogram was constructed to calculate the posttest probability to facilitate the interpretation of results. To confirm the stability of the present study, we conducted a sensitivity analysis by excluding the studies introducing a high risk of bias. If more than 10 studies were included, we would assess the publication bias by using Deeks’ funnel plot asymmetry test [18]. Additionally, we would conduct subgroup analyses according to the baseline lactate and ScvO2 level if sufficient studies were identified because the two variables may reflect what stage is the VO2/DO2 dependency at [19, 20].
Results
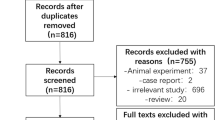
We identified a total of 1381 records from electronic database searching and manually selected additional 30 records from other publications. After deduplication and precluding irrelevant records, we carefully reviewed the full text of 14 candidate studies. Finally, five studies [3,4,5, 21, 22] met the eligibility criteria and were included in the quantitative analysis. Figure 1 depicts the PRISMA flowchart of study selection.
Baseline characteristics
All included studies were prospective designs and published in the past decade, with a sample size ranging from 30 to 88. Among the included studies, 4 [3, 4, 21, 22] were conducted in the intensive care unit (ICU) setting and 1 [5] was performed in the emergency department. In 3 studies [5, 21, 22], all subjects (100%) were mechanically ventilated at the time of fluid challenge; in the remaining 2 studies [3, 4], most subjects (more than 70%) received mechanical ventilation during the study period. Of note, the definitions of fluid responsiveness and fluid challenge varied across the included studies. CO was measured using the thermodilution method [3,4,5, 22] or echocardiography [21]. Table 1 records the baseline characteristics of each included study in detail.
Quality assessment
In the domains of patient selection and index test, all included studies were judged as unclear risk of bias due to lacking sufficient information to support these judgments. However, the reference standard in two studies [4, 22] might introduce bias because they used a cardiac index increase of ≥ 10% to define fluid responsiveness; this threshold, lower than the general threshold [1], might potentially increase the proportion of fluid responder. Furthermore, the study by Nassar et al. [22] had a high concern regarding applicability for reference standard because the duration of the fluid challenge was over 15 min. Table 2 lists the detailed presentation of methodological quality assessment.
Primary analysis
The 5 included studies enrolled a total of 240 participants, of whom 133 (55%) were fluid responders. All included studies reported the cutoff value of ΔScvO2, which ranged from 3.5 to 5%. No between-study heterogeneity was observed, with a Cochran Q statistic of 0.315 (P = 0.420) and an overall I2 of 0%. The pooled results suggested that the ΔScvO2 during fluid challenge exhibited excellent performance for defining fluid responsiveness, with an AUHSROC of 0.86 [95% confidence interval (CI) 0.83–0.89] (Fig. 2). We also found no significant heterogeneity in sensitivity and specificity (Fig. 3), and the small difference between the 95% confidence and prediction regions consistently indicated no substantial heterogeneity between the studies (Fig. 2). The summarized diagnostic accuracies indicated a pooled sensitivity of 0.78 (95% CI 0.69–0.85), a pooled specificity of 0.84 (95% CI 0.72–0.91), and a pooled DOR of 17.7 (95% CI 5.9–53.2). The scatter plot of the cutoff values of ΔScvO2 presented a nearly conically symmetrical distribution (Fig. 4), and the mean and median cutoff values were 4% (95% CI 3–5%) and 4% (95% CI not estimable), respectively. Thus, the range of 3–5% may represent the optimal CI of ΔScvO2 for the evaluation of fluid responsiveness. Accordingly, as shown in the Bayes nomogram (Fig. 5), if an average-risk population has an assumed pretest probability of fluid responder of 50% (as estimated by this meta-analysis), the probability of fluid responder will increase to 83% when the ΔScvO2 is greater than 5% and decrease to 21% when the ΔScvO2 is less than 3%.
