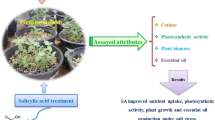
Abstract
Background
Soil salinity is considered as one of the major environmental factors that has reduced plant productivity worldwide. This study investigates the impact of salinity on plant growth attributes, biochemical and physiological leaf characteristics in two cultivars (Adet and Merawi) of Brassica carinata and also explores the role of salicylic acid (SA) in mitigating the effect of salt stress.
Methods
Four-week-old cultivars were treated with NaCl (50, 100 and 150 mM) and SA (0.5 mM) and watered regularly with 100% field capacity. Thus, they were grown under eight different treatments (T1 = no NaCl, no SA; T2 = 0 mM NaCl with 0.5 mM SA; T3 = 50 mM NaCl without SA; T4 = 50 mM NaCl with 0.5 mM SA; T5 = 100 mM NaCl without SA; T6 = 100 mM NaCl with 0.5 mM SA; T7 = 150 mM NaCl without SA; and T8 = 150 mM NaCl with 0.5 mM SA). Nine-week-old cultivars were sampled for analyzing the growth attributes, plant water status, nitrate reductase activity, proline accumulation, photosynthetic traits, lipid peroxidation level and activity of antioxidant enzymes.
Results
Salinity treatments hampered the overall plant growth performance in a dose-dependent manner. Salinity also reduced photosynthetic efficiency by inhibiting chlorophyll synthesis, nitrate reductase activity, chlorophyll fluorescence, stomatal conductance, net photosynthetic and transpiration rates and plant water status. On the other hand, SA application alleviated the adverse effects of salinity and improved the performance of the studied parameters in both the cultivars. Higher dose of salinity increased proline production, but SA application mitigates this impact in both the cultivars studied. The activity of antioxidant enzymes increased under salt stress in a dose-dependent manner. SA treatment to normal or salinity-stressed plants increased the enzymes activity, showing that SA has a crucial role in modulating the cell redox balance and protecting the plants from oxidative damage. SA significantly reduced the salinity-caused effects on the overall performance of plants and their antioxidant systems in both the cultivars. Of the two cultivars, Adet was more tolerant to salinity than Merawi.
Conclusions
Foliar application of SA improved the performance of Ethiopian mustard cultivars and mitigated the damage caused by salt stress.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Background
According to an estimate [1], productivity of food crops needs to be increased by 70% for an extra 2.3 billion individuals up to 2050. Like other abiotic stresses, soil salinity has a remarkable impact on growth, yield and distribution of plants worldwide. Globally, saline soils constitute approximately 10% of the land surface (950 Mha) and 50% of the whole irrigated land (230 Mha) [2], and the influence of salinity is spreading consistently. Maintenance of plant productivity on the saline land will be one of the greatest challenges in the coming years. Salinity stress normally causes reduction in plant growth, which is interceded by an array of physiological, biochemical and molecular changes [2,3,4,5,6]. Salinity hampers the uptake of water, while the consequent high accumulation of Na+ and Cl− together with a decline of K+ leads to a nutrient imbalance [7]. In general, high salinity causes stomatal closure and lowers the level of green pigments and photosynthesis. Further, it increases the generation of reactive oxygen species (ROS), viz. hydrogen peroxide, superoxide, hydroxyl radical and singlet oxygen, which damage the cellular machinery [8,9,10,11,12]. The ROS production leads to chlorophyll deprivation and membrane lipid peroxidation (measured as malondialdehyde content), reducing the membrane fluidity and selectivity [13, 14]. The most affected parameters include the net photosynthetic rate (PN) and water use efficiency (WUE), which ultimately restrict the plant growth rate [3, 15, 16]. The decline in photosynthesis under salinity stress may be due to inhibition of photosystem II complex and a loss of chlorophyll pigments [17]. These alterations are reflected by chlorophyll fluorescence (Fv/Fm), which is conveniently used for detecting and quantifying the plant tolerance to stressful conditions [16, 18,19,20,21].
Plants have exhibited an array of enzymatic and non-enzymatic defense mechanisms to protect cells from oxidative damage [22,23,24,25]. Plant growth regulators such as auxins, brassinosteroid, jasmonates and strigolactones play a vital role in connection with the signaling network and the developmental and adaptive phenomena in plants growing under stress [3, 26, 27]. Salicylic acid (SA), a phenolic growth regulator, fortifies the plant defense against a variety of stresses [28,29,30,31]. Hao et al. [32] reported the SA-induced expression of 59 proteins involved in a variety of cell responses and metabolic processes in Cucumis sativus. SA application stimulated salt tolerance via enhancing the photosynthetic rate and carbohydrate metabolism in maize [33] and induced the NR activity, ATP sulfurylase and antioxidant enzymes in mungbean [34]. In Arabidopsis, SA application suppressed the adverse impact of salt stress by reducing the K+ leakage in root tissues and increasing the activity of H+-ATPase [35], which strengthens the Na+/H+ exchanger at the plasma membrane and reduces the Na+ accumulation in cytosol [65]. SA treatment also reduces lipid peroxidation and may interact with other plant hormones to enhance plant resistance and/or tolerance to salt stress [29, 31, 36, 37].
Soil salinity is common in the Rift Valley, Awash Valley and the lowland areas of Ethiopia [38]. It may become more severe in the following years due to lack of appropriate management practices and a growing interest toward a heavy-irrigation agriculture. In the highlands of Ethiopia, Brassica carinata (Ethiopian mustard) is the third most important oil crop after Guizotia abyssinica (noug) and Linum usitatissimum (linseed) [39, 40]. Among the oil crops of the common ecological niche, B. carinata gives the highest yield. Its leaves are used as vegetable, whereas seeds are used in preparing ‘Injera’, a local food item. The high erucic-acid content of seeds renders them useful for the biodiesel, biopolymers, lubricants, and soap and surfactants industries [41]. The plant displays inter-cultivar/line variation for tolerance to salinity. Twenty-five strains of B. carinata have been examined for salt tolerance at the germination and seedling stages [42], but the role of SA with reference to salt-stress effects in this species has not been investigated so far. The present study attempts to determine the salinity-caused changes in plant growth, leaf water status, photosynthetic efficiency and the enzymatic antioxidant system and then to evaluate the interactive effect of SA application with reference to these parameters in the Adet and Merawi cultivars of B. carinata.
