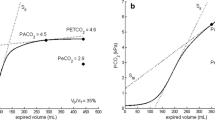
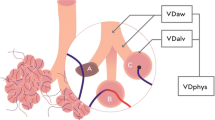
Abstract
Background
The physiological dead space is a strong indicator of severity and outcome of acute respiratory distress syndrome (ARDS). The “ideal” alveolar PCO2, in equilibrium with pulmonary capillary PCO2, is a central concept in the physiological dead space measurement. As it cannot be measured, it is surrogated by arterial PCO2 which, unfortunately, may be far higher than ideal alveolar PCO2, when the right-to-left venous admixture is present. The “ideal” alveolar PCO2 equals the end-tidal PCO2 (PETCO2) only in absence of alveolar dead space. Therefore, in the perfect gas exchanger (alveolar dead space = 0, venous admixture = 0), the PETCO2/PaCO2 is 1, as PETCO2, PACO2 and PaCO2 are equal. Our aim is to investigate if and at which extent the PETCO2/PaCO2, a comprehensive meter of the “gas exchanger” performance, is related to the anatomo physiological characteristics in ARDS.
Results
We retrospectively studied 200 patients with ARDS. The source was a database in which we collected since 2003 all the patients enrolled in different CT scan studies. The PETCO2/PaCO2, measured at 5 cmH2O airway pressure, significantly decreased from mild to mild–moderate moderate–severe and severe ARDS. The overall populations was divided into four groups (~ 50 patients each) according to the quartiles of the PETCO2/PaCO2 (lowest ratio, the worst = group 1, highest ratio, the best = group 4). The progressive increase PETCO2/PaCO2 from quartile 1 to 4 (i.e., the progressive approach to the “perfect” gas exchanger value of 1.0) was associated with a significant decrease of non-aerated tissue, inohomogeneity index and increase of well-aerated tissue. The respiratory system elastance significantly improved from quartile 1 to 4, as well as the PaO2/FiO2 and PaCO2. The improvement of PETCO2/PaCO2 was also associated with a significant decrease of physiological dead space and venous admixture. When PEEP was increased from 5 to 15 cmH2O, the greatest improvement of non-aerated tissue, PaO2 and venous admixture were observed in quartile 1 of PETCO2/PaCO2 and the worst deterioration of dead space in quartile 4.
Conclusion
The ratio PETCO2/PaCO2 is highly correlated with CT scan, physiological and clinical variables. It appears as an excellent measure of the overall “gas exchanger” status.
Similar content being viewed by others
Introduction
The physiological dead space, which includes both the anatomical and alveolar dead space, is a strong indicator of severity and outcome of acute respiratory distress syndrome (ARDS) [1, 2]. The computation of the physiological dead space is based on the dilution of the ideal alveolar PCO2 (PACO2). This ideal PCO2, introduced by Riley, cannot be measured directly and it is assumed to be equal to the capillary PCO2 (PcCO2) [3] which leaves the ventilated/perfused pulmonary units [4]. As the PcCO2 cannot be measured directly, the arterial PCO2 (PaCO2) is assumed to be its surrogate. Therefore, the assumption on which the physiological dead space is computed is that PACO2, PcCO2 and PaCO2 have identical values. While this is nearly correct in the normal lung, in the diseased lung, as in ARDS, the PaCO2 is higher than PcCO2 and PACO2 due to the presence of venous admixture (in Riley’s model, the fraction of blood which flows through “non-aerated lung regions” maintaining the same PO2 and PCO2 of the mixed venous blood). Consequently, PaCO2 is the result of the weighted average of blood coming from the ideal compartment (PcCO2) and of mixed venous PCO2 (PvCO2) [5]. , It is therefore easy to understand why the venous admixture, i.e., a variable which measures the oxygenation impairment, has an effect on variables which describe the wasted ventilation. To compute the alveolar dead space, we may assume that the end-tidal CO2 (PETCO2) is representative of the actual alveolar gases. In this case, the PETCO2 is lower than the PACO2 depending on the amount of alveolar dead space.
