Abstract
Background
In patients with acute respiratory distress syndrome undergoing mechanical ventilation, positive end-expiratory pressure (PEEP) can lead to recruitment or overdistension. Current strategies utilized for PEEP titration do not permit the distinction. Electric impedance tomography (EIT) detects and quantifies the presence of both collapse and overdistension. We investigated whether using EIT-guided PEEP titration leads to decreased mechanical power compared to high-PEEP/FiO2 tables.
Methods
A single-center, randomized crossover pilot trial comparing EIT-guided PEEP selection versus PEEP selection using the High-PEEP/FiO2 table in patients with moderate–severe acute respiratory distress syndrome. The primary outcome was the change in mechanical power after each PEEP selection strategy. Secondary outcomes included changes in the 4 × driving pressure + respiratory rate (4 ΔP, + RR index) index, driving pressure, plateau pressure, PaO2/FiO2 ratio, and static compliance.
Results
EIT was consistently associated with a decrease in mechanical power compared to PEEP/FiO2 tables (mean difference − 4.36 J/min, 95% CI − 6.7, − 1.95, p = 0.002) and led to lower values in the 4ΔP + RR index (− 11.42 J/min, 95% CI − 19.01, − 3.82, p = 0.007) mainly driven by a decrease in the elastic–dynamic power (− 1.61 J/min, − 2.99, − 0.22, p = 0.027). The elastic–static and resistive powers were unchanged. Similarly, EIT led to a statistically significant change in set PEEP (− 2 cmH2O, p = 0.046), driving pressure, (− 2.92 cmH2O, p = 0.003), peak pressure (− 6.25 cmH2O, p = 0.003), plateau pressure (− 4.53 cmH2O, p = 0.006), and static respiratory system compliance (+ 7.93 ml/cmH2O, p = 0.008).
Conclusions
In patients with moderate–severe acute respiratory distress syndrome, EIT-guided PEEP titration reduces mechanical power mainly through a reduction in elastic–dynamic power.
Trial registration This trial was prospectively registered on Clinicaltrials.gov (NCT 03793842) on January 4th, 2019.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Introduction
In acute respiratory distress syndrome (ARDS) management, positive end-expiratory pressure (PEEP) counteracts gravity-dependent alveolar collapse, decreasing shunt and hypoxemia [1], reduces the shearing forces of cyclic alveolar opening/closing, and increases compliance [2]. Due to the heterogeneity of lung injury in ARDS, the application of PEEP can result in recruitment in some lung areas while causing overdistension in others. Suboptimal PEEP may induce ventilator-induced lung injury (VILI) [1].
Randomized controlled trials (RCTs) comparing high vs. low PEEP strategies have not consistently demonstrated the superiority of either [3]. While a network meta-analysis of 18 RCTs suggested a potential mortality benefit of higher PEEP [4], this cumulative analysis failed to consider the impact of individualized PEEP titration and the adverse effects of high PEEP on non-PEEP responders [5].
Electric impedance tomography (EIT) is a bedside imaging technique that identifies changes in lung impedance, a proxy for lung volume [6]. EIT-guided PEEP titration distinguishes PEEP-induced recruitment from overdistension [7,8,9]. Hse et al. demonstrated increased survival with EIT-guided PEEP titration, albeit with higher use of ECMO in the EIT group [10]. Another RCT failed to demonstrate such benefit [11].
Mechanical power (MP) is a physiological construct of the energy transmitted to the patient during invasive mechanical ventilation (IMV). MP integrates the major components of positive pressure ventilation that drive VILI [12]: elastic–static (related to PEEP), elastic–dynamic (related to driving pressure, [ΔP]), and resistive (related to flow and airway resistance). High MP is associated with ARDS mortality [13]. Given the conflicting data regarding the utility of EIT and the need for feasible surrogate endpoints to guide larger multicenter RCT, we performed a randomized crossover trial to explore the effects of EIT-guided PEEP titration on MP in patients with ARDS. We hypothesized that EIT-guided PEEP titration would result in lower MP, compared to the use of the High-PEEP/FiO2 table.