Methods
Experimental set-up
The experiments were conducted at University of Gondar, located at 12°35′14.19″N, 37°26′29.53″E, at 2143 m above mean sea level. The annual average of the maximum and minimum temperature at Gondar lies ~ 27 and ~ 16 °C, respectively, whereas mean relative humidity (RH) and yearly precipitation are ~ 56% and 1161 mm, respectively. During the experimental period (March–April), RH was 50%, the maximum and the minimum daily temperature remained 29 ± 1 and 18 ± 1 °C, respectively, and no rainfall was observed during experimentation.
Seeds of Brassica carinata (A. Br.) cultivars Adet and Merawi (Fig. 1a, b) procured from Gondar Agricultural Research Centre were sterilized with ethanol (80%) for around 15 min, rinsed with distilled water, and then sown in plastic trays containing soil (75%) and farmyard manure (FYM 25%). After 2 weeks of germination, uniform-sized seedlings of each cultivar were shifted in plastic pots (25 cm width × 26 cm height) filled with 8.5 kg media (comprising of soil and FYM in 3:1 ratio) and sown at a depth of ~ 2 cm. Each plastic pot contained three seedlings (Fig. 1c, d). The potted seedlings were irrigated with tap water daily at 100% field capacity (FC) up to 2 weeks, which was considered as the acclimatization period. The sandy loam soil (62.56% sand, 14.88% clay and 22.56% silt) had a pH of 7.23 and an EC of 0.69 ms cm−1. After 4 weeks, a randomized design was adopted with 5 replications per treatment and 3 plants per replication for both the cultivars.
Salt treatment and foliar spray of salicylic acid
From the fifth week (after germination), the soil of each pot was treated with NaCl concentrations (0, 50, 100 and 150 mM) and watered regularly with 100% field capacity. Additionally, salicylic acid (0.5 mM) procured from SD Fine Chem Limited, Mumbai, India, was applied to the aerial plant parts four times (at one-week interval) starting from the fifth week after germination up to the eighth week. The pot soil was covered by a plastic sheet during the SA spray in order to avoid the access of SA solution to the root system. Thus, plants of each cultivar were grown under eight different treatments (T1 = no NaCl, no SA; T2 = 0 mM NaCl with 0.5 mM SA; T3 = 50 mM NaCl without SA; T4 = 50 mM NaCl with 0.5 mM SA; T5 = 100 mM NaCl without SA; T6 = 100 mM NaCl with 0.5 mM SA; T7 = 150 mM NaCl without SA; and T8 = 150 mM NaCl with 0.5 mM SA). NaCl dissolved in tap water was supplied (300 ml per pot) to the seedlings of T3–T8 on every second day, from the fifth week onward, while only tap water was used for T1 and T2. Sampling was done by uprooting the plants gently when they were 9 weeks old.
Measurement of growth variables
Plant growth attributes and some of the characteristic foliar features of both cultivars were recorded for each treatment, i.e., T1 to T8. The length of root and stem was measured in cm and opened leaves were counted. Ground-line basal diameter (mm) of the stem was measured with an electronic digital calliper. In addition, the length, width (each in mm) and area of leaf (mm2) were measured using Leaf Area Meter (AM 300, ADC Bio Scientific Limited, UK). The roots, stems and leaves of plants from each treatment were separated to measure their total dry mass with the help of electronic digital balance (CY510, Citizen Scale, Poland). Five replications were used for each parameter.
Measurement of chlorophyll concentration
Chlorophyll concentration in leaf tissues was estimated following the method of Hiscox and Israelstam [43]. Test tubes containing 0.5 g of green-leaf tissue in 7 ml of dimethyl sulfoxide (DMSO) were kept in oven at 65 °C for 1 h, and DMSO was added to this chlorophyll extract obtained from each sample of each cultivar in order to make a volume of 10 ml. Of this, 3 ml extract was transferred to polystyrene cuvettes and optical density (OD) recorded at 480, 510, 645 and 663 nm, using a T60 UV–Vis spectrophotometer (PG Instruments Limited, England). DMSO was used for the blank. The chlorophyll and carotenoid contents were obtained using the formulae given by Duxbury and Yentsch [44] and MaClachlan and Zalick [45], respectively.
Measurement of relative water content
Water status of leaves of both the cultivars was estimated for each treatment by measuring the relative water content (RWC) of fully developed leaves, which were weighed to obtain the fresh weight (FW) and then kept in distilled water for overnight at about 5 °C in the dark, before obtaining their turgid weight (TW). It was then oven-dried (80 °C) for 12 h and weighed again to obtain the dry weight (DW). RWC was calculated as:
Measurement of foliar functions
Chlorophyll fluorescence and gas exchange measurements were recorded in the morning (10–11 a.m.) from the first, second and third leaves of each cultivar, and means of the values were obtained. Chlorophyll fluorescence was recorded for each treatment by using a portable Multi-Mode OS5P Chlorophyll Fluorometer (Opti-Sciences, Inc., USA). Prior to fluorescence measurements, the upper surface of the leaf was pre-darkened for 30 min by using leaf clips to secure a complete relaxation of all the reaction centers. The basal non-variable chlorophyll fluorescence (Fo), maximal fluorescence induction (Fm), and variable fluorescence (Fv) were determined to calculate the maximum quantum yield of PSII efficiency (Fv/Fm) by using the formula Fv/Fm = (Fm − Fo)/Fm. Moreover, stomatal conductance (gs), net photosynthetic rate (Pn) and transpiration rate (E) were recorded from fully expanded attached leaves with the help of a portable leaf gas exchange system (ADC BioScientific Limited, U.K.). All these measurements were taken on 15 plants from each treatment and with 3 replicates for each plant. Water use efficiency (WUE) in the photosynthesizing leaf was worked out as the ratio of photosynthesis to water loss in transpiration, i.e., WUE = Pn/E.