HSROC curve of the ΔScvO2 for defining fluid responsiveness. The area under the hierarchical summary receiver operating curve was 0.86 (95% CI 0.83–0.89). The size of the circles indicates the weight of each individual study. The summary point (maroon solid square) represents the average sensitivity and specificity estimates from the study results, and its corresponding 95% confidence region (orange dashed line) is illustrated. The 95% prediction region represents the confidence region for a forecast of true sensitivity and specificity in a future study. HSROC hierarchical summary receiver operating characteristic; ΔScvO2 variation in central venous oxygen saturation during the fluid challenge
As two studies [4, 22] used a cardiac index increase of ≥ 10% to define fluid responsiveness, which introduced a high risk of bias in the domain of reference standard (Table 2), we excluded the two studies to conduct a sensitivity analysis. The sensitivity analysis indicated that the AUHSROC (0.89, 95% CI 0.86–0.92), the pooled sensitivity (0.81, 95% CI 0.72–0.88), and the pooled specificity (0.84, 95% CI 0.72–0.91) (Additional file 1: Fig. S1) were comparable to the primary analysis, confirming the robustness of our results. Given the limited included studies, we did not assess the publication bias, and we also abandoned the scheduled plan of conducting stratified analysis on the baseline lactate level or ScvO2 level.
Discussion
This systematic meta-analysis of 5 observational studies evaluated the diagnostic accuracy of the ΔScvO2 during the fluid challenge for fluid responsiveness in mechanically ventilated patients receiving volume expansion. The principal findings indicated that the ΔScvO2 during the fluid challenge is a reliable indicator of fluid responsiveness, and the range of 3–5% may represent its optimal CI for evaluating fluid responsiveness.
To the best of our knowledge, this is the first systematic meta-analysis to explore the diagnostic accuracy of ΔScvO2 in defining fluid responsiveness. As a potential marker of fluid responsiveness, ΔScvO2 has several advantages over conventional variables. First, tracking the changes in ScvO2 during a fluid challenge can not only assess the response of CO to a fluid bolus but also evaluate the metabolic efficacy of fluid administration. Furthermore, unlike other dynamic variables associated with heart–lung interactions, ΔScvO2 would not, in theory, be affected by arrhythmia, tidal volume, or lung compliance [2, 23]. Therefore, this meta-analysis provides an important clinical implication that ΔScvO2 has the potential to become a surrogate marker of fluid responsiveness when CO measurement is unavailable or not applicable. However, before the wide application of ΔScvO2 in clinical practice, some prerequisites should be recognized. According to the simplified formula [24], SvO2 is estimated as arterial oxygen saturation (SaO2)–VO2/(1.34 × hemoglobin × CO), where SvO2 can be substituted by ScvO2 given the good correlation between the two variables [8]. Thus, the changes in ScvO2 after volume expansion can theoretically track the fluid-induced changes in CO as long as SaO2, VO2, and hemoglobin keep unchanged during the fluid challenge. However, the assumption that hemoglobin keeps unchanged during the fluid challenge is not always true. Administering large volumes of fluids within a short period will inevitably result in a decreased hemoglobin concentration. Previous studies have demonstrated that the hemoglobin concentration was decreased by 6–9% after a 500 mL of fluid challenge [4, 19], which was further confirmed by a recent meta-analysis [25]. Accordingly, the potential reduction in hemoglobin concentration might contribute to a decreased ScvO2 after volume expansion (i.e., a lower ΔScvO2). The negative effect of the reduced hemoglobin concentration might result in an underestimation, not overestimation at least, of the diagnostic performance of ΔScvO2 for fluid responsiveness.
Likewise, VO2 is not always constant during volume expansion. Numerous studies found the VO2/DO2 dependency phenomenon in septic patients when DO2 was increased through administrating fluid [26,27,28]. Theoretically, at the VO2/DO2 dependence stage, VO2 and DO2 would change linearly, and ScvO2 would be unchanged despite the fluid-induced increase in DO2 because of the relatively constant oxygen extraction, and thus might not reflect the fluid-induced changes in CO [29]. In other words, ΔScvO2 will not be a reliable marker of fluid responsiveness if oxygen metabolism is in the VO2/DO2 dependency state. However, the above-mentioned theoretical conjecture does not imply the invalidity of ΔScvO2 to evaluate the cardiac response to a fluid bolus, because the pathologic DO2/VO2 dependency phenomenon might be not always common in critically ill patients. For example, in the study by Xu et al. [4], only 2 of 18 included septic shock patients who were fluid responsive exhibited a significant increase in VO2 immediately after fluid challenge; other studies [19, 20, 30] recorded a proportion of approximately 50% of septic patients in whom a fluid-induced DO2 increase did not lead to a significant increase in VO2. In this case, selecting the appropriate cohort to utilize ΔScvO2 to define fluid responsiveness would be an interesting issue. Since DO2 decreases below the critical value and cannot meet oxygen demand, oxygen debt will be produced at the VO2/DO2 dependency stage, resulting in an elevated lactate level [19, 29]. In this consideration, we speculate that lactate might help to discriminate against those patients who can use ΔScvO2 to define fluid responsiveness and those who cannot. We initially planned to perform subgroup analyses based on the baseline lactate level. However, the scheduled plan of conducting stratified analysis was abandoned due to the limited included studies. Hence, this hypothesis should be verified in future research.