Materials and methods
Study design and population
In this single-center randomized crossover trial, we compared EIT-guided PEEP selection vs. High-PEEP/FiO2 tables (NCT 03793842). The University of Michigan Institutional Review Board approved this study (HUM00148126). We obtained informed consent from each patient’s legal representative. We included patients ≥ 18 years receiving IMV for ARDS management for < 72 h with a PaO2/FiO2 ratio < 150 and a PEEP > 8 cm/H2O. Exclusion criteria are provided in Additional file 1.
Study protocol
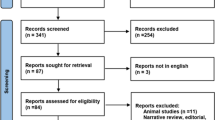
Patients were randomly assigned 1:1 to receive EIT-guided PEEP titration followed by PEEP selection via the High-PEEP/FiO2 table (EIT first) or vice versa (tables first). Randomization was achieved using opaque, sealed envelopes. At randomization, all patients received lung-protective ventilation (LPV). PEEP was selected according to High-PEEP/FiO2 tables. Due to the need to maintain PEEP, a washout period was not feasible. Patients who were proned after randomization were excluded from the analysis due to its effects on PEEP requirement and MP [14].
During the EIT-guided PEEP titration phase, patients underwent a recruitment maneuver. PEEP was then decreased by two cmH2O every 5–10 min until a 10% drop in delta end-expiratory lung impedance in dorsal regions was detected by EIT, five cmH2O PEEP was reached, or hemodynamic instability/hypoxemia developed. PEEP was then set based on the intercept between the lower overdistension and collapse percentages [15].
Patients randomized to the EIT-first group underwent EIT-guided PEEP titration, as above. This was followed by six hours of management per the University of Michigan ARDS protocol (Additional file 1), with PEEP left at the EIT-determined level. Afterward, patients crossed over to a PEEP level set using the High-PEEP/FiO2 tables, which was maintained for 14–18 h. Patients randomized to the tables-first group underwent the same interventions in the reverse order (Fig. 1). After both interventions, FiO2 and respiratory rate (RR) were adjusted for oxygenation > 90% and a pH 7.3–7.45, respectively.
Outcomes
The primary outcome of this study was the change in MP after each PEEP selection strategy. Secondary outcomes included changes in the 4ΔP + RR index (an estimate of ventilator energy transfer to the lung) [13], elastic–static, elastic–dynamic, and resistive powers, as well as ΔP, plateau pressure (Pplat), PaO2/FiO2 ratio, and static compliance (Cstat). We calculated MP with Gattinoni’s simplified formula: 0.098 X RR X TV (Ppeak-[Pplat-PEEP/2]) [12] and analyzed its components.
Statistical analysis
We compared baseline characteristics using Fisher’s test for categorical and a two-sample t test for continuous variables. Changes in ventilator parameters with each intervention were compared using paired t tests. We fit serial linear mixed-effect regression models assessing the association between the interventions and change in MP, adjusting for randomization order and pre-intervention MP in sequential models. We repeated this to determine the association between intervention and the 4ΔP + RR index, MP components, and ΔP. Our small sample size represents a convenience sample similar in scope to other EIT studies. Statistical analyses were performed in StataMP version 17.0 (StataCorp).
Results
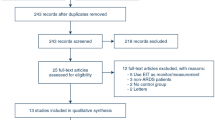
Sixteen patients were enrolled in this study. One patient was withdrawn due to hemodynamic instability; three were proned after randomization and excluded from the analysis (Additional file 2: Fig S1). Baseline characteristics are shown in Table 1. The median baseline MP was 20 J/min (IQR: 19, 28). EIT led to a significant change in PEEP compared to tables (mean difference of change: − 2 cmH2O, 95% CI − 3.95, − 0.05, p = 0.046),
EIT decreased MP compared to PEEP/FiO2 tables (− 4.36 J/min, 95% CI − 6.7, − 1.95, p = 0.002). (Table 2). This difference persisted after adjusting for randomization order and pre-intervention MP. (Additional file 3: Tables S1-2). EIT led to a decrease in the 4ΔP + RR index (− 11.42 J/min, 95% CI − 19.01, − 3.82, p = 0.007) mainly through a decrease in elastic–dynamic power (− 1.61 J/min, 95% CI: − 2.99, − 0.22, p = 0.027), and driving pressure (− 2.92 J/min, 95% CI: − 4.59, − 1.23, p = 0.003) (Table 2 and Fig. 2). These differences persisted after adjusting for randomization order and baseline MP (Additional file 3: Table S3). Elastic–static and resistive powers were unchanged across both interventions.