Measurement of nitrate reductase activity and the proline and TBARS contents
Nitrate reductase (NR; E.C. 1.6.6.1) activity in the intact leaf tissue was estimated for each treatment by the method of Jaworski [46] and expressed in nMNO2 g−1 FW h−1. Leaf proline content for each treatment was estimated following the method of Bates et al. [47] and expressed in µg g−1 FW. The content of total 2-thiobarbituric acid reactive substances (TBARS) was determined by using the method of Cakmak and Horst [48], which involves the use of trichloroacetic acid (TCA) and thiobarbituric acid (TBA), and expressed in nmol g−1 FW.
Measurement of antioxidant enzymes
Leaf material (500 mg) from each cultivars and treatment was homogenized in the extraction buffer {0.5% Triton X-100 [C14H22O(C2H4O)n] and 1% polyvinylpyrrolidone [(C6H9NO)n] in 100 mM potassium phosphate buffer, pH 7.0}. Chilled mortar and pestle were used for preparing the crude extraction. The homogenate was then centrifuged at 15,000 rpm (20 min) at 4 °C, and the supernatant was used for antioxidant enzymes assays. Activity of catalase (CAT; E.C. 1.11.1.6) and superoxide dismutase (SOD; E.C. 1.15.1.1) was measured following a slightly modified method of Chandlee and Scandalios [49] and that of Beauchamp and Fridovich [50], respectively, and expressed in U mg−1 protein. Activity of peroxidase (POX; E.C. 1.11.1.7) was measured using guaiacol as the substrate, as described by Kumar and Khan [51] and modified by Husen [52], and expressed in U mg−1 protein.
Statistical analysis
Analysis of data was done by using the Statistical Package for Social Sciences (SPSS, Version 16.0) software (SPSS Inc., Illinois, USA). Two-way analysis of variance was performed to obtain the significant difference among treatments and cultivars. Significance of difference (P < 0.05) among the mean values was worked out by the Duncan test.
Results
The data regarding the effect of salinity (0, 50, 100 and 150 mM) and salicylic acid (SA) treatments (0 and 0.5 mM) on Adet and Merawi cultivars with reference to various growth attributes are given in Table 1. Salt stress markedly suppressed plant growth in a dose-dependent manner for both the cultivars at P < 0.05 level. High salt concentration (150 mM) showed a comparable effect in Adet and Merawi, as it reduced the root length by 47 and 49%, shoot length by 35 and 38%, number of leaves by 20 and 29%, leaf width by 17 and 19%, leaf length by 19 and 20% and leaf area by 28 and 27%, respectively. The roots of cv Adet were longer than those of cv Merawi under the control as well as saline conditions. The decrease in growth attributes was lesser in Adet than in Merawi. Further, all growth attributes of Adet cultivar except for the root length, basal diameter and leaf length were significantly enhanced by the foliar application of 0.5 mM SA. In the case of Merawi, all growth attributes except for the basal diameter and leaf length were significantly (P < 0.05 level) increased on application of 0.5 mM SA, compared to the control. Further, SA application on salt-stressed condition alleviated the effect of salinity and improved all the growth traits significantly (P < 0.05 level), compared to controls for both the cultivars.
There was a higher biomass production in roots, stem, leaves and the whole plant of the controls, compared to plants treated with salt or SA. Of the various salt concentrations, the highest dose (150 mM) was most effective in reducing the plant biomass production for both the cultivars. In comparison with the controls, the reduction in roots, stem, leaves and whole plant biomass production at 150 mM was up to 54, 46, 41 and 45%, respectively, in cv Adet and up to 55, 49, 42 and 46%, respectively, in cv Merawi. On the whole, Adet was better than Merawi in terms of biomass production. In both cultivars, foliar application of 0.5 mM SA significantly increased (at P < 0.05) the biomass production in all plant parts except roots, compared to the control. It also alleviated the effect of salinity significantly, and enhanced the biomass production of all plant parts in the salt-stressed plants of both the cultivars (Table 2).
Compared with the control, the quantity of leaf pigments (chlorophyll and carotenoids) declined with increase in the salinity level. At the highest concentration (150 mM) used, chlorophylls a and b, total chlorophyll and carotenoids declined by 57, 49, 48 and 50%, respectively in cv Adet, and by 55, 49, 53 and 58%, respectively, in cv Meravi, as compared to the control. The chlorophyll b and carotenoid contents were significantly higher in Adet than in Merawi. Compared with the control, SA application (0.5 mM) to cv Adet caused a significant increase of 5, 10 and 21% in the chlorophyll b, total chlorophyll and carotenoids contents, respectively, while chlorophyll a content increased only nonsignificantly. In cv Merawi, however, only the total chlorophyll increased significantly (by about 7%) over the control. Furthermore, foliar application of SA on salt-stressed plants markedly reduced the damage caused by salinity to chlorophylls a and b, total chlorophyll and carotenoids; thus, T8 showed a significant (P < 0.05 level) improvement over T7 in both the cultivars (Table 3).