The measurement of the PETCO2 is easily performed in intensive care. Therefore, the alveolar dead space may be derived as follows:
As the alveolar dead space, as measured by the equation above, depends both on the “true” alveolar dead space and on the extent of the venous admixture, the PETCO2/PaCO2 ratio may be seen as a direct overall meter of the gas exchanger performance in a scale from 0 to 1. Indeed, a PETCO2/PaCO2 ratio equal to 1 represents the perfect gas exchanger, being, in this condition, the alveolar dead space and the venous admixture equal to 0. The presence of alveolar dead space and/or venous admixture at different extent would progressively decrease this ratio from the unity, reflecting the progressive deterioration of the gas exchanger in its two components, oxygenation and CO2 removal.
The aim of this study is to investigate whether the PETCO2/PaCO2, easily measurable at the bedside, can be an adequate tool to assess the physio-anatomical condition of the gas exchanger.
Materials and methods
Study population
This study population consisted of 200 patients, studied from 2003 and 2016 in two university hospitals (Policlinico Milano, Milan, Italy and University Medical Center Göttingen, Göttingen, Germany). All patients suffered from ARDS according to the Berlin criteria [6]. The ethics committee was notified and permission to use the data was granted (Göttingen Antragsnummer 14/12/12).
Recorded variables
For each patient, the CT scans were acquired at 5, 15 and 45 cmH2O of airway pressure. We reported the anatomical variables derived from the CT quantitative analysis: namely, hyperinflated (− 1000/− 900 HU), well aerated (− 900/− 500 HU), poorly inflated (− 500/− 200 HU) and non-aerated (− 100/ + 100 HU) tissues [7, 8]. Recruitability was computed as the fraction of non-aerated tissue at 5 cmH2O minus the fraction of non-aerated tissue at 45 cmH2O [9]. Lung inhomogeneity was computed on a voxel-by-voxel basis, as the ratio of gas content between acinar size lung units and surrounding lung units [10]. A ratio equal to 1 would indicate perfect homogeneity, a ratio of 2 would indicate an inflation of the central lung unit double than the surrounding units. At 5 and 15 cmH2O of airway pressure, we collected the mechanical ventilation settings and respiratory mechanics variables (tidal volume, respiratory rate, alveolar ventilation, and respiratory system elastance), hemodynamics (systolic and diastolic arterial blood pressures, central venous pressure, heart rate, ScvO2 and arteriovenous O2 content difference) and gas exchange variables (PETCO2, PaO2, PaCO2, PaO2/PaCO2 ratio, SaO2, venous admixture (Qs/Qt), physiological dead space fraction (Vd/Vt)). Tidal volume and FiO2 were kept constant at these two PEEP levels. The volumetric capnography measurements were performed with COSMO (Respironics Novametrix, Wallingford, USA).
The first analysis was done grouping the patients according to the their ARDS severity (mild, moderate–mild, moderate–severe, severe) [11]. An additional analysis was performed dividing the patients into four groups (~ 50 patients per group) based on the equal-count quartiles of their PETCO2/PaCO2 ratios determined during ventilation at 5 cmH2O of PEEP. For details regarding the calculated variables, please refer to Additional File 1.
Statistical methods
The normal distribution of the data was assessed by the Shapiro–Wilk test. Physiological, CT scan variables and PETCO2/PaCO2 ratio were compared among groups with one-way analysis of variance or Kruskal–Wallis test as appropriate. Multiple comparisons were performed with Bonferroni correction. Two tailed, p values < 0.05 were considered statistically significant. These statistical analyses were performed with R (R Foundation for Statistical Computing version 3.7).
Results
The main anthropometric and the physiological characteristics of the study population obtained at 5 cmH2O of PEEP are presented in Table 1. Figure 1, panel A shows the PETCO2/PaCO2 ratio as a function of ARDS severity. The ratio decreased linearly with increasing severity. In Fig. 2, we report the mortality rate observed in the quartiles of PETCO2/PaCO2 ratio.