This meta-analysis presents a major methodological strength that the 95% CI of the optimal cutoff value was estimated to avoid the binary constraint of a “black-or-white” decision of the ROC curve and fit the reality of clinical or screening practice. In a realistic clinical decision-making scenario, a single threshold seemly cannot meet the demand of defining fluid responsiveness. For instance, if the measured ΔScvO2 is slightly higher or lower than the cutoff value, it is difficult to judge whether the patient will benefit from volume expansion. Hence, we estimated the optimal cutoff value as well as its corresponding CI to overcome this limitation. According to the estimated CI and the Bayes nomogram, we established a feasible decision-making algorithm: (1) if the measured ΔScvO2 is greater than 5%, the patient is expected to have a probability of 83% to be a fluid responder; (2) the probability of being fluid responder will decrease to 21% if the measured ΔScvO2 is less than 3%; and (3) if the measured ΔScvO2 is between 3 and 5%, the fluid responsiveness is difficult to determine; in this case, other methods are suggested to assess fluid responsiveness.
Several limitations in our study should be acknowledged. First, the limited included studies and the relatively small sample sizes (240 patients) represent the primary limitation, resulting in a decreased statistical power that hampered us from drawing a firm conclusion. Furthermore, studies with a small sample size may overestimate the effect sizes. Additionally, several subgroup analyses were not performed due to the limited included studies. For instance, the baseline lactate level and ScvO2 level may be associated with the diagnostic performance of ΔScvO2; the fluid type (synthetic colloids, albumin, or crystalloids) might cause a potential bias in the results because of the different volume expansion power. Second, all of the included studies had low methodological quality. Of note, two studies [4, 22] might introduce an important bias in our results because they applied a lower threshold value (10%) to define fluid responsiveness, which might increase the proportion of fluid responders. However, after excluding the two studies [4, 22], the sensitivity analysis confirmed the robustness of our findings. Nevertheless, these methodological shortcomings might intrinsically lead to a potential bias in our results and thereby restrict the validity and applicability of our findings. Third, the range of optimal CI (3–5%) is close to the measurement error of ScvO2 using a point-of-care blood gas analyzer [31], which could degrade the credibility of our findings to some extent. Thus, it necessitates a standardization of blood gas analysis in clinical practice to reduce measurement error. Lastly, the current findings are only applicable to critically ill patients under mechanical ventilation. It is unclear whether ΔScvO2 can evaluate fluid responsiveness in patients with spontaneous breathing. Theoretically, ΔScvO2 can also reliably evaluate fluid responsiveness in patients with spontaneous breathing as long as they stay quiet during the fluid challenge so that VO2 will not be affected by other factors (such as emotion or activity).
Conclusion
In mechanically ventilated patients receiving volume expansion, the measurement of ΔScvO2 during the fluid challenge is a useful and reliable approach to evaluate fluid responsiveness. The range of 3–5% may represent the optimal confidence interval of ΔScvO2 to define fluid responsiveness. Given the low methodological quality of the included studies, larger studies with high methodological quality are warranted to validate the applicability of ΔScvO2 in evaluating fluid responsiveness.