Salt-stress treatments reduced the physiological efficiency of leaves in both the cultivars in comparison with the respective controls. The degree of reduction of RWC was high (around 26%) for both the cultivars at 150 mM salt treatment. However, foliar application of SA (0.5 mM) on salt-stressed plants significantly reduced the salinity-induced loss in both the cultivars (Table 4). Furthermore, in general, chlorophyll fluorescence (Fv/Fm), stomatal conductance (gs), net photosynthetic rate (Pn), transpiration rate (E) and water use efficiency (WUE) were reduced in salinity-affected plants. In both cultivars, the degree of reduction in Fv/Fm, gs, Pn, E and WUE was increased with increase in the level of salinity. At 150 mM, cv Adet exhibited a decrease of 23% in Fv/Fm, 27% in gs, 38% in Pn, 30% in E and 11% in WUE in comparison with the control, whereas these parameters declined by 24, 24, 42, 27 and 21%, respectively, in cv Merawi. The values of all these parameters except gs were higher in Adet than in Merawi cultivar. SA application increased Pn, compared to the control plants, and also alleviated significantly the salinity-induced reduction in Fv/Fm, gs, Pn, E and WUE in both the cultivars (Table 4).
NR activity was reduced under salinity stress; the degree of reduction increasing with the increase in salinity level. Thus, at 150 mM salt concentration, it declined by ~ 19% in both the cultivars. On the contrary, compared to the control, it increased by 6% in Adet and by a nonsignificant 2% in Merawi due to SA application. In general, Adet showed a higher NR activity than Merawi. In both these cultivars, SA application significantly (P < 0.05 level) reduced the salinity-induced decline of NR activity in the salt-stressed plants (Fig. 2a).
Effect of salicylic acid treatments on the a nitrate reductase, b proline and c lipid peroxidation levels in the leaves of B. carinata cultivars grown under salt stress (where T1 = control; T2 = 0 mM NaCl with 0.5 mM SA; T3 = 50 mM NaCl without SA; T4 = 50 mM NaCl with 0.5 mM SA; T5 = 100 mM NaCl without SA; T6 = 100 mM NaCl with 0.5 mM SA; T7 = 150 mM NaCl without SA; and T8 = 150 mM NaCl with 0.5 mM SA). Each value represents the mean ± SE of five replicates
The contents of proline and TBARS increased significantly under salinity stress in both the cultivars in a dose-dependent manner (Fig. 2b, c). Thus, compared to the control plants, the proline and TBARS contents increased by 16 and 57%, respectively, in cv Adet, and up to 15 and 53%, respectively, in cv Merawi under 150 mM salt concentration. The level of increase of both proline and TBARS was higher in Adet than in Merawi. These salinity-induced increases were reduced greatly by SA application (P < 0.05), in both the cultivars (Fig. 2b, c).
The activity of antioxidant enzymes, viz. superoxide dismutase (SOD), catalase (CAT) and peroxidase (POX) increased with increasing salt stress in both the cultivars and went up to 29, 27 and 179%, respectively, in cv Adet, and up to 32, 25 and 194% in cv Merawi, respectively, with 150 mM salt treatment, in comparison with the control. The CAT and POX activity was higher in Adet, while SOD went ahead in Merawi. Moreover, SA application significantly decreased the salinity-induced increase in the activity of SOD, CAT and POX enzymes in both the cultivars, as shown in Fig. 3.
Effect of salicylic acid treatments on antioxidant enzymes, viz. a superoxide dismutase, b catalase and c peroxidase in the leaves of B. carinata cultivars grown under salt stress (where T1 = control; T2 = 0 mM NaCl with 0.5 mM SA; T3 = 50 mM NaCl without SA; T4 = 50 mM NaCl with 0.5 mM SA; T5 = 100 mM NaCl without SA; T6 = 100 mM NaCl with 0.5 mM SA; T7 = 150 mM NaCl without SA; and T8 = 150 mM NaCl with 0.5 mM SA). Each value represents the mean ± SE of five replicates
Discussion
The present study indicates that the salinity-induced loss of the growth and biomass of B. carinata is relatively stronger in cultivar Merawi than in Adet. The saline environment in the soil influences water imbibition by roots due to low osmotic potential of the substrate, besides hampering the phenomena of photosynthesis, protein synthesis, nutrient homeostasis, compatible solutes accumulation and the antioxidant defense mechanisms [5, 8, 25, 53]. The salinity-caused decline in growth and biomass of B. carinata cultivars might be due to reduced leaf area, imbalance in plant water status and low production of photoassimilates [3,4,5, 54]. The beneficial role of various plant hormones, including salicylic acid (SA), in signaling network, and in the developmental and adaptation processes of plant species against the biotic and abiotic stresses has long been known. SA application significantly improved the growth attributes in B. carinata, as observed earlier in maize [33], barley [55], mungbean [56] and mustard [57].
The salt-induced decline in the chlorophylls a and b, total chlorophyll and carotenoids contents in B. carinata is likely to be due to the oxidation of chlorophyll and other chloroplast pigments and the instability of pigment-protein complex under the influence of salinity [58]. The positive influence of SA application substantiates the early findings with certain crops including soybean [59], maize [33] and sunflower [60]. This could possibly involve stimulation of Rubisco activity and leaf pigment biosynthesis.
Relative water content (RWC) of leaf indicates the physiological water status of plants. In our study, the decreased RWC in both the cultivars under increased salinity is indicative of a loss of cell turgor that leads to a limited water availability for the cell extension and expansion. Moreover, the increase in leaf RWC in response to SA application could possibly be an adaptive symptom to improve the degree of moistness and sustain the water balance in plant tissues under the salinity-induced osmotic stress [61, 62].