Changes in mechanical power and its components after each intervention. Changes in PEEP (A and B), MP by the Gattinoni’s Simplified formula (C and D), 4∆PxRR index (E and F), elastic–dynamic power (G and H), elastic–static power (I and J), and resistive power (K and L). Asterisk indicates a statistically significant difference in the change with the EIT versus High-PEEP tables interventions based on p value < 0.05
EIT led to changes in peak pressures (− 6.25 cmH2O, p = 0.003), Pplat (− 4.53 cmH2O, p = 0.006), and Cstat (+ 7.93 ml/cmH2O, p = 0.008) (Additional file 4: Fig S2). There was no significant change in RR or PaO2/FiO2 ratio.
After the EIT phase, one patient developed pneumomediastinum, which did not require additional intervention. Three patients developed hypotension during the RM. In one patient, the protocol was stopped due to persistent hemodynamic instability.
Discussion
In this randomized crossover trial, we found a significant decrease in MP using EIT-guided PEEP titration compared to High-PEEP/FiO2 tables in mechanically ventilated patients with moderate–severe ARDS. This difference persisted after sensitivity analysis and adjustment for randomization order and pre-intervention MP. A reduction in the elastic–dynamic MP mainly drove the decrease in MP.
Zhao and colleagues reported that EIT-guided PEEP titration was associated with improved respiratory mechanics [15]. Similarly, a RCT by Hsu and colleagues reported improvement in ΔP, Cstat, and survival rates with EIT-guided PEEP titration compared to the pressure–volume curves through a decrease in PEEP [11].
He and colleagues compared the effects of EIT-guided PEEP titration vs. a low PEEP/FiO2 table [10] without finding differences in survival, ventilator-free days, or ICU stay. However, this study was limited by using similar PEEP between groups and including mild ARDS. In our study, EIT-guided PEEP titration led to significant changes in PEEP, and we only enrolled patients with moderate–severe ARDS. Using a crossover design allowed us to analyze the effects of each intervention on an individual level by using each patient as their own control. Using the High-PEEP/FiO2 tables as the control intervention permitted comparison with the strategy associated with better ventilation/perfusion matching [16] and outcomes in severe ARDS [4].
In patients with ARDS, persistent elevation of MP > 17 J/min is associated with higher mortality [13, 17]. Although patients in our study received standard LPV per protocol at baseline, MP was elevated (median 20 J/min). EIT-guided PEEP titration led to a mean reduction of MP by 4.36 J/min, a reduction associated with decreased mortality [17, 18], particularly when achieved during the initial hours of IMV [18]. The reduction in MP was achieved through decreased elastic–dynamic power, but not the elastic–static or resistive powers. Although changes in RR were allowed to achieve pH > 7.3, they were not different across groups.
Our findings are consistent with previous observations [13] that propose oscillating mechanical stresses as the main injurious mechanism for VILI. In our study, EIT decreased PEEP levels despite ∆Ps believed to be LPV, suggesting EIT-directed PEEP titration to be a more effective means of optimizing LPV than PEEP/FiO2 tables via a reduction in MP. In addition, observational studies have shown that lung recruitment is not systematically associated with detectable improvements in Cstat nor ∆P, therefore precluding accurate titration of PEEP based exclusively on these parameters [19, 20].
Our study has several limitations; 1) excluding subjects on prone positioning could have introduced post-randomization selection bias. 2) Sample was small, but the effects in MP reduction were significant and occurred despite optimal LPV at baseline. This suggests a strong effect of EIT in reducing MP. 3) We did not include a washout phase. However, our analysis considered the order of interventions to assess for carryover effects. 4) We did not assess for recruitability before the recruitment maneuver. This could have impacted sample’s enrichment. 5) Our intervention focused on titrating PEEP during the initial 24 h. However, PEEP/FiO2 tables are meant to guide continuous changes in PEEP based on FiO2 responses, dead space fraction, and mechanics. This was not assessed. 6) MP was calculated using airway not transpulmonary ΔP rather which could have introduced measurement bias.