Availability of data and materials
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
Abbreviations
- CO:
-
Cardiac output
- DO2 :
-
Oxygen delivery
- VO2 :
-
Oxygen consumption
- ScvO2 :
-
Central venous oxygen saturation
- ΔScvO2 :
-
Variation in ScvO2
- SvO2 :
-
Mixed venous oxygen saturation
- ROC:
-
Receiver operating characteristic
- AUROC:
-
Area under the ROC curve
- HSROC:
-
Hierarchical summary ROC
- AUHSROC:
-
Area under the HSROC curve
- ICU:
-
Intensive care unit
- DOR:
-
Diagnostic odds ratio
- CI:
-
Confidence interval
- SaO2 :
-
Arterial oxygen saturation
References
Messina A, Calabrò L, Pugliese L, Lulja A, Sopuch A, Rosalba D, et al. Fluid challenge in critically ill patients receiving haemodynamic monitoring: a systematic review and comparison of two decades. Crit Care. 2022;26(1):186.
Monnet X, Malbrain MLNG, Pinsky MR. The prediction of fluid responsiveness. Intensive Care Med. 2023;49(1):83–6.
Giraud R, Siegenthaler N, Gayet-Ageron A, Combescure C, Romand JA, Bendjelid K. ScvO(2) as a marker to define fluid responsiveness. J Trauma. 2011;70(4):802–7.
Xu B, Yang X, Wang C, Jiang W, Weng L, Hu X, et al. Changes of central venous oxygen saturation define fluid responsiveness in patients with septic shock: a prospective observational study. J Crit Care. 2017;38:13–9.
Khalil MH, Sekma A, Zhani W, Zorgati A, Ben Soltane H, Nouira S. GREAT Network. Variation in central venous oxygen saturation to assess volume responsiveness in hemodynamically unstable patients under mechanical ventilation: a prospective cohort study. Crit Care. 2021;25(1):245.
Huang H, Wu C, Shen Q, Fang Y, Xu H. Value of variation of end-tidal carbon dioxide for predicting fluid responsiveness during the passive leg raising test in patients with mechanical ventilation: a systematic review and meta-analysis. Crit Care. 2022;26(1):20.
Walley KR. Use of central venous oxygen saturation to guide therapy. Am J Respir Crit Care Med. 2011;184(5):514–20.
Dueck MH, Klimek M, Appenrodt S, Weigand C, Boerner U. Trends but not individual values of central venous oxygen saturation agree with mixed venous oxygen saturation during varying hemodynamic conditions. Anesthesiology. 2005;103(2):249–57.
Yazigi A, Abou-Zeid H, Madi-Jebara S, Haddad F, Hayek G, Jabbour K. Correlation between central venous oxygen saturation and oxygen delivery changes following fluid therapy. Acta Anaesthesiol Scand. 2008;52(9):1213–7.
Osthaus WA, Huber D, Beck C, Roehler A, Marx G, Hecker H, et al. Correlation of oxygen delivery with central venous oxygen saturation, mean arterial pressure and heart rate in piglets. Paediatr Anaesth. 2006;16(9):944–7.
McInnes MDF, Moher D, Thombs BD, McGrath TA, Bossuyt PM, The PRISMA-DTA, et al. Preferred reporting items for a systematic review and meta-analysis of diagnostic test accuracy studies: the PRISMA-DTA statement. JAMA. 2018;319(4):388–96.
Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155(8):529–36.
Macaskill PGC, Deeks JJ, Harbord RM, Takwoingi Y. Analysing and presenting results. Cochrane handbook for systematic reviews of diagnostic test accuracy version 1.0. London: The Cochrane Collaboration; 2010.
Reitsma JB, Glas AS, Rutjes AW, Scholten RJ, Bossuyt PM, Zwinderman AH. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J Clin Epidemiol. 2005;58(10):982–90.
Lijmer JG, Bossuyt PM, Heisterkamp SH. Exploring sources of heterogeneity in systematic reviews of diagnostic tests. Stat Med. 2002;21(11):1525–37.
Rutter CM, Gatsonis CA. A hierarchical regression approach to meta-analysis of diagnostic test accuracy evaluations. Stat Med. 2001;20(19):2865–84.
Deeks JJ. Systematic reviews in health care: Systematic reviews of evaluations of diagnostic and screening tests. BMJ. 2001;323(7305):157–62.
Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol. 2005;58(9):882–93.
Monnet X, Julien F, Ait-Hamou N, Lequoy M, Gosset C, Jozwiak M, et al. Lactate and venoarterial carbon dioxide difference/arterial-venous oxygen difference ratio, but not central venous oxygen saturation, predict increase in oxygen consumption in fluid responders. Crit Care Med. 2013;41(6):1412–20.