As the plant growth is intimately linked to the rate of photosynthesis, any decline in growth due to salt stress is attributable to the suppression of photosynthesis [3, 56, 63, 64]. The dose-dependent salinity-induced reductions in gs, Pn, E and WUE differ considerably between the cultivars, possibly due to their differential optimum requirement of photoassimilates for healthy growth, whereas the alleviative effect of SA might be due to its positive role in chlorophyll biosynthesis and/or nitrate mobilization in the tissue [65]. SA also stimulates Rubisco activity [33]. Ashraf et al. [66] found the influence of SA on photosynthesis to be concentration-dependent; low concentrations (less than 10 µM) mitigated the salinity-induced decline in photosynthesis in various plant species. In Brassica juncea also, SA alleviated the adverse effects of salinity and improved Pn and plant growth by enhancing the enzymes action in ascorbate–glutathione pathway, thus suggesting its role in maintaining the redox balance under salt stress [57]. Arabidopsis mutant with high endogenous SA concentration (siz1) exhibited reduced stomatal apertures and increased salt tolerance [67]. Our observations, showing a dose-dependent decline under salt stress and a rise due to SA treatment, find support from some early reports on tomato [64], Torreya grandis [61] and Vicia faba [16]. The decline of E and WUE under salt stress and their restoration by SA application in both cultivars also go in line with earlier investigation on vegetable crops [64, 68]. Salinity often alters water balance and thus reduces the WUE [3, 69], possibly due to inhibition of water absorption by roots and water translocation from roots to aerial plant parts.
The reduction in the photochemical efficiency of PSII (Fv/Fm) under stressful environment, duly linked with a decline of photosynthetic attributes, including leaf pigments and biomass production, has been used as an indicator for determining the seedling-stock quality [19, 70]. The dose-dependent decrease in Fv/Fm, as recorded in this study indicates that salinity affects the photochemistry of photosynthesis [3, 71]. Reduction in Fv/Fm ratio and a non-photochemical quenching coefficient (qN) under salt stress, and their restoration by SA treatment, were also observed in tomato plants [64]. Nevertheless, Asensi-Fabad and Munné-Bosch [72] have reported that under the salt-stress condition, the SA-deficient and SA-hyperaccumulating Arabidopsis mutants exhibited only insignificant difference in chlorophyll contents and the Fv/Fm ratio.
Nitrate reductase (NR) limits the reaction rate during nitrogen assimilation and hence is important for metabolic regulation and protein synthesis. NR activity was reduced by salinity stress in both the cultivars and increased by SA application due to mitigation of salinity-induced effects possibly by stabilizing the plasma membrane, as also observed in wheat [73]. This, in turn, could enhance the uptake of nutrients including nitrate, which induces NR [74].
In general, elevated levels of TBARS content, a product of lipid peroxidation, indicate the damage caused by free radicals to cell membranes that leads to oxidative stress. Our data depict a salt-concentration-dependent increase in the TBARS content in both the cultivars. High levels of H2O2 possibly damage the membrane, which expedites the generation of hydroxyl radicals and thus leads to lipid peroxidation [75]. SA application was ameliorative, possibly through improved membrane functioning, but the cultivar sensitivity to oxidative stress varied.
Proline detoxifies the excess ROS, improves the osmotic adjustment, lends protection to biological membranes and also stabilizes enzymes and proteins [22, 76]. The leaf proline content increased substantially with increase in salinity but SA application mitigates this impact in both the cultivars studied. Misra and Misra [77] have reported that the up-regulation of proline biosynthesis enzymes (viz. pyrroline-5-carboxylate reductase and γ-glutamyl kinase) and the down-regulation of proline oxidase activity led to an enhanced proline status, which helped in maintaining the cell turgor under salinity stress in Rauwolfia serpentina.
Activation of antioxidant enzymes is a vital strategy adopted by various plants to combat the ROS-induced oxidative damage and increase the stress tolerance. In our study, expression of antioxidant enzymes (SOD, CAT and POX) increased under salt stress in a dose-dependent manner. SA treatment to normal or salinity-stressed plants increased the enzymes activity, showing that SA can have a crucial role in modulating the cell redox balance and protecting the given plants from oxidative damage. The increased SOD activity facilitated the superoxide radical scavenging, which led to increased plant tolerance to oxidative stress. Increase in CAT and POX activity due to salinity as well as SA was also reported by Jini and Joseph [37]. SA pre-treatment mitigates the negative influences of salinity on photosynthesis and plant growth by strengthening the antioxidant system [56], whereas SA deficiency can facilitate the salinity-induced damage and suppress the antioxidant activities, as observed in NahG transgenic of Arabidopsis lines [78]. Li et al. [23] also observed the SA-induced enhanced salt tolerance in wheat through an improved transcript level of antioxidant genes such as GPX1, GPX2, DHAR, GR, GST1, GST2, MDHAR and GS, and a higher activity of the ascorbate (AsA)-GSH pathway enzymes.
Conclusion
Analysis of the data on growth features, photosynthetic efficiency and defense status of the two cultivars of B. carinata has brought out that growth performance of cv Adet was better than that of cv Merawi in terms of size as well as biomass of both root and shoot under salinity stress. Although differences in relative water content, chlorophyll fluorescence and stomatal conductance were nonsignificant, the chlorophyll contents, net photosynthetic rate and water use efficiency were markedly less affected by salinity in cv Adet. Likewise, although NR activity was almost similar and lipid peroxidation in terms of TBARS content was a little more in cv Adet, larger proline content and better modulation of antioxidant enzymes seemingly overcame the adverse impact of stress and displayed a better tolerance capacity and improved the growth of cv Adet, compared to cv Merawi. SA application mitigated the impact of salinity in both the cultivars studied, but was relatively more effective in cultivar Adet. A summarized impact of salinity and the role of SA in stress mitigation is presented in Fig. 4.
References
FAO. 2050: A third more mouths to feed. 2009. http://www.fao.org/news/story/en/item/35571/icode/.
Park HJ, Kim WY, Yun DJ. A new insight of salt stress signaling in plant. Mol Cell. 2016;39:447–59.
Husen A, Iqbal M, Aref IM. IAA-induced alteration in growth and photosynthesis of pea (Pisum sativum L.) plants grown under salt stress. J Environ Biol. 2016;37:421–9.