Conclusion
This study shows that EIT-guided PEEP titration decreases MP in patients with moderate–severe ARDS compared to a high-PEEP/FiO2 table. A decrease in the dynamic–elastic component primarily drives the reduction in MP. The clinical impact of EIT-guided PEEP titration should be tested in large multicenter trials.
Availability of data and materials
The datasets used and analyzed in this study are available from the corresponding author upon reasonable request.
Abbreviations
- ARDS:
-
Acute respiratory distress syndrome
- Cstat:
-
Static compliance
- EIT:
-
Electric impedance tomography
- FiO2:
-
Fraction of inspired oxygen
- IMV:
-
Invasive mechanical ventilation
- MP:
-
Mechanical power
- PEEP:
-
Positive end-expiratory pressure
- Ppeak:
-
Peak pressure
- Pplat:
-
Plateau pressure
- RCTs:
-
Randomized controlled trials
- RR:
-
Respiratory rate
- TV:
-
Tidal volume
- VILI:
-
Ventilator-induced lung injury
- ∆P:
-
Driving pressure
References
Mauri T. Personalized positive end-expiratory pressure and tidal volume in acute respiratory distress syndrome: bedside physiology-based approach. Crit Care Explor. 2021;3(7):e0486.
Sahetya SK, Mancebo J, Brower RG. Fifty years of research in ARDS. Vt selection in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2017;196(12):1519–25.
Walkey AJ, Del Sorbo L, Hodgson CL, et al. Higher PEEP versus Lower PEEP strategies for patients with acute respiratory distress syndrome. A systematic review and meta-analysis. Ann Am Thorac Soc. 2017;14(S4):S297–303.
Dianti J, Tisminetzky M, Ferreyro BL, et al. Association of positive end-expiratory pressure and lung recruitment selection strategies with mortality in acute respiratory distress syndrome: a systematic review and network meta-analysis. Am J Respir Crit Care Med. 2022;205(11):1300–10.
Goligher EC, Kavanagh BP, Rubenfeld GD, Ferguson ND. Physiologic responsiveness should guide entry into randomized controlled trials. Am J Respir Crit Care Med. 2015;192(12):1416–9.
Jimenez JV, Weirauch AJ, Culter CA, et al. Electrical impedance tomography in acute respiratory distress syndrome management. Crit Care Med. 2022;50(8):1210–23.
Scaramuzzo G, Spinelli E, Spadaro S, et al. Gravitational distribution of regional opening and closing pressures, hysteresis and atelectrauma in ARDS evaluated by electrical impedance tomography. Crit Care. 2020;24:622.
Somhorst P, van Der Zee P, Endeman H, et al. PEEP-FiO2 table versus EIT to titrate PEEP in mechanically ventilated patients with COVID-19-related ARDS. Crit Care. 2022;26(1):272.
Sella N, Zarantonello F, Andreatta G, Gagliardi V, Boscolo A, Navalesi P. Positive end-expiratory pressure titration in COVID-19 acute respiratory failure: electrical impedance tomography vs. PEEP/FiO 2 tables. Crit Care. 2020;24(1):540. https://doi.org/10.1186/s13054-020-03242-5.
Hsu HJ, Chang HT, Zhao Z, et al. Positive end-expiratory pressure titration with electrical impedance tomography and pressure-volume curve: a randomized trial in moderate to severe ARDS. Physiol Meas. 2021;42:014002.
He H, Chi Y, Yang Y, et al. Early individualized positive end-expiratory pressure guided by electrical impedance tomography in acute respiratory distress syndrome: a randomized controlled clinical trial. Crit Care. 2021;25:230.
Giosa L, Busana M, Pasticci I, et al. Mechanical power at a glance: a simple surrogate for volume-controlled ventilation. Intensive Care Med Exp. 2019;7:61.
Costa ELV, Slutsky AS, Brochard LJ, et al. Ventilatory variables and mechanical power in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2021;204:303–11.
Katira BH, Osada K, Engelberts D, et al. Positive end-expiratory pressure, pleural pressure, and regional compliance during pronation: An experimental study. Am J Respir Crit Care Med. 2021;203:1266–74.
Zhao Z, Chang MY, Chang MY, Gow CH, Zhang JH, Hsu YL, Frerichs I, Chang HT, Möller K. Positive end-expiratory pressure titration with electrical impedance tomography and pressure-volume curve in severe acute respiratory distress syndrome. Ann Intensive Care. 2019;9(1):7. https://doi.org/10.1186/s13613-019-0484-0.PMID:30656479;PMCID:PMC6336593.