Mallat J, Lemyze M, Meddour M, Pepy F, Gasan G, Barrailler S, et al. Ratios of central venous-to-arterial carbon dioxide content or tension to arteriovenous oxygen content are better markers of global anaerobic metabolism than lactate in septic shock patients. Ann Intensive Care. 2016;6(1):10.
Giraud R, Vujovic B, Assouline B, Neto Silva I, Bendjelid K. Do ScvO2 variations induced by passive leg raising predict fluid responsiveness? A prospective study. Physiol Rep. 2021;9(17):e15012.
Nassar B, Badr M, Van Grunderbeeck N, Temime J, Pepy F, Gasan G, et al. Central venous-to-arterial PCO2 difference as a marker to identify fluid responsiveness in septic shock. Sci Rep. 2021;11(1):17256.
De Backer D, Aissaoui N, Cecconi M, Chew MS, Denault A, Hajjar L, et al. How can assessing hemodynamics help to assess volume status? Intensive Care Med. 2022;48(10):1482–94.
Squara P. Central venous oxygenation: when physiology explains apparent discrepancies. Crit Care. 2014;18(6):579.
Quispe-Cornejo AA, Alves da Cunha AL, Njimi H, Mongkolpun W, Valle-Martins AL, Arébalo-López M, et al. Effects of rapid fluid infusion on hemoglobin concentration: a systematic review and meta-analysis. Crit Care. 2022;26(1):324.
Haupt MT, Gilbert EM, Carlson RW. Fluid loading increases oxygen consumption in septic patients with lactic acidosis. Am Rev Respir Dis. 1985;131(6):912–6.
Gilbert EM, Haupt MT, Mandanas RY, Huaringa AJ, Carlson RW. The effect of fluid loading, blood transfusion, and catecholamine infusion on oxygen delivery and consumption in patients with sepsis. Am Rev Respir Dis. 1986;134(5):873–8.
Vincent JL, De Backer D. My paper 20 years later: effects of dobutamine on the VO2/DO2 relationship. Intensive Care Med. 2014;40(11):1643–8.
Bendjelid K. Cardiac output-ScvO2 relationship during sepsis: a subtle association. J Crit Care. 2017;38:351–2.
Abou-Arab O, Braik R, Huette P, Bouhemad B, Lorne E, Guinot PG. The ratios of central venous to arterial carbon dioxide content and tension to arteriovenous oxygen content are not associated with overall anaerobic metabolism in postoperative cardiac surgery patients. PLoS ONE. 2018;13(10):e0205950.
Mallat J, Lazkani A, Lemyze M, Pepy F, Meddour M, Gasan G, et al. Repeatability of blood gas parameters, PCO2 gap, and PCO2 gap to arterial-to-venous oxygen content difference in critically ill adult patients. Medicine. 2015;94(3):e415.
Acknowledgements
Not applicable.
Funding
This work was supported by the grants from Zhejiang Medicine and Health Science and Technology Project (No. 2023KY1084) and the Project of NINGBO Leading Medical & Health Discipline (No. 2022-F16). The funders had no role in the study design, data collection, and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
JP performed the literature search, interpreted the findings, and drafted the manuscript. YS performed the literature search, assessed the risk of bias, and interpreted the findings. ZX performed the study selection and data extraction and conducted the statistical analysis. PD designed the study, performed the study selection and data extraction, and revised the manuscript. XZ designed the study, helped to perform the study selection, assessed the risk of bias, participated in data interpretation, and revised the manuscript. All the authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Detailed searching strategies in each database. Table S2. Detailed diagnostic accuracy of ScvO2 variation for evaluating fluid responsiveness. Figure S1. Sensitivity analysis to assess the robustness of ScvO2 variation for defining fluid responsiveness by excluding studies introducing a high risk of bias
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Pan, J., Sun, Y., Xu, Z. et al. Variation in central venous oxygen saturation to evaluate fluid responsiveness: a systematic review and meta-analysis. Crit Care 27, 203 (2023). https://doi.org/10.1186/s13054-023-04480-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13054-023-04480-z