Table 2 gives the quantitative CT scan variables obtained at 5 cmH2O PEEP stratified as quartiles of PETCO2/PaCO2. As shown, the well-aerated tissue increased with the PETCO2/PaCO2 ratio. The poorly inflated, non-aerated tissue, the inhomogeneity index [10] and recruitability all significantly decrease throughout the PETCO2/PaCO2 quartiles. As shown in Table 3, the PETCO2/PaCO2 ratio was strongly associated with respiratory system elastance, alveolar ventilation and VCO2. These variables improved when the PETCO2/PaCO2 ratio approached unity. The gas exchange variables under the same conditions are presented in Table 4. As shown, the PaCO2 progressively decreased with the concurrent increase of PETCO2/PaCO2 ratio, while the PaO2/FiO2 ratio and saturation increased. Both venous admixture and dead space significantly decreased throughout the PETCO2/PaCO2 quartiles.
PETCO2/PaCO2 ratio at different airway pressures
Figure 3 illustrates how lung tissue aeration changed in the different PETCO2/PaCO2 ratio quartiles when airway pressure was increased from 5 to 15 and 45 cmH2O. The amount of non-aerated tissue decreased steadily from 5 to 45 cmH2O in all quartiles, while the amount of normally aerated tissue increased.
Portions of lung tissue classified as not aerated, poorly aerated, normally aerated and hyperinflated among PETCO2/PaCO2 ratio quartiles to the response of step increase of PEEP at 5, 15 and 45 cmH2O. The asterisk denotes p < 0.001 among the portions of tissue throughout PETCO2/PaCO2 ratio quartiles for Mann–Whitney test. The dollar denotes p < 0.001 among portions of lung tissue to the response to PEEP for Wilcoxon test
In Table 5, we report the changes of CT scan, respiratory mechanics and gas exchange variables through the quartiles of PETCO2/PaCO2 when PEEP was increased from 5 to 15 cmH2O. As shown, the greatest improvement of non-aerated tissue, PaO2 and venous admixture were observed in quartile 1 of PETCO2/PaCO2 and the worst deterioration of dead space in quartile 4.
Discussion
In this study, we found that the PETCO2/PaCO2 ratio is strongly associated with most of the morphological and physiological characteristics of ARDS, resulting as an easy and appealing measure of the status and the performance of the lung.
The physiological meaning of the PETCO2/PaCO2 ratio may be easily understood when one considers CO2 kinetics through the anatomical space from the pulmonary capillaries to the airway opening. Figure 4 shows that, in the ideal lung, PaCO2 is equal to PcCO2 (venous admixture fraction = 0). Similarly, PETCO2 is equal to PACO2 (alveolar dead space fraction = 0). Therefore, in this “ideal” setting the ratio of PETCO2 to PaCO2 would be 1. This ratio will depart progressively from 1 in the presence of a venous admixture and/or alveolar dead space. Consequently, the PETCO2/PaCO2 ratio is a rather unspecific variable, as it is linked to both CO2 and O2 exchange impairment, but for the same reason it may give an immediate warning of an overall impairment of gas exchange. The potential role of monitoring the PETCO2/PaCO2 ratio in order to follow and understand the disease course is emphasized by its close association with the overall severity of ARDS and the mortality.
The PvCO2 decreases to PcCO2 (the capillary PCO2 pressure) after releasing CO2 in the alveolar space. The partial pressure of CO2 in the alveoli (PACO2) which are perfused is equal to PcCO2. This pressure represents the “ideal” alveolar gas, as it derives from pulmonary units which are both perfused and ventilated. This model ignores other factors possibly affecting the CO2 dynamics, such as the CO2 production from the inflammatory cells residing in the inflamed lung. Actually, this ideal PACO2 may be “diluted” by the gas coming from unperfused but ventilated alveoli (alveolar dead space). The PACO2 then becomes PETCO2. In turn, the PETCO2 may be diluted into the gas ventilating the airways and the apparatus (anatomical dead space) becoming PETCO2. It must be realized that the number of molecules of CO2 involved are exactly the same, but their concentration (and partial pressure) depends on the progressive dilution with alveolar gases and “anatomical” gases. In normal individual, PACO2 and PETCO2 differ only of 1–2 mmHg, being the alveolar dead space negligible. The PvCO2 may also reach the arterial circulation without any modification in presence of venous admixture. In this case, PaCO2 is the result of mixing between PcCO2 and PvCO2. Greater is the venous admixture, greater is the difference between PaCO2 (higher) and PcCO2. In the perfect gas exchanger, PETCO2 equals the PACO2 (no alveolar dead space) and PACO2 equals PaCO2 (no venous admixture). Therefore PETCO2/PaCO2 equals 1