Pavlovsky B, Pesenti A, Spinelli E, Scaramuzzo G, Marongiu I, Tagliabue P, Spadaro S, Grasselli G, Mercat A, Mauri T. Effects of PEEP on regional ventilation-perfusion mismatch in the acute respiratory distress syndrome. Crit Care. 2022;26(1):211. https://doi.org/10.1186/s13054-022-04085-y.PMID:35818077;PMCID:PMC9272883.
Parhar KKS, Zjadewicz K, Soo A, Sutton A, Zjadewicz M, Doig L, et al. Epidemiology, mechanical power, and 3-year outcomes in acute respiratory distress syndrome patients using standardized screening. An observational cohort study. Ann Am Thorac Soc. 2019;16(10):1263–72.
Chi Y, Zhang Q, Yuan S, Zhao Z, Long Y, He H. Twenty-four-hour mechanical power variation rate is associated with mortality among critically ill patients with acute respiratory failure: a retrospective cohort study. BMC Pulm Med. 2021;21(1):331. https://doi.org/10.1186/s12890-021-01691-4.PMID:34696739;PMCID:PMC8543779.
Grieco DL, Bongiovanni F, Dell’Anna AM, Antonelli M. Why compliance and driving pressure may be inappropriate targets for PEEP setting during ARDS. Crit Care. 2022;26(1):234. https://doi.org/10.1186/s13054-022-04109-7.PMID:35918772;PMCID:PMC9345391.
Protti A, Santini A, Pennati F, Chiurazzi C, Cressoni M, Ferrari M, Iapichino GE, Carenzo L, Lanza E, Picardo G, Caironi P, Aliverti A, Cecconi M. Lung response to a higher positive end-expiratory pressure in mechanically ventilated patients with COVID-19. Chest. 2022;161(4):979–88. https://doi.org/10.1016/j.chest.2021.10.012.
Acknowledgements
Not applicable
Funding
This study was funded by the CHEST foundation.
Author information
Authors and Affiliations
Contributions
JVJ, TS, and RH were involved in the study design and conception. JVJ, EM, AW, KF, and CC were involved in data acquisition. JVJ, EM, and HP performed the statistical analysis. JVJ, EM, HP, and RH analyzed and interpreted the data. JVJ drafted the manuscript. EM, AW, CC, KF, WWL, PC, IV, HP, and RH provided critical manuscript reviews. All authors participated in the final manuscript revision and take responsibility for the data’s integrity and the data analysis’s accuracy.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Institutional Review Board of the University of Michigan approved this study (HUM00148126). We obtained informed consent from each patient’s legal representative.
Consent for publication
Not applicable.
Competing interests
EM was supported by Grant Number T32 HL 007749 (Multidisciplinary Training Program in Lung Disease) from the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. AW reports speaking fees from Drager and author fees from Jones and Bartlett Learning, unrelated to this work. WWL reports personal fees from Konica Minolta and Continuing Education Alliance. PC is a consultant for Breas Medical US and performs Medicolegal Expert witness work. HP reported receiving grants from the US Department of Veterans Affairs outside the submitted work, serving on the Surviving Sepsis Campaign Guidelines Panel, and serving as physician lead for a Michigan statewide sepsis collaborative. RH serves on the advisory board for Merck, Boehringer Ingelheim. Consultant for LungPacer and NOTA-Laboratories has textbook royalties from Springer Website and UpToDate. Grants: CHEST Foundation, NHLBI PETAL Network Medicolegal Expert witness work. Dräger loaned equipment (PulmoVista® 500) to RH for research purposes; no monetary funds were provided.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Extended methods and protocol.
Additional file 2.
Flow diagram of the study.
Additional file 3.
Comparison of ventilator parameters.
Additional file 4.
Changes in respiratory mechanics.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Jimenez, J.V., Munroe, E., Weirauch, A.J. et al. Electric impedance tomography-guided PEEP titration reduces mechanical power in ARDS: a randomized crossover pilot trial. Crit Care 27, 21 (2023). https://doi.org/10.1186/s13054-023-04315-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13054-023-04315-x