Husen A, Iqbal M, Aref IM. Plant growth and foliar characteristics of faba bean (Vicia faba L.) as affected by indole-acetic acid under water-sufficient and water-deficient conditions. J Environ Biol. 2017;38:179–86.
Albaladejo I, Meco V, Plasencia F, Flores FB, Bolarin MC, Egea I. Unravelling the strategies used by the wild tomato species Solanum pennellii to confront salt stress: from leaf anatomical adaptations to molecular responses. Environ Exp Bot. 2017;135:1–12.
Negrão S, Schmöckel SM, Tester M. Evaluating physiological responses of plants to salinity stress. Ann Bot. 2017;119:1–11.
Munns R, Tester M. Mechanisms of salinity tolerance. Ann Rev Plant Biol. 2008;59:651–81.
Qureshi MI, Abdin MZ, Ahmad J, Iqbal M. Effect of long-term salinity on cellular antioxidants, compatible solute and fatty acid profile of Sweet annie (Artemisia annua L.). Phytochemistry. 2013;95:215–23.
Srivastava AK, Srivastava S, Lokhande VH, D’Souza SF, Suprasanna P. Salt stress reveals differential antioxidant and energetics responses in glycophyte (Brassica juncea L.) and halophyte (Sesuvium portulacastrum L.). Front. Environ Sci. 2015;3(19):9.
Aref IM, Khan PR, Khan S, El-Atta H, Ahmed AI, Iqbal M. Modulation of antioxidant enzymes in Juniperus procera needles in relation to habitat environment and dieback incidence. Trees Struct Funct. 2016;30:1669–81.
Zhang M, Smith JAC, Harberd NP, Jiang C. The regulatory roles of ethylene and reactive oxygen species (ROS) in plant salt stress responses. Plant Mol Biol. 2016;91:651–9.
Choudhury FK, Rivero RM, Blumwald E, Mittler R. Reactive oxygen species, abiotic stress and stress combination. Plant J. 2017;90:856–67.
Sekmen AH, Türkan I, Takio S. Differential responses of antioxidative enzymes and lipid peroxidation to salt stress in salt-tolerant Plantago maritima and salt-sensitive Plantago media. Physiol Plant. 2007;131:399–411.
Koyro HW, Hussain T, Huchzermeyer B, Khan MA. Photosynthetic and growth responses of a perennial halophytic grass Panicum turgidum to increasing NaCl concentrations. Environ Exp Bot. 2013;91:22–9.
Husen A, Iqbal M, Khanum N, Aref IM, Sohrab SS, Meshresa G. Modulation of salt-stress tolerance of Guizotia abyssinica by application of salicylic acid. J Environ Biol. 2019;40 (in press).
Hussein M, Embiale A, Husen A, Aref IM, Iqbal M. Salinity-induced modulation of plant growth and photosynthetic parameters in faba bean (Vicia faba) cultivars. Pak J Bot. 2017;49:867–77.
Chen TH, Murata N. Glycinebetaine protects plants against abiotic stress: mechanisms and biotechnological applications. Plant, Cell Environ. 2011;34:1–20.
Baker NR. Chlorophyll fluorescence: a probe of photosynthesis in vivo. Ann Rev Plant Biol. 2008;59:89–113.
Getnet Z, Husen A, Fetene M, Yemata G. Growth, water status, physiological, biochemical and yield response of stay green sorghum Sorghum bicolor (L.) Moench varieties: a field trial under drought-prone area in Amhara Regional State, Ethiopia. J Agron. 2015;14:188–202.
Embiale A, Hussein A, Husen A, Sahile S, Mohammed K. Differential sensitivity of Pisum sativum L. cultivars to water-deficit stress: changes in growth, water status, chlorophyll fluorescence and gas exchange attributes. J Agron. 2016;15:45–57.
Husen A, Iqbal M, Aref IM. Growth, water status and leaf characteristics of Brassica carinata under drought and rehydration conditions. Braz J Bot. 2014;37:217–27.
Husen A. Growth characteristics, physiological and metabolic responses of teak (Tectona grandis Linn.f.) clones differencing in rejuvenation capacity subjected to drought stress. Silva Gene. 2010;59:124–36.
Li G, Peng X, Wei L, Kang G. Salicylic acid increases the contents of glutathione and ascorbate and temporally regulates the related gene expression in salt stressed wheat seedlings. Gene. 2013;529:321–5.
Wang Y, Zhang H, Hou P, Su X, Zhao P, Zhao H, Liu S. Foliar-applied salicylic acid alleviates heat and high light stress induced photoinhibition in wheat (Triticum aestivum) during the grain filling stage by modulating the psbA gene transcription and antioxidant defense. Plant Grow Regul. 2014;73:289–97.
Yousuf PY, Ahmad A, Aref IM, Ozturk M, Ganie AH, Iqbal M. Salt-stress-responsive chloroplast proteins in Brassica juncea genotypes with contrasting salt tolerance and their quantitative PCR analysis. Protoplasma. 2015;253:1565–75.
Wani SH, Kumar V, Shriram V, Sah SK. Phytohormones and their metabolic engineering for abiotic stress tolerance in crop plants. Crop J. 2016;4:162–76.
Siddiqi KS, Husen A. Plant response to strigolactones: current developments and emerging trends. Appl Soil Ecol. 2017;120:247–53.
Fahad S, Hussain S, Matloob A, Khan FA, Khaliq A, Saud S, Hassan S, Shan D, Khan F, Ullah N, Faiq M, Khan MR, Tareen AK, Khan A, Ullah A, Ullah N, Huang J. Phytohormones and plant responses to salinity stress: a review. Plant Grow Regul. 2014;75:391–404.
Jayakannan M, Bose J, Babourina O, Rengel Z, Shabala S. Salicylic acid in plant salinity stress signalling and tolerance. Plant Grow Regul. 2015;76:25–40.
Khan MIR, Fatma M, Per TS, Anjum NA, Khan NA. Salicylic acid-induced abiotic stress tolerance and underlying mechanisms in plants. Front Plant Sci. 2015;6:462.