This is not really surprising, as almost all variables characterizing the ARDS are related to the PETCO2/PaCO2 ratio. With regard to the morphological variables, the PETCO2/PaCO2 ratio is related to the extent of non-aerated and aerated tissue, the size of the baby lung as well as to the extent of recruitability. No other gas exchange variable exhibits such a large number of correlations with lung morphology. Indeed, venous admixture and physiological dead space were only related to the non-aerated tissue and to the normally aerated tissues, respectively. Therefore, the relative nonspecificity of the PETCO2/PaCO2 ratio, which reflects the overall gas exchange (both for oxygen and carbon dioxide), explains its correlation with all the morphological components of the lungs, of which some are more related to oxygen while others are more related to CO2 exchange. On the other hand, the linkage between the PETCO2/PaCO2 ratio, alveolar dead space and venous admixture accounts for its high sensitivity in detecting an overall impairment in gas exchange. The PETCO2/PaCO2 ratio was inversely associated with respiratory system elastance and directly correlated with alveolar ventilation, while no relationship was found with hemodynamic variables. The low specificity but high sensitivity of the PETCO2/PaCO2 ratio to reflect an overall impairment of gas exchange is strikingly shown by the correlation we found with the gas exchange variables. Indeed, as shown in Table 4, all measured or computed variables related either with oxygenation or carbon dioxide clearance were strongly associated with the PETCO2/PaCO2 ratio. Therefore, an altered PETCO2/PaCO2 ratio, as such, is associated both with the morphology and, the function of gas exchange, suggesting it as a sensitive, easily available marker, of changes in lung conditions. Interestingly, we found that the VCO2 significantly increased throughout the quartiles. We believe that this is due to an improved alveolar ventilation, with greater elimination of carbon dioxide. Indeed, the low VCO2 measured in quartile 1 possibly represents only a fraction of the metabolic CO2 production which is partly retained. Increased alveolar ventilation throughout the quartiles leads to a normalization or even a higher than normal (metabolic) CO2 clearance [12].
The PETCO2/PaCO2 ratio may be also considered to anticipate the PEEP response. The PEEP response in gas exchange is a balance between the decrease of venous admixture and increase in alveolar dead space. The venous admixture decrease could either be due to recruitment, better mechanical conditions of pulmonary units already open, or to a decrease in cardiac output, while the alveolar dead space increase may be due to an overdistention of pulmonary units relative to their perfusion. An increase in alveolar dead space would tend to reduce PETCO2, while a decrease of right-to-left venous admixture would tend to reduce PaCO2. The PETCO2/PaCO2 ratio is related to the two variables, and its changes may reflect these physiopathological mechanisms. Moreover, we showed that patients starting with a lower PETCO2/PaCO2 ratio had a more favorable response to PEEP.
The PETCO2/PaCO2 ratio is obviously related to the physiological dead space, although it may be considered a “positive variable” (greater the ratio, better the gas exchange) rather than “negative” (greater the dead space, worse the gas exchange). Indeed, any change of the PETCO2/PaCO2 ratio toward the value of 1 indicates an improvement of the whole gas exchanger condition, which may then be easily monitored after any maneuvre on the respiratory system, change of ventilator setting and pharmacological intervention. Indeed, the daily monitoring of this easy to use variable may show a progressive increase towards the unity, giving some evidence that the lung conditions are improving. In contrast, any change of this ratio towards lower values immediately indicates an overall decrease of the gas exchange performances. The PETCO2/PaCO2 ratio also helps the clinician to be more aware of the dynamics of CO2. Too often oxygenation represents the concern at the bedside of an ARDS patient, but it is only considering also PaCO2 that one can have a more complete picture of the lung structure and function [13]. Finally, the PETCO2/PaCO2 ratio finds in its strength as an overall meter of gas exchange impairment also its weakness. Indeed, to fully understand the various components of the of the gas exchange alteration, both dead space and venous admixture must be be measured.