Gharbi E, Martínez JP, Benahmed H, Fauconnier ML, Lutts S, Quinet M. Salicylic acid differently impacts ethylene and polyamine synthesis in the glycophyte Solanum lycopersicum and the wild-related halophyte Solanum chilense exposed to mild salt stress. Physiol Plant. 2016;158:152–67.
Hao JH, Dong CJ, Zhang ZG, Wang XL, Shang QM. Insights into salicylic acid responses in cucumber (Cucumis sativus L.) cotyledons based on a comparative proteomic analysis. Plant Sci. 2012;187:69–82.
Khodary SEA. Effect of salicylic acid on the growth, photosynthesis and carbohydrate metabolism in salt stressed maize plants. Int J Agri Biol. 2004;6:5–8.
Nazar R, Iqbal N, Syeed S, Khan NA. Salicylic acid alleviates decreases in photosynthesis under salt stress by enhancing nitrogen and sulfur assimilation and antioxidant metabolism differentially in two mungbean cultivars. J Plant Physiol. 2011;168:807–15.
Jayakannan M, Bose J, Babourina O, Rengel Z, Shabala S. Salicylic acid improves salinity tolerance in Arabidopsis by restoring membrane potential and preventing salt-induced K+ loss via a GORK channel. J Exp Bot. 2013;64:2255–68.
Horváth E, Brunner S, Bela K, Papdi C, Szabados L, Tari I, Csiszár J. Exogenous salicylic acid-triggered changes in the glutathione transferases and peroxidases are key factors in the successful salt stress acclimation of Arabidopsis thaliana. Funct Plant Biol. 2015;42:1129–40.
Jini D, Joseph B. Physiological mechanism of salicylic acid for alleviation of salt stress in rice. Rice Sci. 2017;24:97–108.
Tsige H, Gebrasellasie T, Mamo T. Assessment of salinity/sodicity problems in Abaya State farm, Southern Rift Valley of Ethiopia. Ethiop J Nat Res. 2000;2:151–63.
CSA (Central Statistics Authority). Agriculture sample survey 2002/2003. Report on area and production for major crops. Addis Ababa: Statistical Bulletin 200, CSA; 2003.
Husen A, Mishra VK, Semwal K, Kumar D. Biodiversity status in Ethiopia and challenges. In: Bharati KP, Chauhan A, Kumar P, editors. Environmental pollution and biodiversity, vol. 1. New Delhi: Discovery Publishing House Pvt Ltd.; 2012. p. 31–79.
Velasco L, Nabloussi A, De Haro A, Fernández-Martínez JM. Development of high-oleic, low-linolenic acid Ethiopian-mustard (Brassica carinata) germplasm. Theor App Genet. 2003;107:823–30.
Ashraf M, Sharif R. Inter-cultivar variation for salt (NaCl) tolerance in a potential oil-seed crop ethiopian mustard (Brassica carinata A. Br.). Arch Agron. Soil Sci. 1997;42:129–36.
Hiscox JD, Israelstam GF. A method for the extraction of chlorophyll from leaf tissue without maceration. Canadian J Bot. 1979;57:1332–4.
Duxbury AC, Yentsch CS. Plankton pigment monographs. J Mar Res. 1956;15:91–101.
MacLachlan S, Zalik S. Plastid structure, chlorophyll concentration and free amino acid composition of a chlorophyll mutant of barely. Can J Bot. 1963;41:1053–60.
Jaworski EG. Nitrate reductase assay in intact plant tissue. Biochem Biophysi Res Commun. 1971;43:1274–9.
Bates LS, Waldren RP, Teare ID. Rapid determination of free proline for water stress studies. Plant Sci. 1973;39:205–7.
Cakmak I, Horst J. Effect of aluminium on lipid peroxidation, superoxide dismutase, catalase and peroxidase activities in root tips of soybean (Glycine max). Physiol Plant. 1991;83:463–8.
Chandlee JM, Scandalios JG. Analysis of variants affecting the catalase development program in maize scutellum. Theor App Genet. 1984;69:71–7.
Beauchamp CO, Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971;44:276–87.
Kumar KB, Khan PA. Peroxidase and polyphenol oxidase in excised ragi (Eleusine coracana cv. PR 202) leaves during senescence. Ind J Exp Bot. 1982;20:412–6.
Husen A. Clonal propagation of Dalbergia sissoo Roxb. and associated metabolic changes during adventitious root primordium development. New For. 2008;36:13–27.
Yousuf PY, Ahmad A, Ganie AH, Iqbal M. Salt stress-induced modulations in the shoot proteome of Brassica juncea genotypes. Environ Sci Pollut Res. 2015;23:2391–401.
Bagheri R, Bashir H, Ahmad J, Iqbal M, Qureshi MI. Spinach (Spinacia oleracea L.) modulates its proteome differentially in response to salinity, cadmium and their combination stress. Plant Physiol Biochem. 2015;97:235–45.
El-Tayeb MA. Response of barley grains to the interactive effect of salinity and salicylic acid. Plant Grow Regul. 2005;45:215–24.
Khan MIR, Asgher M, Khan NA. Alleviation of salt-induced photosynthesis and growth inhibition by salicylic acid involves glycinebetaine and ethylene in mungbean (Vigna radiata L.). Plant Physiol Biochem. 2014;80:67–74.
Nazar R, Umar S, Khan NA, Sareer O. Salicylic acid supplementation improves photosynthesis and growth in mustard through changes in proline accumulation and ethylene formation under drought stress. South Afr J Bot. 2015;98:84–94.
Stepien P, Klobus G. Water relations and photosynthesis in Cucumis sativus L. leaves under salt stress. Biol Plant. 2006;50:610–6.