Conclusions
In this study, we evaluated the PETCO2/PaCO2 ratio as a clinical tool to comprehensively evaluate the gas exchange lung function. The ratio was associated with most of the physiological variables that can be measured at the bedside and, therefore, it can represent a useful parameter for the daily monitoring of the ARDS patient.
Availability of data and materials
Dataset available upon reasonable request.
References
Nuckton TJ, Alonso JA, Kallet RH, Daniel BM, Pittet JF, Eisner MD, Matthay MA (2002) Pulmonary dead-space fraction as a risk factor for death in the acute respiratory distress syndrome. N Engl J Med 346:1281–1286
Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS (2012) Acute respiratory distress syndrome: the Berlin Definition. JAMA 307:2526–2533
Enghoff H (1938) Volumen inefficax. Upsala Lakaref Forh 44:191–218
Riley RL, Cournand A (1949) Ideal alveolar air and the analysis of ventilation-perfusion relationships in the lungs. J Appl Physiol 1:825–847
Fletcher R (1989) Relationship between alveolar deadspace and arterial oxygenation in children with congenital cardiac disease. Br J Anaesth 62:168–176
Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS (2012) Acute respiratory distress syndrome: the Berlin Definition. JAMA
Herrmann P, Nguyen XP, Luecke T, Quintel M (2002) MALUNA 1.03 ein Softwaretool zur analyse computertomographischer Schnittbilder del Lunge. Huethig Verlag, Heidelberg
Gattinoni L, Pesenti A, Avalli L, Rossi F, Bombino M (1987) Pressure–volume curve of total respiratory system in acute respiratory failure. Computed tomographic scan study. Am Rev Respir Dis. https://doi.org/10.1164/ajrccm/136.3.730
Gattinoni L, Caironi P, Cressoni M, Chiumello D, Ranieri VM, Quintel M, Russo S, Patroniti N, Cornejo R, Bugedo G (2006) Lung recruitment in patients with the acute respiratory distress syndrome. N Engl J Med 354:1775–1786
Cressoni M, Cadringher P, Chiurazzi C, Amini M, Gallazzi E, Marino A, Brioni M, Carlesso E, Chiumello D, Quintel M, Bugedo G, Gattinoni L (2014) Lung inhomogeneity in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med 189:149–158
Maiolo G, Collino F, Vasques F, Rapetti F, Tonetti T, Romitti F, Cressoni M, Chiumello D, Moerer O, Herrmann P, Friede T, Quintel M, Gattinoni L (2018) Reclassifying acute respiratory distress syndrome. Am J Respir Crit Care Med 197:1586–1595
Giosa L, Busana M, Bonifazi M, Romitti F, Vassalli F, Pasticci I, Macri MM, D’Albo R, Collino F, Gatta A, Palumbo MM, Herrmann P, Moerer O, Iapichino G, Meissner K, Quintel M, Gattinoni L (2020) Mobilizing carbon dioxide stores: an experimental study. Am J Respir Crit Care Med. https://doi.org/10.1164/rccm.202005-1687OC
Gattinoni L, Bombino M, Pelosi P, Lissoni A, Pesenti A, Fumagalli R, Tagliabue M (1994) Lung structure and function in different stages of severe adult respiratory distress syndrome. JAMA. https://doi.org/10.1097/00132586-199412000-00006
Acknowledgements
We would like to thank Sartorius AG, Göttingen, Germany, for an unrestricted grant for lung injury-related research towards the Department of Anesthesiology of Göttingen University Medical Center.
Funding
Open Access funding enabled and organized by Projekt DEAL. Institutional.
Author information
Authors and Affiliations
Contributions
Conception and design: MB, MQ, DC, LG. Data collection: FR, MB, MMP, IS, SG. Analysis and interpretation: KM, MMQ, DC. Drafting the manuscript for important intellectual content: all authors. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The ethics committee was notified and permission to use the data was granted (Göttingen Antragsnummer 14/12/12).
Consent for publication
All authors have approved the manuscript.
Competing interests
The authors have no conflict of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Additional methods and formulas.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bonifazi, M., Romitti, F., Busana, M. et al. End-tidal to arterial PCO2 ratio: a bedside meter of the overall gas exchanger performance. ICMx 9, 21 (2021). https://doi.org/10.1186/s40635-021-00377-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40635-021-00377-9