Zhao HJ, Lin XW, Shi HZ, Chang SM. The regulating effect of phenolic compounds on the physiological characteristics and yield of soybeans. Acta Agron Sin. 1995;21:351–5.
Noreen S, Ashraf M, Akram NA. Does exogenous application of salicylic acid improve growth and some key physiological attributes in sunflower plants subjected to salt stress? J Appl Bot Food Qual. 2011;84:169–77.
Li T, Hu Y, Du X, Tang H, Shen C, Wu J. Salicylic acid alleviates the adverse effects of salt stress in Torreya grandis cv. Merrillii seedlings by activating photosynthesis and enhancing antioxidant systems. PLoS ONE. 2014;9(10):e109492.
Rady MM, Mohamed GF. Modulation of salt stress effects on the growth, physio-chemical attributes and yields of Phaseolus vulgaris L. plants by the combined application of salicylic acid and Moringa oleifera leaf extract. Sci Hortic. 2015;193:105–13.
Syeed S, Anjum NA, Nazar R, Iqbal N, Masood A, Khan NA. Salicylic acid-mediated changes in photosynthesis, nutrients content and antioxidant metabolism in two mustard (Brassica juncea L.) cultivars differing in salt tolerance. Acta Physiol Plant. 2011;33:877–86.
Mimouni H, Wasti S, Manaa A, Gharbi E, Chalh A, Vandoorne B, Lutts S, Ahmed HB. Does salicylic acid (SA) improve tolerance to salt stress in plants? A study of SA effects on tomato plant growth, water dynamics, photosynthesis, and biochemical parameters. OMICS J Integr Biol. 2016;20:180–90.
Shi Q, Bao Z, Zhu Z, Ying Q, Qian Q. Effects of different treatments of salicylic acid on heat tolerance, chlorophyll fluorescence, and antioxidant enzyme activity in seedlings of Cucumis sativa L. Plant Grow Regul. 2006;48:127–35.
Ashraf M, Akram NA, Arteca RN, Foolad MR. The physiological, biochemical and molecular roles of brassinosteroids and salicylic acid in plant processes and salt tolerance. Crit Rev Plant Sci. 2010;29:162–90.
Miura K, Okamoto H, Okuma E, Shiba H, Kamada H, Hasegawa PM, Murata Y. SIZ1 deficiency causes reduced stomatal aperture and enhanced drought tolerance via controlling salicylic acid induced accumulation of reactive oxygen species in Arabidopsis. Plant J. 2013;73:91–104.
Sajjad M, Siddiqi EH, Bhatti KH, Nawaz K, Hussain K, Talat K, Anxar S, Munir M, Afzal A. Foliar application of salicylic acid as potent inducer of salt tolerance radish (Raphanus sativus L.). Middle East J Sci Res. 2013;14:1098–102.
Huez-López MA, Ulery AL, Samani Z, Picchioni G, Flynn RP. Response of chile pepper (Capsicum annuum L.) to salt stress and organic and inorganic nitrogen sources: II. Nitrogen and water use efficiencies, and salt tolerance. Trop Subtrop Agroecosys. 2011;14:757–63.
Husen A. Growth characteristics, biomass and chlorophyll fluorescence variation of Garhwal Himalaya’s fodder and fuel wood tree species at the nursery stage. Open J For. 2013;3:12–6.
Kalaji HM, Bosa K, Kościelniak J, Źuk-Gołaszewskae K. Effects of salt stress on photosystem II efficiency and CO2 assimilation of two Syrian barley landraces. Environ Exp Bot. 2011;73:64–72.
Asensi-Fabado MA, Munne-Bosch S. The aba3-1 mutant of Arabidopsis thaliana withstands moderate doses of salt stress by modulating leaf growth and salicylic acid levels. J Plant Grow Regul. 2011;30:456–66.
Agarwal S, Sairam RK, Srivastava G, Meena R. Changes in antioxidant enzyme activity and oxidative stress by abscisic acid and salicylic acid in wheat genotypes. Biol Plant. 2005;49:541–50.
Campbell WH. Nitrate reductase structure, function and regulation: bridging the gap between biochemistry and physiology. Ann Rev Plant Physiol Plant Mol Biol. 1999;5:277–303.
Mittler R. Oxidative stress, antioxidants and stress tolerance. Trend Plant Sci. 2002;7:405–10.
Iqbal N, Umar S, Khan NA, Khan MIR. A new perspective of phytohormones in salinity tolerance: regulation of proline metabolism. Environ Exp Bot. 2014;100:34–42.
Misra N, Misra R. Salicylic acid changes plant growth parameters and proline metabolism in Rauwolfia serpentina leaves grown under salinity stress. Am Eurasian J Agric Environ Sci. 2012;12:1601–9.
Cao WH, Liu J, He XJ, Mu RL, Zhou HL, Chen SY, Zhang JS. Modulation of ethylene responses affects plant salt-stress responses. Plant Physiol. 2007;143:707–19.
Authors’ contributions
AH made major contribution to data collection, experimental work and drafted the manuscript. AH and MI wrote and reviewed the manuscript. SS and MKAA assisted in enzyme assays and statistical analyses, respectively. All authors read and approved the final manuscript.
Acknowledgements
The authors are thankful to Gondar Agricultural Research Centre and the departments of Biology and Chemistry at University of Gondar, Ethiopia, for providing authentic seeds, laboratory/nursery assistance and chemicals, respectively.
Competing interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Availability of data and materials
The datasets supporting the results of this article are included in the article.
Consent to publish
Not applicable.
Ethics approval and consent to participate
Not applicable.
Funding
Not applicable.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Husen, A., Iqbal, M., Sohrab, S.S. et al. Salicylic acid alleviates salinity-caused damage to foliar functions, plant growth and antioxidant system in Ethiopian mustard (Brassica carinata A. Br.). Agric & Food Secur 7, 44 (2018). https://doi.org/10.1186/s40066-018-0194-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40066-018-0194-0